Indexed In
- Open J Gate
- Genamics JournalSeek
- CiteFactor
- Cosmos IF
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- Directory of Abstract Indexing for Journals
- OCLC- WorldCat
- Proquest Summons
- Scholarsteer
- ROAD
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2020) Volume 12, Issue 4
Predictive Medicine Evolution
Vladislav Baranov1* and Helen Baranova22European Institute of Personalised Prevention and Health. 29 rue du Portier, 98000, Monaco
Received: 30-Apr-2020 Published: 30-Jun-2020, DOI: 10.35248/0974-8369.20.12.467
Abstract
Genetic Health Chart (GHCh) also known in the Russia as Genetic Pass (GP) is now considered as a major practical guide for predictive, preventive and personalized medicine (PPPM) which reflects basic achievements of human genome studies and makes major advances in line with spectacular advances of molecular technologies of human genome endeavor. Since its birth in 2000 GP has been treated as a bank for collection of personal genetic data important for prediction, prevention and for treatment of inherited and inborn disorders. Major steps of PPPM, its main problems, disappointments and advances as well as their impact in evolution of GP are briefly outlined. The basic goal of the currant genomic studies as emphasized concerns the urgent need for correct interpretation of the clinical value of genetic testing and its applicability for routine clinical practice. Feasible paths towards the gradual implementation of personal genetic data, in line with other laboratory tests, for the individualized clinical trials are discussed.
Keywords
Genetic Health Chart; Personalized medicine
Introduction
The review is devoted to the impact of human genome studies in the progress of modern medicine. Basic achievements of genome research have resulted in the deciphering human genome (2003) [1] and molecular landmarks dispersed throughout haploid genome (HapMap Project) (2004) [2], has made a tremendous contribution into our knowledge of common genetic and complex disorders. Initial genetic researches were mostly devoted to identification the genes and their mutations responsible for monogenic disorders. Current genome studies mainly focus on genetic testing and its associations with common diseases for their efficient diagnostics, prevention and personalized treatment [3].
Identification of candidate ( “ predisposition ” ) genes in the functional genetic modules underlying each common disorder and the use the genetic background for their prevention constitutes a major goal of personalized molecular medicine [4,5].
The concept of genetic pass (GP) as an personal DNA databank reflecting inherited human predisposition to different complex and monogenic disorders, with special emphasis on the state of art, and numerous difficulties related to the practical implementation of personalized medicine has appeared in early 2000 in conjunction with already recognized predictive, preventive personalized medicine initially called 3P Medicine [6,7]. One more “P” was donated from “participatory” suggested in 2008 by Leroy Hood (USA) thus giving rise to 4P Medicine (Figure 1).
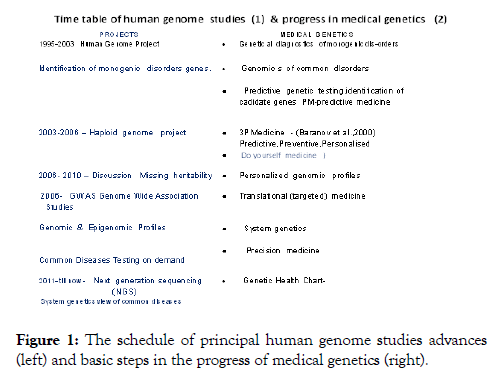
Figure 1: The schedule of principal human genome studies advances (left) and basic steps in the progress of medical genetics (right).
Predictive medicine
In 2004 National Institutes of Health recommended replacing “ personalized medicine ” with “ precision medicine, ” later converted into genomic or individualized medicine. All these names rather similar by sense - medical care should stem from the unique patient’s biology, determined by the genome. The concept of 4PM was suspected to revolutionize clinical and preventive patient care.
The problems related to the uncertainness of the results of genetic testing could be overcome at least partly by means of new molecular technologies, such as genome-wide association studies (GWAS), massive parallel DNA sequencing, next generation sequencing (NGS), genetic and epigenetic profiling. The basic tasks of genomic today could be attributed to proper evaluation the practical value of genetic testing and its applicability for the clinical benefit. Successive steps of progress in genome technologies as well as these ones in medical genetics are shown in Figure 1.
Over 1500 candidate genes associated with common diseases were picked up in human genome by functional gene mapping in many European populations summarized by Gendia (http://www.gendia.net (Gennovation (www.gennovations.com), Genosense (http://www.genosense.com), as well as by many genetic centers in the Russia (Saint-Petersburg; Tomsk, Ufa, Moscow e.a. ,[7-9]). Hundreds candidate genes associated with many common and monogenic disoders were also tested in our laboratory at the Ott’s Institute of Obstetrics, Gynecology & Reproductology in Saint-Petersburg. Original Copy of the Gene Pass as a natural synopsis of predictive genetic testing is shown in Figure 2.
