Indexed In
- Open J Gate
- Genamics JournalSeek
- Ulrich's Periodicals Directory
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Proquest Summons
- Scholarsteer
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2024) Volume 14, Issue 3
Preparation and Evaluation of Acebutolol Mucoadhesive Films
Nalini Kanta Sahoo*, D. Kusuma and S. Jyothi SriReceived: 10-Aug-2020, Manuscript No. JMST-24-5955; Editor assigned: 13-Aug-2020, Pre QC No. JMST-24-5955 (PQ); Reviewed: 27-Aug-2020, QC No. JMST-24-5955; Revised: 01-Aug-2024, Manuscript No. JMST-24-5955 (R); Published: 29-Aug-2024, DOI: 10.35248/2155-9589.24.14.395
Abstract
Mucoadhesive drug delivery offers a safe and easy method of drug utilization, because drug absorption can be promptly terminated in case of toxicity by removing the dosage form. A mucoadhesive film for systemic administration of acebutolol has been developed using HPMC K4M, HPMC E5, HPMC E15, Carbopol and Eudragit and ethanol by solvent casting method. The prepared films characterized by means of film thickness, swelling capacity, disintegration, drug release, weight variation, folding endurance, etc. The in vitro disintegration time and dissolution time of the optimized formulation (F12) was found to be 9 seconds and 99.23% within 8 mins respectively. FTIR studies showed no drug polymer interaction takes place. These results revealed that mucoadhesive films of acebutolol could be formulated for immediate drug release to ensure symptomatic relief which leads to improved patient compliance in the management of hypertension.
Keywords
Acebutolol; Solvent casting; HPMC E15; FTIRs; Mucoadhesive; Carbopol
Introduction
Over the last two decades mucoadhesion becomes of interest for its potential to optimize localized drug delivery, by retaining a dosage form at the site of action (with in gastro intestinal tract) or systemic delivery, by retaining a formulation in intimate contact with absorption site (in the buccal cavity). Mucoadhesion may be defined as a state in which two materials, one of which mucus or a mucous membrane, is held together for extended period of time. These mucoadhesive drug delivery systems improve the bioavailability of the drugs by bypassing the first pass effects and avoiding the presystemic elimination of the drug within the GI tract. Out of the various sites available for mucoadhesive drug delivery, buccal mucosa is the most suited one for local as well as systemic delivery of drugs [1]. It's anatomical and physiological features like presence of smooth muscles with high vascular perfusion, avoidance of hepatic first pass metabolism and hence can potentially improve bioavailability are the unique features which make it as an ideal route for mucoadhesive drug delivery.
In the present investigation, the drug acebutolol has been selected for the formulation mucoadhesive films. Acebutolol is one of the commonly prescribed angiotensin drugs. It has low bioavailability (40%-60%) due to hepatic first pass metabolism. Hence to improve its therapeutic efficacy and bioavailability the drug may be administered by buccal route through buccal films. Mucoadhesive delivery of acebutolol may circumvent hepatic first pass metabolism and improve bioavailability. Hence the present work deals with the formulation and characterization of mucoadhesive buccal film of acebutolol using mucoadhesive polymer.
Materials and Methods
Acebutolol procured from goldfish pvt. Ltd, HPMC K4M, HPMC E15, HPMC E5, Eudragit procured from S.D. Fine chem. Ltd., Mumbai. Carbopol, PEG 200 procured from LOBA Chemie Pvt. Ltd. Mumbai. Aspartame, citric acid procured from Thermo Fisher Scientific India Pvt. Ltd. Mumbai. Straw berry procured from MSN Labs Ltd., Hyderabad [5].
Preparation
The mucoadhesive films were prepared by the method of solvent casting technique employing ‘O’ shape ring placed on a glass surface as substrate by using different polymers. The polymeric solutions are levigation which served the purpose of plasticizer as well as penetration enhancer [2]. The solution was mixed occasionally to get semisolid consistency. Then the solution was subjected to sonication in a bath sonicator to remove the air bubbles.
