Indexed In
- RefSeek
- Hamdard University
- EBSCO A-Z
- Publons
- Euro Pub
- Google Scholar
- Quality Open Access Market
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Mini Review - (2021) Volume 7, Issue 4
Prevention of Opioid Induced Nausea and Vomiting During Treatment of Acute Pain with Combination Antiemetic Opioid Medication: An Update and Review
Timothy W. Neal1* and John R. Zuniga22Departments of Surgery and Neurology, Division of Oral and Maxillofacial Surgery, UT Southwestern/Parkland Memorial Hospital, Dallas, USA
Received: 01-Jul-2021 Published: 22-Jul-2021, DOI: 10.35248/2684-1320.21.7.155
Abstract
Patients treated with opioids for the management of acute pain often experience opioid induced nausea and vomiting (OINV). OINV compromises pain control, leads to sleep disturbances, and increase in health care costs. Currently, there is no approved therapeutic agent to treat acute pain while preventing and reducing OINV. This has led to the production of Hydexor (CL-108), which is a combination agent containing opioid and an antiemetic. The purpose of this review is to revisit the use of CL-108 as a modality in the reduction of OINV, and to provide an update for use.
Keywords
Opioid; Acute pain; Pharmacologic; Promethazine
Introduction
Opioid medications are commonly used in the treatment of moderate-to-severe acute pain. The pharmacologic effects of these medications, in particular nausea and vomiting, can negatively impact daily function and quality of life in the postoperative period. Opioid induced nausea and vomiting (OINV) has been reported to develop in approximately 40% of patients treated with opioids for acute pain [1-3]. In order to avoid these distressing symptoms, patients may skip doses leading to inadequate pain control [4,5]. Not only does OINV hinder acute pain management, but these symptoms can also lead to more hospital, emergency department, and physician office visits. Patients that experience OINV while taking opioids for acute pain represent a much larger health care cost burden as compared to those who do not experience these symptoms [6,7].
