PMC/PubMed Indexed Articles
Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- CiteFactor
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2020) Volume 11, Issue 7
Potential Role of Native Arbuscular Mycorrhizal Fungi (AMF) in the Restoration of Laurisilva
Catarina Drumonde-Melo1*, Paulo Borges1, Helena Freitas2 and Luis Nunes32CFE – Centre for Functional Ecology, Department of Life Sciences, University of Coimbra, 3001-401, Coimbra, Portugal
3MICOazorica Lda, Canada das Vinhas n 39, Vila de Sao Sebastiao, 9700-625 Angra do Heroísmo, Terceira, Acores, Portugal
Received: 17-Jun-2020 Published: 22-Jul-2020, DOI: 10.35248/2157-7471.20.11.503
Abstract
The beneficial association of seedlings with arbuscular mycorrhizal fungi (AMF) is thought to improve early tree establishment through increased uptake of poorly labile soil nutrients (particularly P) and enhancing plant tolerance to biotic and abiotic factors. Seedlings of Juniperus brevifolia, an endemic woody plant of the Azores archipelago with potential commercial value, was grown in the nursery with and without inoculation by a commercial plant growth promoter consisting of AMF isolated from the Azores (MICOazorica). Treatments were arranged in a randomized complete block design in a greenhouse. At six months after planting, all AMF-inoculated plants were colonized. The percentage of colonization varied between 46% and 96% (Mean 70%). At harvest, all physical parameters were significantly greater in AMF-inoculated plants relative to uninoculated plants. Based on the obtained results, we strongly advise the use of native AMF, in strategies used in restoration programs in the Azores.
Keywords
Arbuscular Mycorrhizal Fungi (AMF); Endemic plant; Ecological restoration; Facilitation; Inoculation
Introduction
The Azorean native forests despite their current small area [1] harbour a greater and more diverse pool of endemic plants and animals than any other native and human-modified habitats of this archipelago. Juniperus brevifolia (Cupressaceae) is the dominant tree species in native Azorean mountain woodlands, e.g. Juniperus-Laurus forests, Juniperus-Ilex forests and Juniperus- Sphagnum woods [2]. Agriculture is important in the cultural cycle of Azores, and its intensification over recent decades, has resulted in a loss of biological diversity and the degradation of soil structure [3]. Consequently, most of Azorean native forest has been converted to agricultural lands, contributing to a considerable decrease of endemic vegetation of up to 90% in the Juniperus population, though the situation varies among islands [4,5]. Consequently, J. brevifolia is classified as vulnerable (VU) on the IUCN Red List [6]. Several efforts have been made to restore these unique ecosystems, but success has been limited because of the difficulty of multiplication and establishment of these endemic species due to their adaptation to natural habitats [7,8]. Thus, urgent action to restore and expand native forest is required to avoid continued loss of endemic species [1,3,9].
Mycorrhizas are symbiotic associations that involve a great diversity of plants (> 80%) and fungi from ascomycetes, basidiomycetes, and the Glomeromycota, the last all of which are thought to form arbuscular mycorrhizal fungi (AMF) being of greatest agronomic interest. AMF live in symbiosis with the roots of most terrestrial plants, increasing the update of water and nutrients, especially phosphorus, in exchange for carbohydrates from the plant [10]. This symbiotic relationship may improve host plant productivity [11], drought resistance [12], tolerance to soil pathogens [13,14] and heavy metals [15], and establishment and survival as a crop [16-22]. Beyond benefitting the growth of their host, AMF confer other ecosystem services, such as reducing soil erosion by promoting soil aggregation through the production of the glycoprotein glomalin [23].
AMF have also been shown to influence plant community structure [24-26], to drive plant community succession [27,28] and to regulate plant invasive success [29]. Given the different benefits that plant communities can either directly or indirectly receive through associating with AMF, the reintroduction of native AMF has the potential to promote native plant growth in restorations and to improve soil health and ecosystem quality [30-32].
The application of AMF inoculants in agriculture is increasing, but its success is limited because AMF show a broad range of functional diversity [33,34] and their effect is within the mutualism-parasitism continuum [35,36]. Therefore, deciding on the appropriate inoculum for native plants is a very important step. Native inocula, adapted not only to the local environment conditions but also to a particular host, may perform better than exotic inocula [21,37-39]. A basic understanding of the biology of AMF and an improvement in inoculum production and inoculation technology are required to advance the management of these fungi.
Here we aimed to determine the potential role of AMF native inoculum on survival and physiological aspects of J. brevifolia seedling plants under nursery conditions, to improve the success of native plant establishment in restored ecosystems.