The dried films were separated and the backing membrane used was aluminium foil. Then the formulations were stored in desiccators until further use. The formulation of mucoadhesive of films is shown in Tables 1-3.
| Ingredients | F1 | F2 | F3 | F4 | F5 |
|---|---|---|---|---|---|
| Acebutolol | 200 | 200 | 200 | 200 | 200 |
| HPMC K4M | 100 | 100 | 100 | 100 | 100 |
| Carbopol | - | 50 | - | 50 | - |
| Eudragit | 50 | - | 50 | - | 50 |
| PEG 200 | 20 | 20 | 20 | 20 | 20 |
| Aspartame | 5 | 5 | 5 | 5 | 5 |
| Citric acid | 25 | 25 | 25 | 25 | 25 |
| Straw berry | Q.S | Q.S | Q.S | Q.S | Q.S |
| Water | Q.S | Q.S | Q.S | Q.S | Q.S |
Table 1: Formulation of mucoadhesive films by using HPMC K4M.
| Ingredients | F6 | F7 | F8 | F9 | F10 |
|---|---|---|---|---|---|
| Acebutolol | 200 | 200 | 200 | 200 | 200 |
| HPMC E 15 | 100 | 100 | 100 | 100 | 100 |
| Carbopol | 50 | - | 50 | - | 50 |
| Eudragit | - | 50 | - | 50 | - |
| PEG 200 | 20 | 20 | 20 | 20 | 20 |
| Aspartame | 5 | 5 | 5 | 5 | 5 |
| Citric acid | 25 | 25 | 25 | 25 | 25 |
| Straw berry | Q.S | Q.S | Q.S | Q.S | Q.S |
| Water | Q.S | Q.S | Q.S | Q.S | Q.S |
Table 2: Formulation of acebutolol by using HPMC E15.
| Ingredients | F11 | F12 | F13 | F14 | F15 |
|---|---|---|---|---|---|
| Acebutolol | 200 | 200 | 200 | 200 | 200 |
| HPMC E 5 | 100 | 100 | 100 | 100 | 100 |
| Carbopol | - | 50 | - | 50 | - |
| Eudragit | 50 | - | 50 | - | 50 |
| PEG 200 | 20 | 20 | 20 | 20 | 20 |
| Aspartame | 5 | 5 | 5 | 5 | 5 |
| Citric acid | 25 | 25 | 25 | 25 | 25 |
| Straw berry | Q.S | Q.S | Q.S | Q.S | Q.S |
| Water | Q.S | Q.S | Q.S | Q.S | Q.S |
Table 3: Formulation of acebutolol by using HPMC E5.
Evaluation parameters
Thickness and weight variation: The thickness of the film at three different points was determined using thickness gauge and the films were then weighed individually using digital balance to determine the weight of each film taken out from the casted film. The films were subjected to weight variation by individually weighing ten randomly selected films. Such determinations were carried out for each formulation.
Folding endurance: Strip of prepared film (4 cm × 4 cm) was folded repeatedly at the same place till it broke. The number of times the film could be folded at the place without breaking or cracking is equal to the value of folding endurance.
Tensile strength (Kg/cm2): The instrument used to measure the tensile strength designed in our laboratory especially for this project work. The instrument is a modification of chemical balance used in normal laboratory. One pan of the balance was replaced with one metallic plate having a hook for attaching the film [3]. The equilibrium of the balance was adjusted by adding weight to the pan of balance. The instrument was modified in such a way that the film can be fixed up between two hooks of horizontal beams to hold the test film. A film of 2.5 cm length was attached to one side hook of the balance and the other side hook was attached to plate fixed up to the pan.

Surface pH: To determine surface pH, 42 films of each formulation were allowed to swell for two hours on the surface of an agar plate. Surface pH was measured by using pH paper placed on the surface of the swollen film as per reported method. A mean of three readings was recorded.
Swelling index: Mucoadhesive film of 4 cm × 4 cm area from each formulation was taken. Initial weight of the film was taken by using single pan balance (w1 gm) and it was placed in a petri dish containing 50 ml of water. After definite interval film was removed and blotted with filter paper and weighed again (w2 gm) [4].The swelling index was calculated from the formula.
