Indexed In
- Open J Gate
- Academic Keys
- JournalTOCs
- ResearchBible
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2020) Volume 8, Issue 1
Psychosomatic Health in Elderly People for Preventing Frailty: The role of IGF-1 and BDNF in the Muscle and the Brain
Mitsugu Hachisu1,2*, Masayuki Obayashi2, Mari Kogo3 and Kazushige Ihara1,22Showa University Research Institute for Sport and Exercise Sciences, Yokohama, Japan
3Department of Social Medicine, Hirosaki University, School of Medicine, Hirosaki, Japan
Received: 19-Mar-2020 Published: 16-Apr-2020, DOI: 10.35248/2329-8847.20.08.223
Abstract
The elderly population of people aged > 65 years is rapidly growing up in developed countries, including Japan, and becoming a burden by increasing caregiving and social security costs. Prolonging a healthy life span without caregiver help would reduce the social burden. Frailty in elderly people can cause accidental falls causing them to be bed ridden, but constant exercise could help prevent such accidents. Various exercises enhance the synthesis and release of neurotrophic factors such as IGF-1 and BDNF; these neurotrophic factors enhance neuronal growth and survival in the brain prevent muscle atrophy and sometimes contribute to muscle hypertrophy in elderly people. These exercise-induced neurotrophic factors can thereby help prevent the lowering of cognitive performance, processing speed and mood in elderly people including those with Alzheimer’s disease or Parkinson’s disease.
In this review, we emphasize that IGF-1 and BDNF work as anabolic growth factors for skeletal muscle, play a role in the neurogenesis, synaptogenesis, and neuronal survival in the brain, ameliorate cognition, and stabilize psychiatric mood. IGF-1, further, has activities in the elimination of amyloid-β protein by activating protein transportation at the choroid plexus and angiogenesis acting on brain vessels. Thus, elderly people are recommended to exercise regularly for their health and avoid the condition of frailty.
Keywords
Elderly people; Muscle; Exercise; Insulin-like growth factor 1; Brain-derived neurotrophic factor
Introduction
The elderly population of people aged > 65 years is growing in developed countries, including Japan, and the mean life expectancy has increased to more than 80 years. With a prolonged life expectancy, being independent of caregivers is beneficial to one’s well-being and reduces the social burden. Extending a healthy life expectancy may be accomplished by a good lifestyle that includes a healthy diet, exercising, low stress, and positive thinking.
Exercise promotes neurotrophic factors such as brain-derived neurotrophic factor (BDNF) and insulin-like growth factor 1 (IGF-1) [1,2]. Neurotrophic factors mediate the prevention of muscle mass deterioration, as well as the deterioration of cognitive function, by enhancing neuronal survival and growth. BDNF is a protein with a molecular weight of 12,300 Da, consisting 119 amino acids [3] originally purified from the porcine brain, and its expression is increased in the brain by exercise [4].
IGF-1 has a molecular weight of 7,600 Da protein and consists of 70 amino acids produced by the stimulation of growth hormone in the liver [5,6]. Further, it is produced in the muscle by exercise and has activities promoting the proliferation and differentiation of myoblasts [7], leading to muscle hypertrophy.
We have reported a relationship between serum BDNF levels and quadriceps muscle thickness in 805 elderly healthy people aged 65–84 years, as investigated by the health examinations of people residing in the Tokyo area [8]. Although we did not have data regarding the exercise habits or other lifestyle factors and their effect on the thickness of quadriceps in the report, it is assumed that subjects having thick quadriceps do exercise or have a more active life than their counterparts. Wasting of the skeletal muscle mass with aging is often attributable to a decrease in the secretion of growth factors and steroid hormones [9]. Decrease of muscle mass by aging is more prominent in the lower limbs [10], and individuals with weak lower limbs tend to do less walking or stair climbing. The function of gaiting or climbing up and down the stairs, which uses the lower limb muscles, is more rapidly aggravated by aging than other functions [11,12]. The deterioration of the lower limb muscles tends to lead to frailty with decreasing muscle mass, body weight, hand grip strength, and deterioration of cognitive function. Recommendations for preventing frailty include consuming proper nutrition and exercising in order to increase neurotrophic factors such as BDNF and IGF-1, which affect muscle mass and neural cognitions.
In this paper, we have discussed the roles of the neurotrophic factors, BDNF and IGF-1, and exercises and nutrition in promoting a healthy life in elderly people by preventing frailty.
Physiology of BDNF and IGF-1 proteins
BDNF is processed from precursor of BDNF (pro-BDNF) in Golgi bodies in cells. BDNF and its receptor, tropomyosin-related receptor kinase B (TrkB), are present in the brain tissue including the hippocampus and are involved in neurogenesis, neuronal survival, and synaptic plasticity [13].
The pro-BDNF protein also has an activity on neurons, but the actions are contrary to those of BDNF: the BDNF/TrkB pathway facilitates persisting activity, whereas the pro-BDNF/ pan-neurotrophin receptor p75 (p75NTR) pathway inhibits the mnemonic property of entorhinal pyramidal neurons [14]. Further, Sun et al. [15] reported that pro-BDNF inhibits neurite outgrowth (synaptogenesis) of primary cultured dorsal root ganglion (DRG) and cortical neurons of rats in vitro and the effect was reversed by the anti-pro-BDNF antibody.