The effort to adequately manage OINV in the setting of moderate-to-severe acute pain has led to the production of combination agents containing opioid and antiemetic. One such agent is Hydexor (CL-108), which contains 7.5 mg hydrocodone/325 mg APAP and 12.5 mg promethazine. Promethazine is an effective antiemetic with antihistamine, anticholinergic, and antidopaminergic properties approved for prevention or treatment of postoperative nausea and vomiting [8]. CL-108 works predominantly through a mechanism of delivery designed for the rapid-release formulation of promethazine prior to the eventual release of the opioid hydrocodone. The proposed benefit being that the antiemetic centers involving the vestibular system, chemoreceptor trigger zone (CTZ), and the gastrointestinal tract receptors are activated prior to the Mu-receptor activation from hydrocodone [9].
The purpose of this mini review is to revisit the primary end points (nausea/vomiting and analgesia) and the secondary end points (adverse events and quality of life) of CL-108, and to provide an update for use.
Review and Update
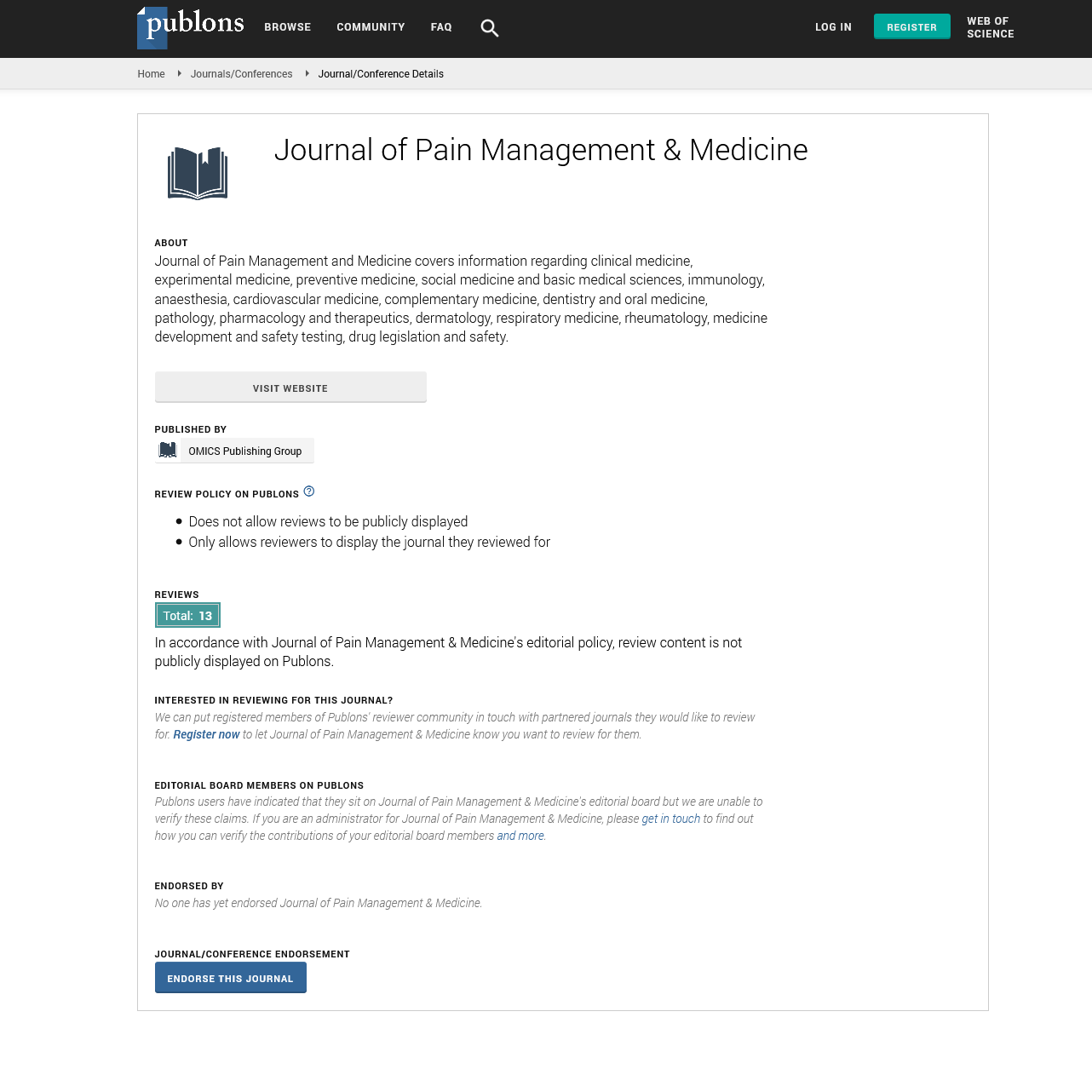
CL-108 effectively reduced nausea and vomiting in two clinical trials. In a randomized, doubleblind, placebo-controlled study comparing the emetic effects of HC/APAP (hydrocodone 7.5 mg/acetaminophen 325 mg) and CL-108 following orthopedic surgery, CL-108 treated patients experienced fewer episodes of retching and vomiting [10] (Figure 1).
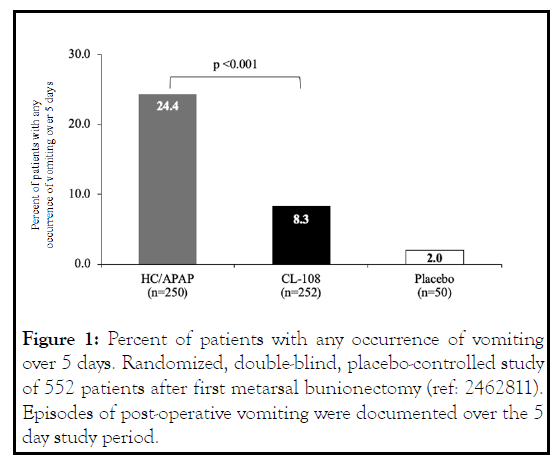
Figure 1: Percent of patients with any occurrence of vomiting over 5 days. Randomized, double-blind, placebo-controlled study of 552 patients after first metarsal bunionectomy (ref: 2462811). Episodes of post-operative vomiting were documented over the 5 day study period.