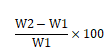
Where, w2=wet weight of the film, w1=dry weight of the film.
Drug content uniformity: A film of 4 cm × 4 cm area equal diameter were taken in separate buffer was added and continuously stirred. The solutions were filtered, suitably diluted and analyzed in a UV spectrometer. The average of drug content of three films was taken as final reading.
In vitro dissolution studies
The in-vitro dissolution studies were conducted using buffer (300 mL). The dissolution studies were carried out using USP dissolution apparatus XXIV (Electrolab, Mumbai, India) at 37 ± 0.5°C and at 50 rpm using specified dissolution media. Each film with dimension (4 cm2 of each) was placed on a stainlesssteel wire mesh with sieve opening 700 μm. The film sample placed on the sieve was submerged into dissolution media. Samples were withdrawn at regular time intervals and filtered through 0.45 μm Whatman filter paper and were analyzed spectrophotometrically. To maintain the volume, an equal volume of fresh dissolution medium maintained at same temperature was added after withdrawing samples. The absorbance values were converted to concentration using standard calibration curve previously obtained by experiment. The dissolution testing studies were performed in triplicate for all the batches.
Stability studies
The stability study of the optimized mucoadhesive films was carried out under different conditions according to ICH guidelines. The film was packed in the aluminium foil and stored in a stability chamber for stability studies. Accelerated stability studies were carried out at 40°C/75% RH for the best formulations for 6 months [5]. The patches were characterized for the drug content and other parameters during the stability study period.
Results and Discussion
Preparation of mucoadhesive films of acebutolol
Mucoadhesive films of acebutolol were prepared by solvent casting technique is shown Figure 1 with the use of mucoadhesive polymers such as carbopol, eudragit. The prepared films were evaluated for different physicochemical tests such as weight variation, thickness, content uniformity, swelling index, surface pH, in vitro disintegration time and in vitro drug release studies [6].

Figure 1: Preparation of acebutolol mucoadhesive films.
Evaluation of mucoadhesive films
Thickness of all mucoadhesive films was measured with digital vernier calliper (Table 4). The optimized film has thickness of 0.221 ± 0.03 mm. A result of thickness measurement showed that as the concentration of polymer increases, thickness of mucoadhesive film also increases. A result showed that as the concentration of polymer increases weight of film also increases. The weight variation of the optimized formulation was in the range of 21 ± 0.60 mm, which was acceptable [7].
The swelling of the films were observed in pH 6.8 phosphate buffer solution F12. Swelling was more pronounced in films F12 which containing HPMC and carbopol it is shown in Table 4.
| Formulation code | Weight (mg) | Thickness (mm) | Disintegration time (sec) | Swelling index (%) |
|---|---|---|---|---|
| F1 | 24 ± 0.65 | 0.224 ± 0.05 | 11 ± 0.22 | 27 ± 0.30 |
| F2 | 27 ± 0.68 | 0.223 ± 0.05 | 15 ± 0.26 | 29 ± 0.30 |
| F3 | 25 ± 0.65 | 0.225 ± 0.06 | 14 ± 0.24 | 40 ± 0.45 |
| F4 | 26 ± 0.67 | 0.226 ± 0.06 | 13 ± 0.24 | 41 ± 0.45 |
| F5 | 23 ± 0.64 | 0.224 ± 0.05 | 11 ± 0.22 | 36 ± 0.38 |
| F6 | 24 ± 0.65 | 0.223 ± 0.05 | 12 ± 0.22 | 38 ± 0.40 |
| F7 | 25 ± 0.65 | 0.226 ± 0.06 | 10 ± 0.22 | 27 ± 0.18 |
| F8 | 22 ± 0.63 | 0.224 ± 0.05 | 16 ± 0.25 | 20 ± 0.21 |
| F9 | 27 ± 0.68 | 0.223 ± 0.05 | 14 ± 0.24 | 22 ± 0.24 |
| F10 | 24 ± 0.65 | 0.225 ± 0.06 | 11 ± 0.22 | 24 ± 0.26 |
| F11 | 26 ± 0.67 | 0.223 ± 0.05 | 12 ± 0.22 | 28 ± 0.30 |
| F12 | 21 ± 0.60 | 0.221 ± 0.03 | 8 ± 0.21 | 48 ± 0.35 |
| F13 | 23 ± 0.64 | 0.224 ± 0.05 | 10 ± 0.22 | 29 ± 0.30 |
| F14 | 24 ± 0.65 | 0.225 ± 0.06 | 11 ± 0.22 | 27 ± 0.29 |
| F15 | 22 ± 0.63 | 0.226 ± 0.06 | 13 ± 0.24 | 24 ± 0.26 |
Table 4: Evaluation parameters of acebutolol mucoadhesive films.