BDNF was purified from the porcine brain by Barde et al. as a neurotrophic factor, such as nerve growth factor (NGF), and is active in the neuronal functioning of the brain [3]. BDNF mRNA is distributed in all brain areas including the hippocampus, cerebral cortex, medial preoptic area, pontine nuclei, thalamus, hypothalamus, and olfactory bulb [16]. Lower serum BDNF levels are found in those with depression and Alzheimer’s disease in humans, but they may be restored by the treatment of medicines [17,18]. Malberg et al. showed an increase of neurogenesis in the adult rat hippocampus by a selective serotonin reuptake inhibitor (SSRI), suggesting the upregulation of activity or expression of BDNF by SSRIs [19]. Chronic antidepressant treatment increases neurogenesis in the adult rat hippocampus. Similarly, it has been reported that the anti-Alzheimer’s drug, donepezil, enhances the survival of newborn neurons in the DG of the adult rat hippocampus and also shows an increase in the phosphorylation of the cAMP response element binding protein (CREB), suggesting the role of BDNF [20]. However, stress inhibits neurogenesis paralleled with reduced BDNF expression, inducing depression/ anxiety-like behavior in mice [21].
Duman [22] showed that animals housed in an enriched environment with low stress have higher BDNF concentrations in the brain than animals housed in normal cages. The enriched environment consisted of tunnels, a wheel cage, food, and housing, with several animals in the cage. The animals enjoy pursuing each other, running in the wheel cage, hiding in tunnels, and looking for food, and therefore live with activity and low stress in the cage. Activity in the daily life of humans may also influence the BDNF levels. These results demonstrate that BDNF is expressed in the brain and plays a role in neurogenesis, neuronal survival, and synaptogenesis in the brain. Matthews et al. [23], however, reported that BDNF is also produced in skeletal muscle, especially in satellite cells, by exercise. The increased BDNF in the periphery can penetrate into the brain by passing through the blood brain barrier (BBB) [24].
Mousavi and Jasmine showed that BDNF is expressed in satellite cells, but not in mature myofibers or the neuromuscular junction area, along with the expression of p75NTR, and emphasized the role of BDNF in the muscle in maintaining the population of muscle progenitor cells [25].
IGF-1 has an activity as an autocrine and/or paracrine signal for cell growth, differentiation, and maintenance in various tissues such as muscle, bone, liver, kidney, neurons, skin, and lungs [26, 27]. In skeletal muscle, IGF-1 can cause muscle hypertrophy with the proliferation and differentiation of myoblasts [7], and it also enhances nutrient uptake and inhibits protein breakdown [28-30].
Exercise and BDNF in the brain and the muscle
Many animal studies have reported that exercise increases the expression of hippocampal BDNF and/or neurogenesis (Figure 1). BDNF mRNA expression in the DG of the mouse hippocampus is increased by voluntary wheel running after a few days [31]. The increased BDNF protein expression is reported to remain for several weeks [32]. Neurogenesis is observed in the DG of these mice [33]. The exercise not only increased proliferation of the progenitor cells but also increased their survival rate as they differentiated and matured [33-34]. The expression of BDNF in mice is increased by just one session of exercise, and the effect is amplified by repeated exercise [35,36].
In humans, the circulation BDNF levels are elevated by acute [37] or chronic exercise [38]. Exercise increases peripheral BDNF levels, and a greater duration of exercise is associated with a greater increase in BDNF levels [37, 39], Increased levels of BDNF are more pronounced by aerobic exercises such as jogging than by resistance training [38]. Memory performance is also ameliorated by chronic endurance exercise in middle-aged male subjects with metabolic syndrome [38]. Concerning amelioration of memory performance in humans, two possibilities are considered: one is that exercise directly expresses BDNF in the brain and promotes neurogenesis as reported in animal experiments; another is that BDNF is produced by the muscle satellite cells, penetrates into the brain, and then acts on the hippocampus. It may depend on a multiplier effect of both actions. However, Yao et al. observed that running exercise reverses cognitive deficit and restores hippocampal cell proliferation in chronic corticosterone-treated rats, but does not change the plasma BDNF levels, suggesting that exercise directly acts on the brain [40].
Exercise and IGF-1 in the brain and the muscle
It is considered that exercise promotes IGF-1 by the repeated contraction of the muscle and/or muscle injury with an excessive load of contraction [2]. Although injured muscle releases various cellular components, Kravchenko et al. suggested that contractile muscle releases potassium ions, which promote IGF-1 synthesis and the IGF-1 is released by the activated satellite cells and muscle cells [41]. They observed that the expression of IGF-1 mRNA and protein are increased in cultured murine myoblasts and myotubes by the 7–12 mM KCl stimulation. The released IGF-1 activates differentiation of satellite cells to myoblasts. The myoblasts are proliferated by IGF-1, and the proliferated cells fuse to the muscle fiber, causing muscle hypertrophy [42].
In the brain, IGF-1 is synthesized in neural precursor cells, astrocytes, microglial cells, and neurons following brain injury during neurodegeneration [43-46]. Ploughmean et al. showed hippocampal IGF-1 is elevated in the ischemic hemisphere in sedentary animals, suggesting an intrinsic restorative response after focal ischemia [46]. Although exercise did not increase IGF-1 in the ischemic site, it increased the IGF-1 level in the intact site of the hippocampus and in the serum. Moreover, they observed that the serum IGF-1 level decrease was correlated with increasing IGF-1 in the ischemic hippocampus depending on the time of voluntary running in rats, suggesting the possibility that the protein enters the brain from circulation by the time [46]. Similarly, Trejo et al. showed that exercise increases the new neurons (neurogenesis) in the hippocampus and IGF-1 protein in the cerebrospinal fluid in adult rats [47]. Moreover, subcutaneously injected IGF-1 enhanced neurogenesis in the hippocampus, and the increased neurogenesis was more pronounced in rats after exercise compared to nonexercised rats, suggesting that the penetration of the circulating IGF-1 into the brain is increased by exercise and mediates the neurogenesis in the adult hippocampus. The authors further showed that when the entry of circulating IGF-1 into the brain is blocked with an injection of IGF-1 antiserum in rats undergoing exercise training, the exercise-induced increase in the number of new neurons in the hippocampus was inhibited [49]. Consequently, circulation IGF-1 is increased by exercise, and exercise might ameliorate degenerative diseases such as Alzheimer’s disease, Parkinson’s disease, as well as multiple sclerosis, by the penetration of circulation IGF-1 into the brain.