The number of CL-108 treated patients that reported retching was nearly half that of HC/APAP treated patients, and there was a 65.8% relative reduction in risk of vomiting. CL-108 reduced post-discharge nausea and vomiting (PDNV), the relative reduction in risk of PDNV was 47%, and the incidence was indistinguishable from placebo [10]. The 5-day postoperative incidence of nausea was significantly reduced (Figure 2).
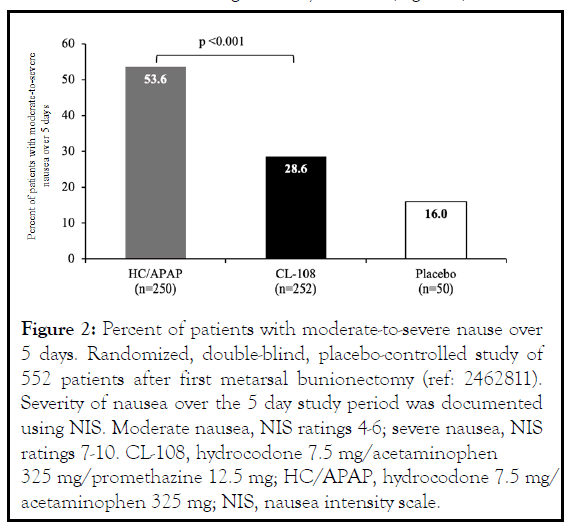
Figure 2: Percent of patients with moderate-to-severe nause over 5 days. Randomized, double-blind, placebo-controlled study of 552 patients after first metarsal bunionectomy (ref: 2462811). Severity of nausea over the 5 day study period was documented using NIS. Moderate nausea, NIS ratings 4-6; severe nausea, NIS ratings 7-10. CL-108, hydrocodone 7.5 mg/acetaminophen 325 mg/promethazine 12.5 mg; HC/APAP, hydrocodone 7.5 mg/ acetaminophen 325 mg; NIS, nausea intensity scale.
In a randomized, double-blind, placebo-controlled study comparing HC/APAP and CL-108 following ambulatory third molar odontectomy/oral surgery there was a 52% relative reduction in the risk of vomiting during the first day, and a 49% reduction over a postoperative 5-day study period [11] (Figure 3 ). Patients reported significantly milder nausea or no nausea and used less rescue antiemetics.
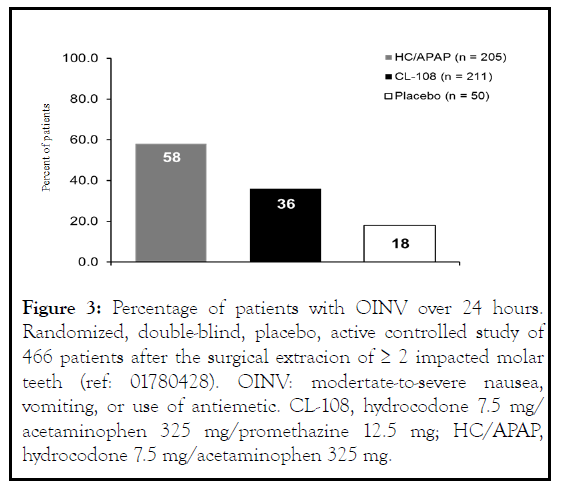
Figure 3: Percentage of patients with OINV over 24 hours. Randomized, double-blind, placebo, active controlled study of 466 patients after the surgical extracion of ≥ 2 impacted molar teeth (ref: 01780428). OINV: modertate-to-severe nausea, vomiting, or use of antiemetic. CL-108, hydrocodone 7.5 mg/ acetaminophen 325 mg/promethazine 12.5 mg; HC/APAP, hydrocodone 7.5 mg/acetaminophen 325 mg.
In the randomized clinical trial oral surgery model comparing CL-108, hydrocodone/APAP, and placebo, CL-108 significantly reduced post-operative acute pain compared with placebo [11] (Figure 4 ). The time to pain relief and summed pain reduction was equivalent to active control. Patients treated with HC/APAP who developed OINV had higher pain intensity scores [12]. In the safety and effectiveness study of patients dissatisfied with NSAID treatment for osteoarthritis, 80% of patients reported less severe pain with CL-108 [13].