The disintegrating time of all the formulations was ranges from 8 to 16 sec. The disintegration time of optimized formulation (F12) was found to be 8 sec, which was very less and desirable for quick onset of action it is shown in Figure 2.
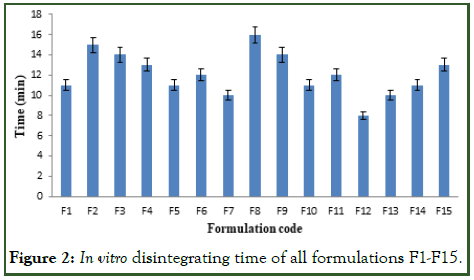
Figure 2: In vitro disintegrating time of all formulations F1-F15.
Drug content in the mucoadhesive films was evaluated and the values were found to be between 91.45 ± 0.45 to 99.23 ± 0.55%. Surface pH of all mucoadhesive films prepared by using different polymers was found to be in the range of 6.14 to 6.94 pH. Results revealed that optimized formulation (F12) showed better tensile strength (11.7g/cm2) and moderate% elongation (9.8) [8]. Folding endurance of mucoadhesive film increases. The optimized film (F12) has folding endurance value of 119 ± 4, which was desirable. Moisture content of mucoadhesive films ranges from 4.08% to 4.89% these are shown in Tables 5 and 6.
| Formulation code | Drug content (%) | Moisture content (%) | Folding endurance (count) | Surface pH |
|---|---|---|---|---|
| F1 | 92.02 ± 0.45 | 4.87 ± 0.48 | 97 ± 1 | 6.23 ± 0.3 |
| F2 | 91.45 ± 0.45 | 4.70 ± 0.32 | 95 ± 2 | 6.14 ± 0.2 |
| F3 | 94.63 ± 0.48 | 4.72 ± 0.33 | 92 ± 3 | 6.20 ± 0.3 |
| F4 | 95.24 ± 0.48 | 4.64 ± 0.30 | 91 ± 1 | 6.32 ± 0.4 |
| F5 | 97.17 ± 0.52 | 4.34 ± 0.33 | 101 ± 2 | 6.30 ± 0.4 |
| F6 | 93.89 ± 0.46 | 4.75 ± 0.34 | 105 ± 5 | 6.45 ± 0.5 |
| F7 | 96.36 ± 0.47 | 4.66 ± 0.31 | 96 ± 1 | 6.56 ± 0.6 |
| F8 | 94.78 ± 0.48 | 4.54 ± 0.28 | 98 ± 2 | 6.74 ± 0.8 |
| F9 | 93.45 ± 0.46 | 4.38 ± 0.19 | 110 ± 3 | 6.84 ± 0.9 |
| F10 | 92.28 ± 0.45 | 4.66 ± 0.31 | 114 ± 1 | 6.79 ± 0.8 |
| F11 | 91.79 ± 0.45 | 4.89 ± 0.48 | 106 ± 2 | 6.67 ± 0.7 |
| F12 | 99.23 ± 0.55 | 4.08 ± 0.11 | 119 ± 4 | 6.94 ± 0.9 |
| F13 | 94.66 ± 0.48 | 4.66 ± 0.31 | 101 ± 2 | 6.54 ± 0.6 |
| F14 | 95.20 ± 0.48 | 4.52 ± 0.24 | 108 ± 1 | 6.79 ± 0.8 |
| F15 | 98.37 ± 0.52 | 4.68 ± 0.32 | 99 ± 3 | 6.37 ± 0.4 |
Table 5: Evaluation parameters of acebutolol mucoadhesive films.