IGF-1 has additional activities such as clearance of Aβ proteins and brain vascularization [48]. Carro et al. showed that IGF-1 enhances the clearance of brain Aβ by over expression of the choroid plexus endocytic receptor, megalin, which participates as an Aβ carrier in the brain. Moreover, they found that exercise increases the levels of megalin by increasing brain IGF-1 in rats and mice [49]. In a different study, it was shown that IGF-1 protects against neuronal cell death induced by Aβ(1-40) in the cell culture of neuronlike SH-SY5Y human neuroblastoma cells [50]. Therefore, it is hypothesized that IGF-1 protects against the accumulation of Aβ protein and tau protein by inducing choroid plexus megalin and ameliorating Alzheimer’s disease [49].
Moreover, IGF-1 enhances angiogenesis acting on brain vessels and ameliorates multiple sclerosis [48] (Figure 1). Lopez-Lopez et al. showed that IGF-1 promotes the proliferation of cultured brain endothelial cells and increases vascularization of the adult mouse brain, and can increase vascular endothelial growth factor (VEGF) protein after subcutaneous injection [51].
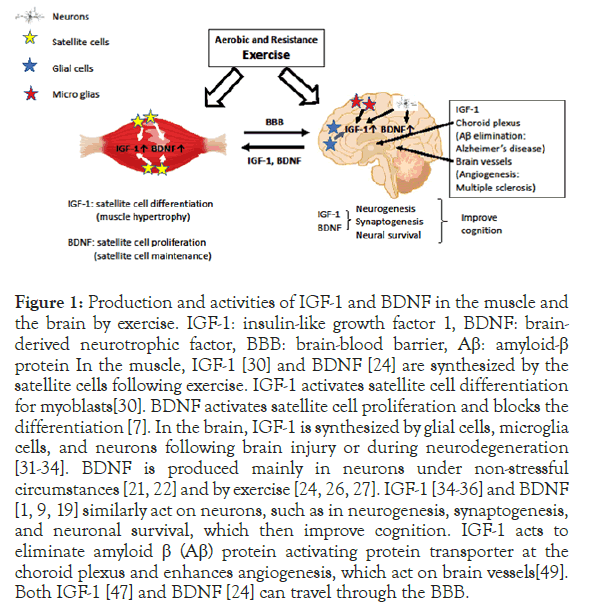
Figure 1. Production and activities of IGF-1 and BDNF in the muscle and the brain by exercise. IGF-1: insulin-like growth factor 1, BDNF: brainderived neurotrophic factor, BBB: brain-blood barrier, Aβ: amyloid-β protein In the muscle, IGF-1 [30] and BDNF [24] are synthesized by the satellite cells following exercise. IGF-1 activates satellite cell differentiation for myoblasts[30]. BDNF activates satellite cell proliferation and blocks the differentiation [7]. In the brain, IGF-1 is synthesized by glial cells, microglia cells, and neurons following brain injury or during neurodegeneration [31-34]. BDNF is produced mainly in neurons under non-stressful circumstances [21,22] and by exercise [24, 26, 27]. IGF-1 [34-36] and BDNF [1,9,19] similarly act on neurons, such as in neurogenesis, synaptogenesis, and neuronal survival, which then improve cognition. IGF-1 acts to eliminate amyloid β (Aβ) protein activating protein transporter at the choroid plexus and enhances angiogenesis, which act on brain vessels [49]. Both IGF-1 [47] and BDNF [24] can travel through the BBB.
Frailty
Pathophysiology of frailty
When people get older, some lose weight or lean body mass and experience fatigue and loss of strength or endurance. Frailty is an age-related physiologic change but not a disease (e.g., age-related sarcopenia or anorexia); therefore, it often causes a higher rate of poor health status and/or a greater extent of subclinical physiologic changes. Low circulating BDNF [52,53] or IGF-1 [54,55] is reported in frail people and is argued as a possible frailty biomarker. Fried studied frailty in more than 5,000 U.S. communities of men and women aged ≥ 65 years and presented the criteria of frailty [56,57]. The five criteria of frailty are as follows:
1. Shrinking: weight loss of 4.5 kg or ≥5% of body weight in the last 12 months.
2. Weakness: lowering hand grip strength.
3. Poor endurance and energy: lack of energy and fatigue.
4. Slowness: lowering gait speed and slow performance.
5. Low physical activity level: physical activity, less than 20 min. per week; and walking, less than 90 min. per week.
An individual is defined as being frail upon meeting at least three frailty criteria. People fulfilling one or two frailty criteria are defined as pre-frail. A mental change in will power and low social activity are also present in the frail population in addition to the above five criteria.
When elderly people become frail, they may easily fall and fracture bones, thus becoming hospitalized and bedridden, thereby requiring nursing care by a caregiver. Otherwise, they may have psychiatric problems such as deterioration of cognition or depression. Furthermore, frailty increases the risk of morbidity for various diseases, including infectious diseases, mortality due to low physical ability, or weakness to endogenous and exogenous stressors. As the social burden from morbidity or being bedridden by elderly people increases, prevention of frailty becomes increasingly necessary.
Presence of preliminary frailty in the cohort of healthy elderly people
We have reported a relationship between body mass index (BMI) and serum BDNF contents in a previous paper, as well as a relationship between the thickness of the quadriceps muscle and serum BDNF contents studied in a cohort of elderly people aged 65–84 years; these are significantly correlated by regression analysis [8].
As the criteria of frailty included weight loss or decrease of muscle mass, i.e., sarcopenia, we classified BMI according to the guidelines of Japan Society for the Study of Obesity. The mean serum BDNF level at the thinness class BMI of ≤18.4 was significantly (p<0.05) lower than that of normal class BMI of 18.5–24.9, obese-1 BMI of 25–29.9 and obese-2 BMI of 30–34.9. BMI is a value computed as “body weight divided by square of height”; thus, the value includes muscle mass, body fat mass, and bone mass, where body fat mass and muscle mass are not distinguished. As the low BMI class has low muscle mass and/or low fat mass, the thinness class of BMI ≤18.4 in this cohort may be interpreted as a preliminary frail class with lower serum BDNF levels than those in the normal BMI class. In general, people exercising for good health and well-being have higher serum BDNF levels.