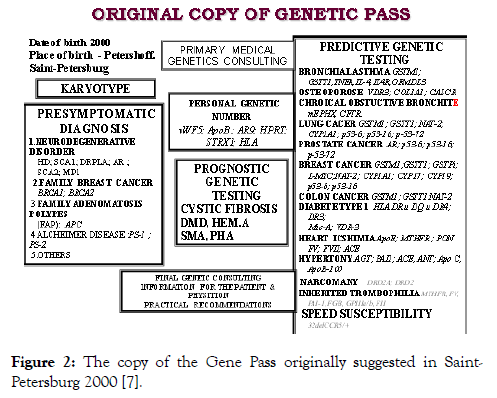
Figure 2: The copy of the Gene Pass originally suggested in Saint- Petersburg 2000 [7].
Personal Genetic Chart (Pass)
Much more enriched candidate gene charts were later suggested by many commercial genetic centers in the Europe, North America, also in the Russia and widely used for genetic profiling of direct to consumer testing. Unfortunately high rise of enthusiastic wave quickly subsides and converted to the plateau of deep disappointment provoked by negligible prognostic values of routine genetic testing. Contrary to monogenic disorders with their mutation testing precision close to 100% the values of genetic testing of common disorders usually does not exaggerate 1-2% thus being far from sufficient to cogent prediction.
Two main faults of comparative gene testing have been suspected: shortage of candidate genes identified by physiologic gene mapping (1), insufficiency of cohort quantity (2).
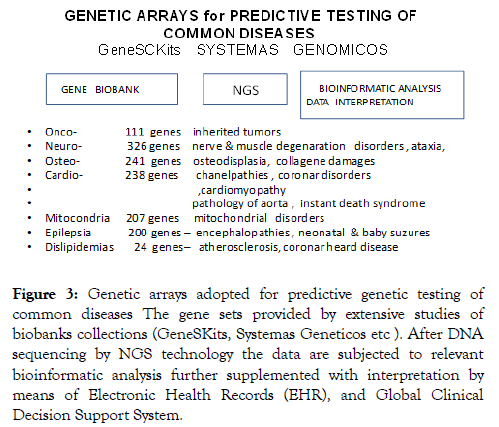
According to our data Gene Pass testing can at its best predict whether the patient belongs to the risk group of some common disease but they are not sufficient to predict the onset of the disorder in particular person. Thus the results of paired candidate testing in the patients if compared to these ones in healthy specimen looked very scanty. Illusion to overcome this obstacle come with implementation of new molecular technology – Genome Wide Association Studies (GWAS) which for the first time provided whole genome screening of candidate genes in abandon cohorts of patients and the control counterparts. Thus it looked very plausible that GWAS technology will solve principal task of predictive testing, making much more reliable prognostic values. But expected miracle has not happened. Actually application of GWAS technology significantly increased the number of candidate genes for each of 300 CD studied by this technology but the prognostic values of GWAS tests still were within merger ranges 10-25% at the best (Figure 3).
Figure 3: Genetic arrays adopted for predictive genetic testing of common diseases The gene sets provided by extensive studies of biobanks collections (GeneSKits, Systemas Geneticos etc ). After DNA sequencing by NGS technology the data are subjected to relevant bioinformatic analysis further supplemented with interpretation by means of Electronic Health Records (EHR), and Global Clinical Decision Support System.
Predictive value of these complex amplisec-arrays technology still remains unknown and needs further verification.
The obvious phenotypic shortage of hereditary information gained the term “ missing heritability ” [10,11]. Several main reasons of this discrepancy are suspected. Small risk values of unfavorable alleles (OR -1.1-1.5) (1) (1). GWAS does not detect SNP polymorphisms with low frequency (<0,5%) (2). Inter genetic SNP associated with common disorders (CD) are not correctly interpreted (3). Gene-gene interactions (epistatic effect) not properly considered (4). Epigenetic & VNTR variations were not taken into account (5); Underestimation of exogenetic damages (6). Taken into account listed limitations makes clear that GWAS by itself a priory is not sufficient for objective predictive testing. Moreover it looks that DNA analysis by itself will never be sufficient to reach 100% confidence of predictive value [12].
These considerations are in line with genetic twin studies. In spite of genetic identity monozygote twins differ in frequency of many complex traits (f.e.height) as well as in frequency of CD. It means the development of complex traits and CD should be attributed to both genetic and epigenetic interactions. Thus the search for dominant unfavorable alleles, relevant considerations of CNV input, gene-gene interactions and epigenetic regulation of genome functions are obvious mainstreams in missing heritability study [12].