| Formulation code | Tensile strength (g/cm2) | Percent elongation (%) |
|---|---|---|
| F12 | 11.7 | 9.8 |
Table 6: Tensile strength and percent elongation.
The cumulative% drug release for the formulations F1 to F15 are tabulated in Tables 7-9 and Figures 3-5.
The optimized formulation (F12) shows highest percent of drug release 99.89 ± 5.25 by the end of 9 min [9].
| Time (min) | F1 | F2 | F3 | F4 | F5 |
|---|---|---|---|---|---|
| 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 1 | 23.17 ± 2.05 | 28.19 ± 2.15 | 32.67 ± 2.16 | 35.66 ± 2.20 | 38.16 ± 2.30 |
| 3 | 33.64 ± 2.16 | 39.46 ± 2.20 | 45.67 ± 2.86 | 48.19 ± 2.89 | 52.18 ± 2.98 |
| 5 | 48.96 ± 2.89 | 52.19 ± 2.98 | 62.19 ± 3.42 | 59.11 ± 3.20 | 67.11 ± 3.46 |
| 7 | 66.71 ± 3.45 | 70.20 ± 4.08 | 80.16 ± 4.38 | 70.62 ± 4.08 | 79.61 ± 4.37 |
| 9 | 74.88 ± 4.10 | 92.16 ± 5.04 | 90.16 ± 5.02 | 94.11 ± 5.10 | 88.21 ± 4.90 |
| 10 | 89.17 ± 4.98 | 96.18 ± 5.12 | 98.19 ± 4.19 |
Table 7: In vitro drug release studies of formulation F1 to F5.
| Time (min) | F6 | F7 | F8 | F9 | F10 |
|---|---|---|---|---|---|
| 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 1 | 23.81 ± 2.09 | 37.66 ± 2.30 | 28.11 ± 2.10 | 34.19 ± 2.24 | 25.61 ± 2.10 |
| 3 | 36.42 ± 2.15 | 56.19 ± 3.06 | 39.64 ± 2.31 | 54.66 ± 3.04 | 38.19 ± 2 .31 |
| 5 | 48.19 ± 2.89 | 64.11 ± 3.42 | 58.66 ± 3.19 | 77.19 ± 4.18 | 56.18 ± 3.06 |
| 7 | 55.61 ± 3.05 | 83.61 ± 4.50 | 75.14 ± 4.12 | 85.18 ± 4.89 | 68.20 ± 3.51 |
| 9 | 69.24 ± 3.52 | 93.42 ± 5.04 | 96.66 ± 5.11 | 90.16 ± 5.02 | 74.62 ± 4.11 |
| 10 | 89.72 ± 4.98 | 92.45 ± 5.04 | 97.66 ± 5.15 |
Table 8: In vitro drug release studies of formulation F6 to F10.
| Time (min) | F11 | F12 | F13 | F14 | F15 |
|---|---|---|---|---|---|
| 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 1 | 28.11 ± 2.10 | 48.13 ± 2.90 | 37.64 ± 2.30 | 39.16 ± 2.32 | 33.61 ± 2.27 |
| 3 | 34.61 ± 2.28 | 56.74 ± 3.06 | 42.11 ± 2.82 | 56.17 ± 3.06 | 58.12 ± 3.08 |
| 5 | 48.19 ± 2.90 | 79.66 ± 4.21 | 69.46 ± 3.53 | 65.18 ± 3.42 | 75.64 ± 4.11 |
| 7 | 52.71 ± 3.02 | 90.14 ± 5.02 | 75.66 ± 4.11 | 72.34 ± 4.08 | 82.19 ± 4.49 |
| 9 | 78.66 ± 4.20 | 99.89 ± 5.25 | 82.19 ± 4.49 | 89.99 ± 4.98 | 98.02 ± 5.08 |
| 10 | 90.14 ± 5.02 | 93.44 ± 5.04 | 91.24 ± 5.03 |
Table 9: In vitro drug release studies of formulation F7 to F15.