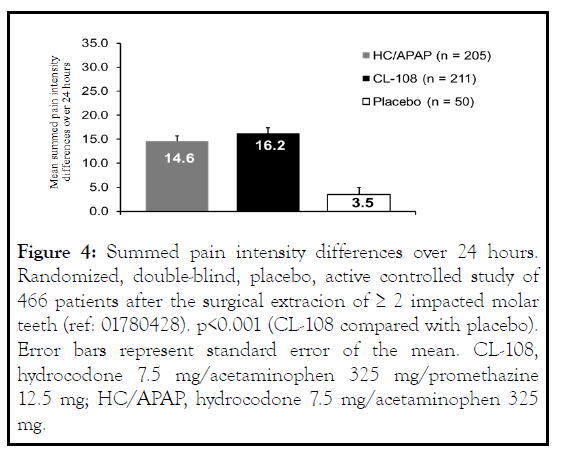
Figure 4: Summed pain intensity differences over 24 hours. Randomized, double-blind, placebo, active controlled study of 466 patients after the surgical extracion of ≥ 2 impacted molar teeth (ref: 01780428). p<0.001 (CL-108 compared with placebo). Error bars represent standard error of the mean. CL-108, hydrocodone 7.5 mg/acetaminophen 325 mg/promethazine 12.5 mg; HC/APAP, hydrocodone 7.5 mg/acetaminophen 325 mg.
The physician investigators rated CL-108 as a good-to-excellent treatment for 84.7% of the patients and concluded CL-108 was a safe and effective treatment of acute pain [13]. Following orthopedic surgery, quality of pain scores were significantly reduced compared to placebo. In CL-108 treated patients with severe pain at baseline, there was greater pain relief over 12 hours compared to placebo [14,15] (Figure 5).
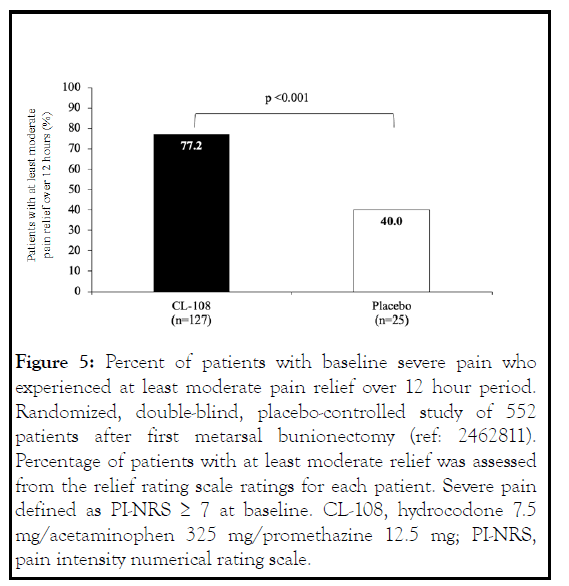
Figure 5: Percent of patients with baseline severe pain who experienced at least moderate pain relief over 12 hour period. Randomized, double-blind, placebo-controlled study of 552 patients after first metarsal bunionectomy (ref: 2462811). Percentage of patients with at least moderate relief was assessed from the relief rating scale ratings for each patient. Severe pain defined as PI-NRS ≥ 7 at baseline. CL-108, hydrocodone 7.5 mg/acetaminophen 325 mg/promethazine 12.5 mg; PI-NRS, pain intensity numerical rating scale.
The safety profile of CL-108 was consistent with its individual components. Drowsiness was the most common adverse event, and both the incidence and severity were dose related. Other common adverse events were dizziness and dry mouth. In the randomized controlled trial following oral surgery, 18% of CL-108 treated patients experienced drowsiness, 8% experienced dizziness, and 6% experienced dry mouth [11] (Figure 6) . There was no difference in abuse potential when compared to hydrocodone [16].