Reduced hand grip strength is cited as one of the five Fried criteria for frailty [56]. We investigated the relationship between the hand grip strength and BMI; there was significant (p=0.0000) positive correlation between them as shown by the regression analysis. When the relationship between hand grip strength and BMI classification was analyzed, the thinnest class of BMI ≤ 18.4 (21.4 ± 6.4 kg) was found to have significantly (p<0.01) lower hand grip strength than the normal (25.4 ± 7.7 kg), obese-1 (27.4 ± 8.2 kg), and obese-2 (27.9 ± 9.2 kg) class BMI. The obese-3 class had very few and the hand grip strength was 27.8 ± 6.4 kg, although this was not significantly different from that of the thinness class. This indicates a pattern similar to that of BDNF level distribution in the BMI classes. This result also supports that people with BMI ≤18.4 possibly belong to the preliminary frail class (but not pre-frailty), although our cohort consisted of healthy elderly people volunteering to undergo health examinations. It has also been reported the frailty in the obese class with a BMI of 30 kg/m2 and higher in the U.S. shows slowness, weakness, and low activity [58]. The serum BDNF levels and hand grip strengths in the obese-2 class with a BMI of 30–34.9 and in the obese-3 class with a BMI of 35-39.9 were not different from the values in the normal class with a BMI of 18.5-24.9 in our study. Therefore, it might be difficult to extract preliminary frailty from the obese classes by checking the levels of serum BDNF or hand grip strength. To extract preliminary frailty from the obese classes with a BMI over 30, other parameters, such as slowness, walking distance, or weekly activity time, should be employed.
Intervention for frailty with nutrition and exercise
Frailty is a condition of deteriorated functional status, such as lowered activity, poor exercise tolerance, and reduced weight with loss of lean body mass. Intake of a balanced nutritional diet with carbohydrate and fatty acid for main energy, protein for composition of body, and vitamins and minerals for conditioning of the body is generally important for remaining healthy. In Japan, the Ministry of Agriculture, Forestry and Fisheries and the Ministry of Health, Labor and Welfare issued the “Japanese Food Guide Spinning Top” in 1995 so that all Japanese people would consume a well-balanced diet [59]. This food guide supports balanced nutrition, not for the prevention of frailty in elderly people, but for obese adults aged 30–60 years, thin females at child-bearing age, and obese children. Effective intake of nutrition in frail and elderly people is necessary as they may have a decreased appetite and eating volume, as well as lower absorption of the available nutrition. Therefore, intake of nutrition in elderly people is different from that in young adults. Volpi et al. showed that muscle protein metabolism in elderly healthy subjects (72 ± 1 years) is blunted compared to that in young subjects (30 ± 3 years) using the measurement of the net balance of phenylalanine across leg after amino acid and glucose intake [60]. Proper nutrition is also needed to reverse decreased weight and muscle mass. Decrease in appetite and/or food intake in elderly people is a major contributing factor to under-nutrition, which leads to frailty. Therefore, Lorenzo-Lopez et al. emphasized the importance of diet quality in preventing frailty [61]. Concerning micronutrients and frailty, the authors [61] reported that a higher protein intake is associated with a lower risk of frailty; a high dietary antioxidant intake is also recommended. Nichols et al. investigated published reports on the effects of the dietary protein and/or essential amino acid (EAA) supplementation on muscle strength and performance and concluded that protein and/or EAA supplementation alone does not improve strength, but does increase muscle mass and the quality of life, as well as the walking distance.
We will describe about amino acids and dipeptides, which possibly useful for prevention of frailty, with increasing expression of BDNF or IGF-1 [62]. Arginine [64] and taurine [65] increase IGF-1 production in cultured cells and in the serum protein of malnourished mice, respectively. Oh et al. observed an increase of IGF-1 gene-expression and protein in cultured CH3 pituitary epithelium cells and HepG2 hepatocytes by L-arginine, through activation of mitogen-activated protein kinase (MAPK) signaling cascade [63]. Moon et al. reported that taurine increases IGF-1 mRNA in cultured HepG2 hepatocytes and IGF-1 mRNA and serum protein levels in a low protein (4%) diet (vs. 20% protein diet in control) administered to mice for 12 weeks [64]. The tibial growth plate thickness and the body weight in the low protein diet mice are recovered by 12-weeks taurine administration, close to those in normal protein diet mice, showing that taurine has possible anti-frailty activity. Increased expression of BDNF mRNA is also reported in the hippocampus of diabetic rats administered taurine for 30 days, and short-term memory is improved in the rats [65]. Soybean-derived glycine-arginine dipeptide, although different from arginine itself, is reported to promote BDNF mRNA expression in the mouse brain and mature neurons in the hippocampus and cerebral cortex of mice [66]. It is reported that the branched-chain amino acids (BCAA), leucine (Leu), isoleucine (Ile), and valine (Val), main components of the skeletal muscle, increase the expression of BDNF mRNA and protein in the hippocampus and tropomyosin receptor kinase B (TRKB) signaling in social-defeat stressed mice and promote resilience to chronic social-defeat stress [68]. Ile and Val activate the BDNF/ TRKB signaling by inducing BDNF gene expression, whereas Leu activates the pathway at the TRKB signaling independent of significant effects on BDNF levels [67]. Carnosine and anserine are imidazol dipeptides composed of beta-alanine and histidine or methylhistidine, respectively, and they are present in high amounts in the avian breast having antioxidant activity [68]. Yamashita et al. observed that neurite growth in human neuroblastoma cells and BDNF mRNA and protein expression in human glioblastoma cells are increased by carnosine in vitro [69]. Tomonaga et al. found increases of the imidazole peptides in the rat brain after the oral administration of chicken breast extract, suggesting activity on the brain [70]. The administration of beta-alanine to rats for 30 days expressed BDNF protein in the hippocampus and enhanced spatial learning and ameliorated anxiety-like behavior [71]. Murakami and Furuse observed that beta-alanine supplementation increased carnosine levels in the cerebral cortex and hypothalamus in mice and BDNF levels in the hippocampus, suggesting that beta-alanine is converted to carnosine in the mouse body [72].