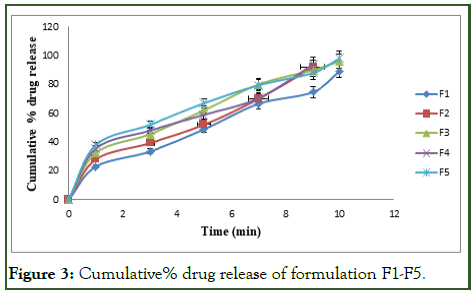
Figure 3: Cumulative% drug release of formulation F1-F5.
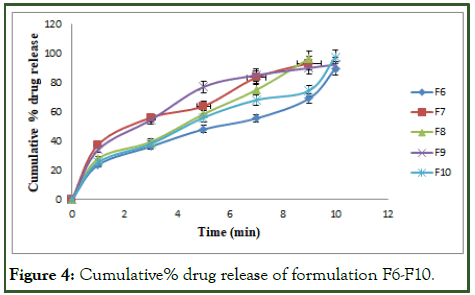
Figure 4: Cumulative% drug release of formulation F6-F10.
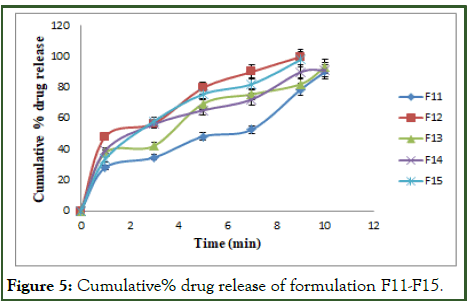
Figure 5: Cumulative% drug release of formulation F11-F15.
The optimized formulation of acebutolol mucoadhesive film (F12) was best explained by first order, it is shown in Table 10 as the plots showed the highest linearity (r2=0.994), followed by, Higuchi (r2=0.974), Korsmeyer Peppas (r2=0.969) and then zero order (r2=0.928). The corresponding plot for the Korsmeyer- Peppas equation of the optimized formulation F12 indicated good linearity [10]. The release exponent ‘n’ was found to be for F12 is 0.71, which appears to indicate Fickian diffusion and may indicate that the drug release was controlled by first order release are shown in Figures 6-9.
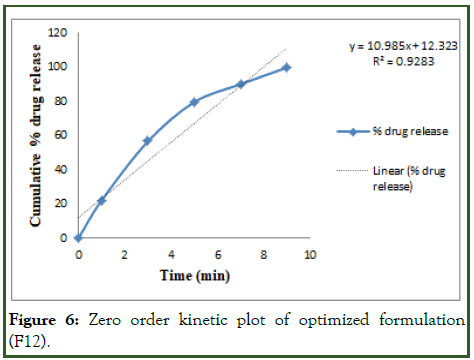
Figure 6: Zero order kinetic plot of optimized formulation (F12).
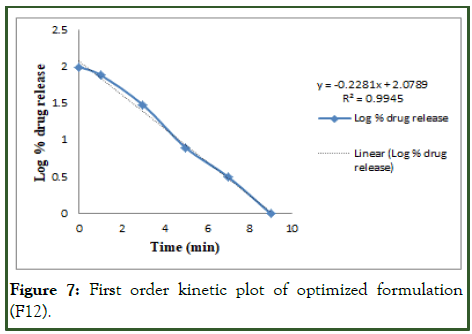
Figure 7: First order kinetic plot of optimized formulation (F12).