System Genetics & Integrative Omics
New option for the further advancement of PM should be addressed to implementation of DNA sequencing. According to Eric Lander (Massachussets Inst. of Technology) implementation of NGS in conjunction with abandon cohorts of patients should be the most efficient way for cutting the Gordiev helix of ‘missing heritability” [13]. The accuracy of nucleotide variation detection supplemented with relevant bioinformatic analysis provides an ample opportunities for precise detection of all nucleotide fluctuations both meaningful and senescent. In 2017г FDA Committee (USA) discarded its former order to seize prognostic genetic testing by American genetic company 23andMe. The company resumed commercial gene testing for 10 common CD (Crone, Alzheimer, Parkinson diseases, prostate and breast cancers [13]. Genetic testing is also used for detection of inherited predisposition to over 100 CD by means of system genetics approach [14]. The precision of genetic testing increased to 20% for prostate cancer and up to 80% for Crone disease. Testing of hundreds genes with assistance of high density arrays was a main prerequisite of this obvious success [15-17].
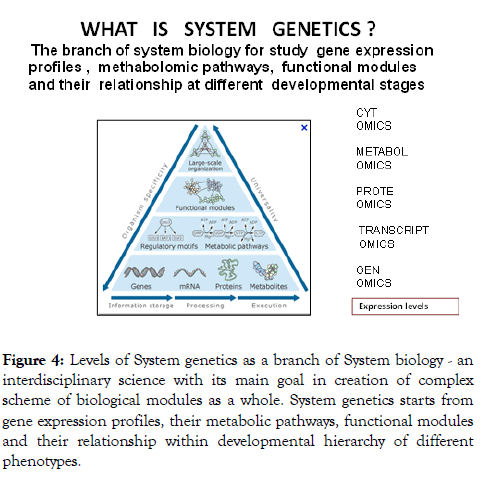
Massive gene testing gained more knowledge in the mapping of many new CD genes and gene regulation loci of substantial commercial value. So called “black-chain technology” now gets big financial support from many commercial companies and pharmaceutical industry destined for production of targeted drugs and personalized treatment [18]. But increase in number of tested genes does not much improved predictive gene testing efficiency. Comparative analysis of functional activity of candidate genes expression in normal and abnormal development should be taken as the best way to understand the genetic architecture and thus the pathogenomic of common disease [9]. Each separate technology cannot give objective view of pathology but their integrative approach might be rather fruitful. The integration of different omics data (genome, transcriptom, proteome, metabolome) also known as system genetic approach (Figure 4) is now widely used in molecular medicine [19].
Figure 4: Levels of System genetics as a branch of System biology - an interdisciplinary science with its main goal in creation of complex scheme of biological modules as a whole. System genetics starts from gene expression profiles, their metabolic pathways, functional modules and their relationship within developmental hierarchy of different phenotypes.
Thorough Network analysis of different omics significantly increases the chances to find new biomarkers and new candidate genes [20]. Integration of protein data, NGS and SNP –GWAS results is now tried in studies of heart diseases, diabetes type 2, autism. System genetic approach is now considered the best to understand integrative genetic architecture of CD and it becomes dominated in PM on its way to Precision Medicine [21].
Evolution of Predictive Medicine
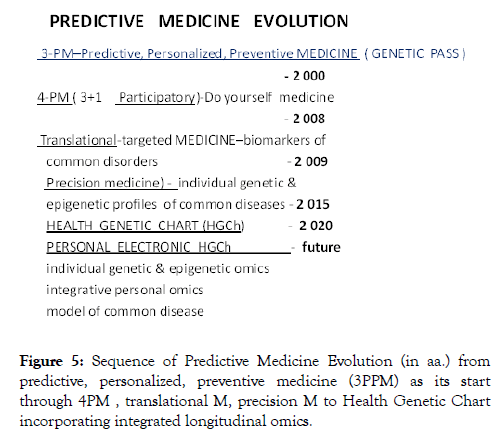
Developmental chronology of PM is presented in Figure 5.
Figure 5: Sequence of Predictive Medicine Evolution (in aa.) from predictive, personalized, preventive medicine (3PPM) as its start through 4PM , translational M, precision M to Health Genetic Chart incorporating integrated longitudinal omics.
Thus started with Human Genome project PM and its synopsis GP has successfully overcome several scientific and practical barriers which logically put them within 20 years in the embrace of the Health Genetic Chart and its Personal Electronic HGCh (Figure 5).