In case of fatty acid, intake of medium-chain fatty acid and polyunsaturated fatty acid (PUFA), which easily metabolite to energy, are recommended, but saturated fatty acid is not recommended. Omega-6 polyunsaturated FAs (ω-6 PUFAs) such as arachidonic acid (AA) are so-called inflammatory FAs producing prostaglandins (PGs), such as PGE2 and thromboxane A2, which play a role in inflammation. On the contrary, omega-3 polyunsaturated FAs (ω-3 PUFAs) such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are called anti-inflammatory FAs as these inhibit the synthesis of PGs from arachidonic acid. DHA enriched (1.2%) diet supplement for 12 days enhanced exercise-induced hippocampal BDNF levels and spatial cognition in traumatic brain injury rats [73].
In human study of frailty, Kim et al. observed low ω-3 PUFAs, DHA and EPA contents in the erythrocyte than healthy communitydwelling older adults aged 70-84 years [74]. Especially, slow walking speed is associated with erythrocyte levels of DHA and EPA. Similarly, Hutchins-Wiese et al. reported low erythrocyte DHA content and DHA/AA ratio in frailty than that in the healthy postmenopausal women aged 60-84 years [75]. Further, the authors reported higher erythrocyte DHA content and DHA/AA ratio and improvement in walking speed by the intervention of EPA and DHA supplementation for 6 months to frailty postmenopausal women compared to placebo group.
Omega-6 fatty acid is also important for neurogenesis, although it does not enhance BDNF production. Arachidonic acid, an omega-6 fatty acid, ameliorates age-dependent decreases in the number of neural stem/progenitor cells in the hippocampus, while DHA increases the number of new neurons and/or their maturation in rats aged 18 months [76]. Therefore, it is important to consume the appropriate ratio of omega-3 and omega-6 fatty acids (1:2). Further, DHA has important activities in the brain, such as antiinflammatory activity to inhibit inflammatory microglia [77], induction of phagocytosis Aβ, and activation of anti-inflammatory microglia [78], in addition to BDNF production.
For vitamins and minerals, intake of vegetables (more than 350 g/ day) and dairy products (milk, yogurt, and cheese) is recommended. These amounts, however, are sometimes not enough for a day; at such times, it is recommended to take supplements. Furthermore, fruits, vegetables, and food legumes have phytochemicals, i.e., polyphenols (included flavonoids), carotenoids, and sulfurcontaining compounds having anti-oxidative activity, enhancement of blood circulation, and potent anti-bacterial effects [79,80]. Polyphenols have antioxidant activity and activity for the prevention of frailty by inhibition of injures to various organs, cell apoptosis, and DNA damages by age-dependent production of free radicals.
There are various reports on preventing the progression of frailty by the intervention of exercise [81-83]. Exercise reverses or mitigates frailty and can preserve the quality of life and various functions including mood, health, and social engagement. Aguirre and Villareal reported that resistance training increases muscle strength and muscle mass and that aerobic endurance training improves peak oxygen consumption [81]. Therefore, they emphasized the importance of both training for muscle strength and aerobic endurance for preventative treatment of frailty. Yamada et al. showed an improvement in skeletal muscle mass index and an increase of circulating IGF-1 levels by a six-month intervention combining exercise and nutrition in community-dwelling older Japanese adults [84]. Combining exercise and proper nutrition is important for the prevention of frailty, as it not only mends the body composition of muscle mass but also retains cognition through the effects of IGF-1 and BDNF.
Conclusion
We have referred importance of appropriate exercise and intake of proper nutrition in the elderly people for prevention of frailty. Physical exercise and intake of proper nutrition enhance production of IGF-1 and BDNF levels in the periphery and the brain. In the periphery, IGF-1 and BDNF are synthesized in the muscle satellite cells by the exercise. IGF-1 act as muscle hypertrophy differentiate the satellite cells to myoblast and fusing to myotubes, and BDNF act as maintenance of satellite cells. Both factors act on the brain passing through the BBB from periphery. Further, exercise directly enhances production of IGF-1 and BDNF in the brain and potentiates neurogenesis, synaptogenesis and neuronal survival. IGF-1, further, has activities Aβ elimination activating protein transporter at choroid plexus and angiogenesis acting on brain vessels.
Intervention of nutrition, protein, amino acids, ω-3 PUFAs and antioxidants (polyphenols, imidazole dipeptides) increase IGF-1 and BDNF protection from oxidation and inflammation of the aging process.
Therefore, exercise and intake of proper nutrition act on muscle atrophy and neural deterioration of frailty, increasing IGF-1 and BDNF.
Acknowledgment
We would like to thank Editage (www.editage.com) for English language editing.
Author Contributions
Mitsugu Hachisu and Kazushige Ihara wrote and edited the paper, Masayuki Obayashi and Mari Kogo wrote the paper.
REFERENCES
- Jeon YK, Ha CH. The effect of exercise intensity on brain derived neurotrophic factor and memory in adolescents. Environ Health Prev Med. 2017;22(1): 27.
- Carro E, Nunez A, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor 1 mediates effects of exercise on the brain. J Neurosci. 2000;20(8): 2926-2933.
- Barde YA, Edgar D, Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1(5): 549-553.
- Liu PZ, Nusslock R. Exercise-mediated neurogenesis in the hippocampus via BDNF. Front Neurosci. 2018;12: 52.