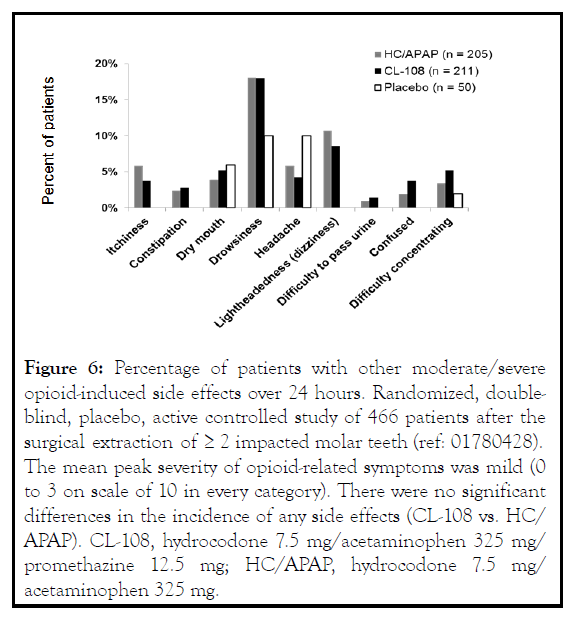
Figure 6: Percentage of patients with other moderate/severe opioid-induced side effects over 24 hours. Randomized, doubleblind, placebo, active controlled study of 466 patients after the surgical extraction of ≥ 2 impacted molar teeth (ref: 01780428). The mean peak severity of opioid-related symptoms was mild (0 to 3 on scale of 10 in every category). There were no significant differences in the incidence of any side effects (CL-108 vs. HC/ APAP). CL-108, hydrocodone 7.5 mg/acetaminophen 325 mg/ promethazine 12.5 mg; HC/APAP, hydrocodone 7.5 mg/ acetaminophen 325 mg.
Discussion
Current indications for CL-108 are for management of acute post-operative pain that is: 1. severe enough to require an opioid analgesic; 2. for a maximum of 3 days; and 3. in adults at high risk for nausea and vomiting with hydrocodone-containing products in a certified medically supervised setting. Regulatory approval is pending [17-19]. Opioid analgesic medications play an essential role in the management of acute pain. Opioids increase the risk of nausea and vomiting in a dose dependent manner, and the effects last for the duration of use. These adverse effects can compromise pain control, recovery, and can lead to worse surgical outcomes. This can be seen in both the oral surgery and orthopedic surgery randomized controlled trials. In the randomized controlled trial following orthopedic surgery, episodes of OINV awakened patients from sleep resulting in reduced quality of life reports. OINV caused sleep disturbance and interfered with pain relief, evidenced when the measured summed pain intensity difference over 48 hours in patients without OINV were twice those who had OINV with HC/APAP exposures
Currently, there is no approved therapeutic to treat acute pain while preventing and reducing OINV. In clinical trials CL-108 reduced the risk of nausea and vomiting when compared to hydrocodone. The reduction in risk of nausea and vomiting has clinical relevance and compares favorably to the risk reduction reported when parenteral antiemetics were administered to prevent PONV. CL-108 also adequately managed pain with no significant increase in abuse potential. A limitation of combination antiemetic opioid medication is identifying a specific patient population that predictably requires combination therapy. Predicting OINV in an individual patient is difficult. In the randomized controlled trial following oral surgery, 20% of the patients reported nausea after a hydrocodone challenge and had no history of prior OINV or motion sickness [19]. OINV can occur in approximately half the time in patients who by history are not at risk of developing OINV but report nausea after a test dose of an opioid.
Conclusion
This brief update reports the results of clinical trials demonstrating that CL-108 is a safe and effective treatment for acute pain and the prevention of OINV in a combined formulation so that analgesia, quality of life, and function are enhanced. Selective patient populations and acute pain control environments and conditions are being identified for safe use.
REFERENCES
- Chang DJ, Desjardins PJ, Bird SR, Black P, Chen E, Petruschke RA, et al. Comparison of rofecoxib and a multidose oxycodone/ acetaminophen regimen for the treatment of acute pain following oral surgery: a randomized controlled trial. Curr Med Res Opin. 2004; 20:939-949.
- Daniels S, Casson E, Stegmann JU, Oh C, Okamoto A, Rauschkolb C, et al. A randomized, double-blind, placebo-controlled phase 3 study of the relative efficacy and tolerability of tapentadol IR and oxycodone IR for acute pain. Curr Med Res Opin. 2009;25:1551-1561.
- Kalso E, Edwards JE, Moore RA, McQuay HJ. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain. 2004;112:372-380.