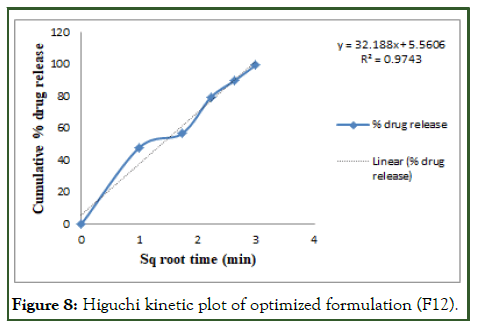
Figure 8: Higuchi kinetic plot of optimized formulation (F12).
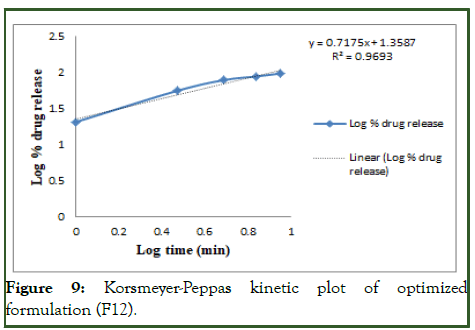
Figure 9: Korsmeyer-Peppas kinetic plot of optimized formulation (F12).
Stability studies
Optimized formulation was selected for stability studies on the basis of high cumulative% drug release. Disintegrating time, drug content and in vitro drug release studies were performed for 6 months according to ICH guidelines [11]. From these results it was concluded that, optimized formulation F12 is stable and retained their original properties with minor differences which depicted in the Tables 10 and 11 and Figures 10-12.
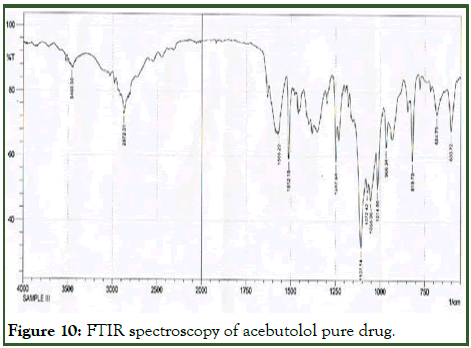
Figure 10: FTIR spectroscopy of acebutolol pure drug.
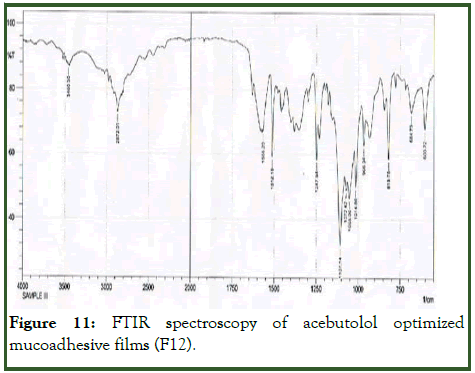
Figure 11: FTIR spectroscopy of acebutolol optimized mucoadhesive films (F12).
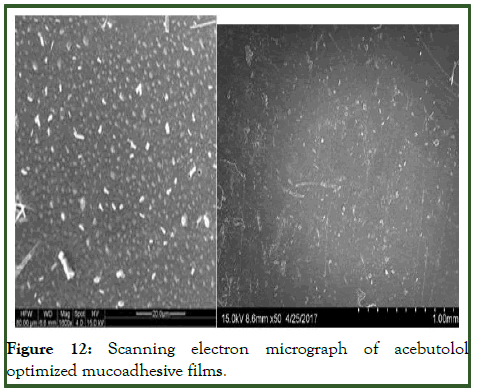
Figure 12: Scanning electron micrograph of acebutolol optimized mucoadhesive films.
| Formula code | Zero order | First order | Higuchi | Korsmeyer-Peppas | ||||
|---|---|---|---|---|---|---|---|---|
| R2 | K | R2 | K | R2 | K | R2 | N | |
| F12 | 0.928 | 10.98 | 0.994 | 0.228 | 0.974 | 32.18 | 0.969 | 0.717 |
Table 10: Release order kinetics for optimized release.
| Retest time for optimized formulation (F12) | Disintegrating time (sec) | Drug content | In vitro drug release profile (%) |
|---|---|---|---|
| 0 days | 8 | 99.23 | 99.89 |
| 30 days | 8 | 99.02 | 99.26 |
| 60 days | 9 | 98.89 | 98.74 |
| 90 days | 10 | 98.1 | 98.36 |
| 120 days | 10 | 97.24 | 98.12 |
| 180 days | 11 | 97.03 | 97.79 |
Table 11: Physicochemical characteristics of optimized formulation stored at 40 ± 2°C/75 ± 5%RH.