- Babaeipour V, Vahidi H, Alikhani S, Ranjbari J, Alibakhshi A, et al. Effect of acyl homoserine lactone on recombinant production of human insulin-like growth factor-1 in batch culture of Escherichia coli. Protein Pept Lett. 2018;25(11): 980-985.
- Rinderknecht E, Humbel RE. The amino acid sequence of human insulin-like growth factor 1 and its structural homology with proinsulin. J Biol Chem. 1978;253(8): 2769-2776.
- Florini JR, Ewton DZ, Falen SL, Van Wyk JJ. Biphasic concentration dependency of stimulation of myoblast differentiation by somatomedins. Am J Physiol. 1986;250(5 pt1): C771-C778.
- Hachisu M, Hashizume M, Kawai H, Hirano H, Kojima M, Fujiwara Y, et al. Relationships between serum brain-derived neurotrophic factor concentration and parameters for health scores in community-dwelling older adults. Geriatr Gerontol Int. 2017;18(3): 456-461.
- Baumgartner RN, Waters DL, Gallagher D, Morley JE, Garry PJ . Predictors of skeletal muscle mass in elderly men and women. Mech Aging Dev. 1999;107(2): 123-136.
- Tanabe K, Kuno S. Muscle mass and the power. Shindan Chiryo. 2010;98(11): 1779-1784.
- Tanimoto Y, Watanabe M, Kono R, Hirota C, Takasaki K, Kono K. Aging changes in muscle mass of Japanese. Nippon Ronen Igakkai Zasshi. Nihon Ronen Igakki Zasshi. 2010;47(1): 52-57.
- Abe T, Loenneke JP, Thiebaud RS, Fukunaga T. Age-related site-specific muscle wasting of upper and lower extremities and trunk in Japanese men and women. Age (Dordr). 2014;36(2): 813-821.
- Numakawa T, Odaka H, Adachi N. Actions of brain-derived neurotrophin factor in the neurogenesis and neuronal function, and its involvement in the pathophysiology of brain diseases. Int J Mol Sci. 2018;19(11): 3650.
- Gibon J, Barker PA, Seguela P. Opposing presynaptic roles of BDNF and proBDNF in the regulation of perdidtent activity in the entohinal cortex. Mol Brain. 2016;9: 23.
- Sun Y, Lim Y, Li F, Liu S, Lu J-J, Haberberger R, et al. (2012) ProBDNF Collapses Neurite Outgrowth of Primary Neurons by Activating RhoA. PLoS One. 2012;7(4): e35883.
- Hofer M, Pagliusi SR, Horn A, Leibrock J, Barde YA. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO J. 1990;9(8): 2459-2464.
- Yoshimura R, Mitoma M, Sugita A, Hori H, Okamoto T, Umene W, et al. Effects of paroxetine or milnacipran on serum brain-derived neurotrophic factor in depressed patients. Prog Neuropsycho-pharmacol Biol Psychiatry. 2007;31(5): 1034-1037.
- Leyhe T, Stransky E, Eschweiler GW, Buchkremer G, Laske C. Increase of BDNF serum concentration during donepezil treatment of patients with early Alzheimer's disease. Eur Arch Psychiatry Clin Neurosci. 2008;258(2): 124-128.
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic Antidepressant Treatment Increases Neurogenesis in Adult Rat Hippocampus. Neurosci. 2000;20(24): 9104-9110.
- Kotani S, Yamauchi T, Teramoto T, Ogura T. Donepezil, an acetylcholine inhibitor, enhances adult hippocampus neurogenesis. Chem Biol Interact. 2008;175(1): 227-230.
- Ping G, Qian W, Song G, Zhaochun S. Valsartan reverses depressive/anxiety-like behavior and induces hippocampal neurogenesis and expression of BDNF protein in unpredictable chronic mild stress mice. Pharmacol Biochem Behav. 2014;124: 5-12.
- Duman RS. Neurotrophic factors and regulation of mood: role of exercise, diet and metabolism. Neurobiol Aging. 2005;26 Suppl 1: 88-93.
- Matthews VB, Aström MB, Chan MH, Bruce CR, Krabbe KS, Prelovsek O, et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia. 2009;52: 1409-1418.
- Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacol. 1998;37(12): 1553-1561.
- Mousavi K, Jasmin BJ. BDNF is expressed in skeletal muscle satellite cells and inhibits myogenic differentiation. J. Neurosci. 2006;26(21): 5739-5749.
- Lui JC, Colbert M, Cheung CSF, Ad M, Lee A, Zhu Z, et al. Cartilage-targeted IGF-1 treatment to promote longitudinal bone growth. Mol Ther. 2019;27(3): 673-680.
- Dicarlo M, Bianchi N, Ferretti C, Orciani M, Di Primio R, Mattiolo-Belmonte M. Evidence Supporting a Paracrine Effect of IGF-1/VEGF on Human Mesenchymal Stromal Cell Commitment. Cells Tissues Organs. 2016;201(15): 333-341.
- Beguinot F, Kahn CR, Moses AC, Smith RJ. Distinct biologically active receptors for insulin, insulin-like growth factor I, and insulin-like growth factor II in cultured skeletal muscle cells. J Biol Chem. 1985;260(29): 15892-15898.
- Shimizu M, Webster C, Morgan DO, Blau HM, Roth RA. Insulin and insulin like growth factor receptors and responses in cultured human muscle cells. Am J Physiol. 1986;251: E611-E615.
- Ewton DZ, Falen SL, Florini JR. The type II insulin-like growth factor (IGF) receptor has low affinity for IGF-I analogs: pleiotypic actions of IGFs on myoblasts are apparently mediated by the type I receptor. Endocrinology. 1987;120(1): 115-123.
- Neeper SA, Gómez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. nature. 1995;373(6510): 109.