According to European PPPM Organization Program [6] as the first step of its pathway PPPM should rely on massive NGS of human genomes to clarify their population, ethnic, social and inter-tissue specificities. Concomitantly integrated longitudinal omics profiles should be compared with relevant clinical traits and laboratory analysis of the same patients thus creating integrative personal gene sets of affected organs and physiological systems. All these data as expected much enrich the capacity of PPPM. Moreover each patients in this system becomes a source of valuable information for the further in depth studies [6]. On the other hand the pathway program prefer to deal not with the patient himself but only with his models in virtual world does not looks robust as no any model can ever completely mimic all features of original subject [22,23]. To our mind the term “ precision medicine ’ is illusive and misleading as the medicine according to classical definition should be treated an ART not a SCIENCE (If not for the great variability among individuals, medicine might have been a science and not an art. ” Sir William Osler (1849-1919). Probability not Determinism governs basic concepts of Medicine and the same stands true for Predictive Medicine. But whatever happens the Genome is always a solid ground of the Personal Life. The Era of Predictive Medicine has arrived!
Funding
This research was funded by the Russian Science Foundation, grant number 18-75-10046.
REFERENCES
- Baltimore D (2002) Our genome unveiled. Nature. 409:814-815
- Frazer KA, Ballinger DG, Cox DR, Hinds DA, Stuve LL, et al. A second generation human haplotype map over 3.1 million SNPs. Nature. 2007;449(7164):851-861.
- Baranov VS. Genome Paths: A Way to Personalized and Predictive Medicine. Acta Naturae. 2009;3:70-80.
- Jain KK. Text book of personalized medicine 2nd edn. Springer 2015;732.
- Hall JA, Gertz R, Amato J, Pagliari C. Transparency of genetic testing services for ‘health, wellness and lifestyle’: analysis of online prepurchase information for UK consumers Europ J Human. Genetics. 2017;25:908-917.
- Personalized Medicine for the European Citizen Towards more precise medicine for the diagnosis, treatment and prevention of disease. Europ Sci Found. 2015.
- Baranov VS, Baranova H, Ivaschenko T, Aseev MV (2000) Human genome and predisposition genes. Introduction into predictive medicine. Intermedica.
- Baranov VS, Ivaschenko T, Baranova H, Glotov AS, Bespalova ON. Genetic pass –as the solid base of individual and predictive medicine « N-L Edit.”. 2009:452.
- Puzyrev VP (2014) Medical pathogenetics. Vavilov’s Genetic & Selection J. 2014;18:7-21.
- Eicher EE, Flint J, Gibson G, Kong A. Missing heritability and strategies for finding the underlining causes of complex diseases. Nat Rev Genet. 2010;11:446-450.
- Manolio TA, Collins FrS, Cox NJ. Finding the missing heritability of complex. Nature. 2010;461:747-753.
- Lander E. Cutting the Gordian Helix. Regulating of Genomic testing in the Era of Precision Medicine. New Egland J Med. 2015;13:1-7.
- The FDA Grant approval for 23 and me direct to consumer Health Risk report April 7, 2017. Source PeJ Getty Images.
- Baranov VS, Baranova H. Personal genetic chart: state of art, today and tomorrow .Vestn Roszdravadzor. 2018;2:16-23.
- Williams Sh. Companies are building platforms based on blockchain technology to let individuals control and directly profit from their genomic ad medical inflammation. The Scientist. 2018.
- Ritchi MD, Andrade M, Kuivaniemi H. The foundation of precision medicine^ integration of electronic health records with genomics through basic, clinical and translational research. Foundation in Genet. 2015.
- van El CG, Cornel MC, Borry P, Hastings RJ, Fellman F, Hodgson SV, et al. (2018). Whole –genome sequencing in health care. Recommendations in the European Society of Human Genetics. Europ J Hum Genet. 2018;21(6):580-584.
- Sieberts SK, Schadt EE. Moving toward a system genetics view of disease. Mamm Genome. 2007;18(6-7):389-401.
- Ritchi MD, Andrade M, Kuivaniemi H. The foundation of precision medicine, integration of electronic health records with genomics through basic, clinical and translational research. Front Genet. 2015;6:104.
- Podkolodny UV, Podkolodnaya OG. Ontologies in bioinformatics and systems biology. Russian Journal of Genetics: Applied Research. 2016;6(7):749-758.
- Baranov VS, Baranova H. Human genome, epigenetic of common diseases, personified medicine. Biosphera. 2012;4:77-85.
- Karczewski KJ, Snyder MP. Integrative omics for health and disease Nature Reviews Genetics 19: Jain K.K. 2015, Text book of personalized medicine 2d edition. Springer 732p299-310.
- Grant B. Moving towards individualized medicine for all. The Scientist. 2019.
Citation: Baranov VS, Baranova H (2020) Predictive Medicine Evolution. Biol Med (Aligarh) 12:467. doi: 10.35248/0974-8369.20.12.467
Copyright: © 2020 Baranov VS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.