Conclusion
The present study indicates a good potential of erodible mucoadhesive films containing acebutolol for systemic delivery with an added advantage of circumventing the hepatic first pass metabolism. The results of the study show that therapeutic level of acebutolol can be delivered by buccal cavity. It may concluded that the formulation F12 shows good swelling, good flexibility, a convenient residency time and promising sustained drug release, thus seems to be a potential candidate for development of mucoadhesive film for effective therapeutic use. The mechanism of drug release was diffusion followed by first order kinetics. FTIR studies showed no drug polymer interaction takes place. These results revealed that mucoadhesive films of acebutolol could be formulated for immediate drug release to ensure symptomatic relief which leads to improved patient compliance in the management of hypertension.
References
- Avachat AM, Gujar KN, Wagh KV. Development and evaluation of tamarind seed xyloglucan-based mucoadhesive buccal films of rizatriptan benzoate. Carbohydr Polym. 2013;91(2):537-542.
[Crossref] [Google Scholar] [PubMed]
- de Vries ME, Bodde HE, Verhoef JC, Junginger HE. Developments in buccal drug delivery. Crit Rev Ther Drug Carrier Syst. 1991;8(3):271-303.
[Google Scholar] [PubMed]
- Kusum Devi V, Saisivam S, Maria GR, Deepti PU. Design and evaluation of matrix diffusion controlled transdermal patches of verapamil hydrochloride. Drug Dev Ind Pharm. 2003;29(5):495-503.
[Crossref] [Google Scholar] [PubMed]
- Jug M, Maestrelli F, Bragagni M, Mura P. Preparation and solid-state characterization of bupivacaine hydrochloride cyclodextrin complexes aimed for buccal delivery. J Pharm Biomed Anal. 2010;52(1):9-18.
[Crossref] [Google Scholar] [PubMed]
- Nappinnai M, Chandanbala R, Balaijirajan R. Formulation and evaluation of nitrendipine buccal films. Indian J Pharm Sci. 2008;70(5):631.
[Crossref] [Google Scholar] [PubMed]
- Nafee NA, Boraie MA, Ismail FA, Mortada LM. Design and characterization of mucoadhesive buccal patches containing cetylpyridinium chloride. Acta Pharm (Zagreb, Croatia). 2003;53(3):199-212.
[Google Scholar] [PubMed]
- McCullough MJ, Savage NW. Oral viral infections and the therapeutic use of antiviral agents in dentistry. Aust Dent J. 2005;50:S31-S35.
[Crossref] [Google Scholar] [PubMed]
- Sahni J, Raj S, Ahmad FJ, Khar RK. Design and in vitro characterization of buccoadhesive drug delivery system of insulin. Indian J Pharm Sci. 2008;70(1):61.
[Crossref] [Google Scholar] [PubMed]
- Satishbabu BK, Srinivasan BP. Preparation and evaluation of buccoadhesive films of atenolol. Indian J Pharm Sci. 2008;70(2):175.
[Crossref] [Google Scholar] [PubMed]
- Shojaei AH, Chang RK, Guo X, Burnside BA, Couch RA. Systemic drug delivery via the buccal mucosal route. Pharm Technol. 2001;25(6):70-81.
- Bhowmik D, Kumar KP, Deb L. Buccal drug delivery system-a novel drug delivery system. Res J Sci Technol. 2016;8(2):90-89.
Citation: Sahoo NK, Kusuma D, Sri SJ (2024) Preparation and Evaluation of Acebutolol Mucoadhesive Films. J Membr Sci Technol. 14:395.
Copyright: © 2024 Sahoo NK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