- Russo-Neustadt A, Beard RC. Cotman C.W. Exercise, antidepressant medications, and enhanced brain derived neurotrophic factor expression. Neuropsychopharmacology. 1999;21(5): 679-682.
- Seri B, García-Verdugo JM, McEwen BS, Alvarez-Buylla A. astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21: 7153-7160.
- Van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2(3): 266-270.
- Van Paag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25(38); 8680-8685.
- Rasmussen P, Brassard P, Adser H, Pedersen MV, Leick L, Hart E, et al. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp Physiol. 2009;94(10); 1062-1069.
- Dinoff A, Herrmann N, Swardfager W, Lanctôt KL. The effect of acute exercise on blood concentrations of brain-derived neurotrophic factor in healthy adults: a meta-analysis. Eur J Neurosci. 2017;46(1): 1635-1646.
- Babaei P, Azali Alamdari K, Soltani Tehrani B, Damirchi A. Effect of six weeks of endurance exercise and following detraining on serum brain derived neurotrophic factor and memory performance in middle aged males with metabolic syndrome. J Sports Med Phys Fitness. 2013;53(4): 437-443.
- Jeon YK, Ha CH. The effect of exercise intensity on brain derived neurotrophic factor and memory in adolescents. environ. Health Prevent Med. 2017;27: 22-29.
- Yau SS-Y, Lau B W-M, Zhang E-D, Lee J C-D, Li A et al. Effects of voluntary running on plasma levels of neurotrophins, hippocampal cell proliferation and learning and memory in stressed rats. Neuroscience. 2012;222: 289-301.
- Kravchenko IV, Furalyov VA, Popov VO. Potassium chloride released from contracting skeletal muscle may stimulate development of its hypertrophy. Biochem Biophys Rep. 2019;18: 100627.
- Clemmons DR. Role of IGF-1 in skeletal muscle mass maintenance. Trend Endocrinol Metab. 2009;20(7): 349-356.
- Arsenijevic Y, Weiss S. Insulin-like Growth factor-1 is a differentiation factor for postmitotic CNS stem cell-derived neuronal precurcors: Distinct actions from those of brain-derived neurotrophic factor. J Neurosci. 1998;18(6): 2118-2128.
- Myhre CL, Thygesen C, Villadsen B, Vollerup J, Ilkjær L, Keohn KT, et al. Microglia express insulin-like growth factor-1 in the hippocampus of aged appswe/ps1⊿e9 transgenic mice. Front Cell Neurosci. 2019;13: 308.
- Gluckman P, Klempt N, Guan J, Sirimanne E, Dragunow M, Klempt M, et al. A role of IGF-1 in the rescue of CNS neurons following hypoxic-ischemic injury. Biochem Biophys Res Commun. 1992;182(2): 593-599.
- Ploughman M, Granter-Button S, Chernenko G, Tucker BM, Mearow KM, Corbett D. Endurance exercise regimens induce differential effects on brain-derived neurotrophic factor, synapsin-1 and insulin-like growth factor 1 after focal ischemia. Neurosci. 2005;136(4): 991-1001.
- Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21(5): 1628-1634.
- Carro E, Torres-Aleman I. Serum insulin-like growth factor 1 in the brain function. Keio J Med. 2006;55(2): 59-63.
- Carro E, Spuch C, Trejo JL, Antequera D, Torres-Aleman I. Choroid plexus megalin is involved in neuroprotection by serum insulin-like growth factor I. J Neurosci. 2005;25(47): 10884-10893.
- Nagai Y, Ogasawara A, Heese K. Possible mechanisms of A beta(1-40)- or A beta(1-42)-induced cell death and their rescue factors. Nihon Yakurigaku Zasshi. 2004;124(3): 135-143.
- Lopez-Lopez C, LeRoith D, Torres-Aleman I. Insulin-like growth factor I is required for vessel remodeling in the adult brain. PNAS. 2004;101(26): 9833-9838.
- Inglés M, Gambini J, Mas-Bargues C, García-García FJ, Viña J et al. (2017) Brain-derived neurotrophic factor as a marker of cognitivefrailty. J Gerontol Ser A. 2016;72(3): 450-451.
- Coelho FM, Pereira DS, Lustosa LP, Silva JP, Dias JMD, et al. Physical therapy intervention (PTI) increases plasma brain-derived neurotrophic factor (BDNF) levels in non-frail and pre-frail elderly women. Arch Gerontol Geriatr. 2012;54(3): 415-420.
- Sasako T, Ueki K. Aging-related frailty and sarcopenia. Frailty/sarcopenia and insulin/IGF-1 signaling. Clin Calcium. 2018;28(9): 1221-1228.
- Doi T, Makizako H, Tsutsumimoto K, Hotta R, Nakakubo S, Makino K, et al. Association between insulin-like growth factor-1 and frailty among older adults. J Nutr Health Aging. 2018;22(1): 68-72.
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontolgy Med Sci. 2001;56(3): M146-M156.
- Walston T, Fried LP. Frailty and the older man. Med Clin North Am. 1999;83(5); 1173-1194.
- Blaum CS, Xue QL, Michelon E, Semba RD, Fried LP. The association between obesity and the frailty syndrome in older women: The women's health and aging studies. J Am Geriatr Soc. 2005;53(6): 927-934.
- https://www.mhlw.go.jp/bunya/kenkou/pdf/eiyou-syokuji5.pdf 2020.
- Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab. 2000;85(12): 4481-4490.
- Lorenzo-López L, Maseda A, de Labra C, Regueiro-Folgueira L, Rodríguez-Villamil JL, Millan-Calenti. Nutritional determinants of frailty in older adults: a systematic review. BMC Geriatr. 2017;17(1): 108.
- Nichols S, McGregor G, Al-Mohammad A, Ali AN, Tew G, Alasdair GT, et al. The effect of protein and essential amino acid supplementation on muscle strength and performance in patients with chronic heart failure: a systematic review. Eur J Nutr. 2019.