- Moskovitz BL, Benson CJ, Patel AA, Chow W, Mody SH, McCarberg BH, et al. Analgesic treatment for moderate-to-severe acute pain in the United States: patients' perspectives in the Physicians Partnering Against Pain (P3) survey. J Opioid Manag. 2011;7:277–286.
- Coluzzi F, Rocco A, Mandatori I, Mattia C. Non-analgesic effects of opioids: opioid-induced nausea and vomiting: mechanisms and strategies for their limitation. Curr Pharm Des. 2012; 18:6043–6052.
- Marrett E, Kwong WJ, Frech F, Qian C. Health Care Utilization and Costs Associated with Nausea and Vomiting in Patients Receiving Oral Immediate-Release Opioids for Outpatient Acute Pain Management. Pain Ther. 2016;5(2):215-226.
- Suh DC, Kim MS, Chow W, Jang EJ. Use of medications and resources for treatment of nausea, vomiting, or constipation in hospitalized patients treated with analgesics. Clin J Pain. 2011;27(6):508-517.
- Promethazine HCI prescribing information (Phenergan®), Wyeth Pharmaceuticals Inc. Philadelphia, PA 19101. 2012.
- Smith HS, Laufer A. Opioid induced nausea and vomiting. Eur J Pharmacol. 2014;722:67-78.
- Daniels S, Patrick K, Richardson S, Zhang B, Schachtel B. Efficacy of CL-108 in preventing emetic episodes during the treatment of acute pain. 17th World Congress on Pain. 2018. Boston, MA.
- Zuniga JR, Papas AS, Daniels SE, Patrick K, Muse DD, Oreadi D, et al. Prevention of Opioid-Induced Nausea and Vomiting During Treatment of Moderate to Severe Acute Pain: A Randomized Placebo-Controlled Trial Comparing CL-108 (Hydrocodone 7.5 mg/Acetaminophen 325 mg/Rapid-Release, Low-Dose Promethazine 12.5 mg) with Conventional Hydrocodone 7.5 mg/Acetaminophen 325 mg. Pain Med. 2019;20(12):2528-2538.
- Schachtel B, Daniels S, Patrick K, Royall S, Zhang B, Richardson S, et al. Does opioid-induced nausea and vomiting (OINV) interfere with pain control? J Pain. 2018;19(3):S80.
- Schachtel B, Marino M, Lazar J, Aazami H, Burnette M, Kivitz A, et al. Safety and Effectiveness of CL-108 for the Treatment of Moderate-to-Severe Pain Associated with Flares of Osteoarthritis of the Knee or Hip. JPain 2016;17(2):429.\
- Schachtel B, Daniels S, Patrick K, Richardson S, Royall S, Schachtel E, et al. Clinically Meaningful Pain Relief in Patients with Moderate to Severe Acute Pain Treated with CL-108. 16th World Congress on Pain. 2016.
- Schachtel B, Daniels S, Patrick K, Richardson S, Royall S, Schachtel E, et al. Measurement of affective, sensory, and evaluative dimensions of pain using a Qualities of Pain Index (QPI) JPain 2017;18(4):S60.
- FDA HYDEXOR Briefing Document Joint Meeting of Anesthetic and Analgesic Drug Products Advisory Committee and Drug Safety and Risk Management Advisory Committee, November 2, 2020.
- Gan TJ, Belani KG, Bergese S, Chung F, Diemunsch P, Habib AS, et al. Fourth Consensus Guidelines for the Management of Postoperative Nausea and Vomiting. Anesth Analg. 2020;131(2):411-448.
- Gan T, Schachtel B. What is the Effect on Acute Pain When a Patient’s Sleep is Disturbed by Opioid-induced Nausea and Vomiting (OINV)? JPain 2019;20(4):S54.
- Zuniga JR, Papas A, Schachtel B, Maibach H, Hersh E. The incidence of opioid-induced nausea and vomiting (OINV) in patients who by history are assumed to be not at risk of developing OINV. 16th World Congress on Pain. 2016.
Citation: Neal TW, Zuniga JR (2021) Prevention of Opioid Induced Nausea and Vomiting During Treatment of Acute Pain with Combination Antiemetic Opioid Medication: An Update and Review. J Pain Manage Med. 7:155.
Copyright: © 2021 Neal TW, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.