- Oh H-S, Oh SK, Lee JS, Wu C, Lee S-J. Effects of L-arginine on growth hormone and insulin-like growth factor 1. Food Sci Biotechnol. 2017;26(6): 1749-1754.
- Moon P-D, Kim M-H, Lim H-S, Oh H-S, Nam S-Y, Han N-R, et al. Taurine, a major amino acid of oyster, enhances linear bone growth in a mouse model of protein malnutrition. Biofactors. 2015;41(3): 190-197.
- Caletti G, Almeida FB, Agnes G, Nin MS, Maria H, Barros T, et al. Antidepressant dose of taurine increases mRNA expression of GABAA receptor α2 subunit and BDNF in the hippocampus of diabetic rats. Behav Brain Res. 2015;283: 11-15.
- Shimizu A, Mitani T, Tanaka S, Fujii H, Maebuchi M, Amiya Y, et al. Soybean-derived glycine-arginine dipeptide administration promotes neurotrophic factor expression in the mouse brain. J Agric Food Chem. 2018;66(30): 7935-7941.
- Nasrallah P, Haidar EA, Stephan JS, Hayek LEI, Karnib N, Khalifeh M, et al. Branched-chain amino acids mediate resilience to chronic social defeat stress by activating BDNF/TRKB signaling. Neurobiol Stress. 2019;11: 100170.
- Sato M, Karasawa N, Shimizu M, Morimatsu F, Yamada R. Safety evaluation of chicken breast extract containing carnosine and anserine. Food Chem Toxicol. 2008;46(2): 291-295.
- Yamashita S, Sato M, Matsumoto T, Kadooka K, Hasegawa T et al. Mechanisms of carnosine-induced activaton of neuronal cells. Biosci Biotechnol Biochem. 2018;82(4): 683-688.
- Tomonaga S, Hayakawa T, Yamane H, Maemura H, Sato M, Takahata Y, et al. Oral administration of chicken breast extract increases brain carnosine and anserine concentrations in rats. Nutr Neurosci. 2007;10(3): 181-186.
- Hoffman JR, Ostfeld I, Stout JR, Harris RC, Kaplan Z, Cohen H. Beta-alanine supplemented diets enhance behavioral resilience to stress exposure in an animal model of PTSD. Amino Acid. 2015;47(6): 1247-1257.
- Murakami T, Furuse M. The impact of Taurine- and Beta-alanine-supplemeted diets on behavioral and neurochemical parameters in mice: antidepressant versus antiaxiolytic-like effects. Amino Acid. 2010;39(2): 427-434.
- Wu A, Ying Z, Gomez-Pinilla F. Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neurosci. 2008;155(3): 751-759.
- Kim D, Won C-W, Park Y. Association between erythrocyte levels of n-3 polyunsaturated fatty acids and risk of frailty in community-dwelling older adults: The Korean frailty and aging cohort study. J Gerontol A Biol Sci Med Sci. 2020.
- Hutchins-Wiese HL, Kleppinger A, Annis K, Liva E, Lammi-Keefe CJ, Durham HA, et al. The impact of supplemental n-3 long chain polyunsaturated fatty acids and dietary antioxidants on physical performance in postmenopausal women. J Nutr Health Aging. 2013;17(1): 76-80.
- Tokuda H, Kontani M, Kawashima H, Kiso Y, Shibata H, Osumi N. Differential effect of arachidonic acid and docosahexaenoic acid on age-related decrease in hippocampal neurogenesis. Neurosci Res. 2014;88: 58-66.
- Heras-Sandoval D, Pedraza-Chaverri J, Pérez-Rojas JM. Role of docosahexaenoic acid in the modulation of glial cells in alzheimer's disease. j neuro inflammation. 2016;13(1): 61.
- Hjorth E, Zhu M, Toro VC, Vedin I, Palmblad J, Cederholm T, et al. Omega-3 fatty acids enhance phagocytosis of Alzheimer's disease-related amyloid-β42 by human microglia and decrease inflammatory markers. J Alzheim Dis. 2013;35(4): 697-713.
- Krupp D, Remer T, Penczynski KJ, Bolzenius K, Wudy SA, Buyken AE. Relevance of fruits, vegetables and flavonoids from fruits and vegetables during early life, mid-childhood and adolescence for levels of insulin-like growth factor (IGF-1) and its binding proteins IGFBP-2 and IGFBP-3 in young adulthood. Br J Nutr. 2016;115(3): 527-537.
- Zhu F, Du B, Xu B. Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: A review. Crit Rev Food Sci Nutr. 2018;58(8): 1260-1270.
- Aguirre LE, Villareal DT. Physical exercise as therapy for frailty. Nestle Nutr Inst Workshop Ser. 2015;83: 83-92.
- Bray NW, Smart RR, Jakobi JM, Jones GR. Exercise prescription to reverse frailty. Appl Physiol Nutr Metab. 2016;41(10): 1112-1116.
- Hsieh TJ, Su S-C, Chen C-W, Kang Y-W, Hu M-H, Hsu L-L, et al. Individualized home-based exercise and nutrition interventions improve frailty in older adults: A randomized controlled trial. Int J Behav Nutr Phys Act. 2019;16(1): 119.
- Yamada M, Nishiguchi S, Fukutani N, Aoyama T, Arai H. Mail-based intervention for sarcopenia prevention increased anabolic hormone and skeletal muscle mass in community-dwelling Japanese older adults: The INE (Intervention by Nutrition and Exercise) Study. J Am Med Dir Assoc. 2015;16(8): 654-660.
Citation: Hachisu M, Obayashi M, Kogo M, Ihara K (2020) Psychosomatic Health in Elderly People for Preventing Frailty: The role of IGF-1 and BDNF in the Muscle and the Brain. J Aging Sci. 8:223. DOI:10.35248/2329-8847.20.08.223.
Copyright: © 2020 Hachisu M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