Materials and Methods
Effectiveness of the native AM fungi
J. brevifolia seedling plants donated by nurseries of Direcao Regional dos Recursos Florestais (Azores Government) were inoculated with the native AMF inoculum produced by MICOazorica Lda. composed of a mixture of Cetraspora sp., Claroideoglomus etunicatum, Rhizophogus sp., Funneliformis mosseae and Gigaspora sp.. MICOazorica inoculum (PCT/ PT2020/050001) consists of rhizospheric samples containing spores, hyphae and mycorrhizal root fragments.
The mycorrhizal potential in this inoculum determined by the serial dilution technique [40] and estimated by the most probable number (MPN) is greater than 85 infective mycorrhizal propagules per gram of substrate. The experiment included two treatments with 16 replicates per treatment: an uninoculated control and AMF-inoculated plants. Initially, seedlings were grown in a disinfested substrate recommended for forest plants (Siro Florestal) mixed with sterilised volcanic soil (3:1). From each seedling plant, five 1-cm root fragments were collected and stained to check AMF colonisation.
Seedling found to be mycorrhiza-free were individually transplanted to 1-litre pots of the same substrate. At transplantation, each pot for AMF inoculation received 7 g of AMF native inoculum and, each uninoculated control pot received the same quantity of disinfested (autoclaved) inoculum. Pots were disposed in the greenhouse in a completely randomised design for 6 months. Pots were watered every 2 days and fertilized once a month with 100 ml of half-strength modified (P-free) Hoagland’s solution.
Harvest and data collection
Plants were harvested 6 months after inoculation. Plant fresh weight, separated into shoot and root, shoot and root length, number of branches, number of secondary roots, and shoot and root dry weights were measured. Measurements of shoot height, root length, number of branches and number of secondary roots were taken at the beginning and the end of the experiment. The shoot and root systems were separated, and the fresh weights measured only at the end of the experiment. Shoot and root dry weights were measured after oven-drying at 72°C for 48 hours.
Analysis of mycorrhizal colonization
A sample was taken of fresh roots (± 5% in fresh weight) for estimation of mycorrhizal colonization level by staining and subsequent microscopic evaluation. Root fragments were cleared and stained as described by Melo et al. [41]. AMF colonization rates were determined by the magnified intersection method [42] under a compound microscope (Axioimager A1, Zeiss) at 400x magnification.
Data analyses
Comparisons of growth measurements between inoculated and non-inoculated plants were tested by one-way ANOVA using MINITAB Release 13.31 [43]. Mycorrhizal dependency (MD) was calculated as:
Percentage mycorrhizal responsiveness = [(Dry Mass mycorrhizal plant - Dry mass non-mycorrhizal plant)/Dry mass mycorrhizal plant] × 100 [44].
Result
Mycorrhizal colonization
All non-mycorrhizal controls remained uncolonised. All inoculated plants were mycorrhizal with colonisation ranging from 46% to 96% with a mean of 70 ± 3.95%. Hyphae and arbuscules predominated, and approx. 15% of sample fields of view contained spores (Table 1 and Figure 1).
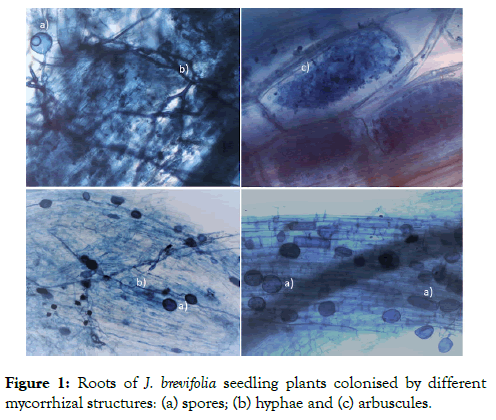
Figure 1: Roots of J. brevifolia seedling plants colonised by different mycorrhizal structures: (a) spores; (b) hyphae and (c) arbuscules.
| Main effects | F | P-value | Control plants | AMF plants |
|---|---|---|---|---|
| Initial shoot height | 0.14 | n.s | 4.44 ± 0.13 a | 4.39 ± 0.08 a |
| Final shoot height | 2.67 | n.s | 13.27 ± 0.65 a | 14.55 ± 0.46 a |
| Increase shoot height | 4.12 | * | 8.81 ± 0.57 a | 10.16 ± 0.42 b |
| Initial root length | 2.08 | n.s | 4.06 ± 0.12 a | 3.666 ± 0.13 a |
| Final root length | 21.25 | *** | 20.25 ± 0.44 a | 23.56 ± 0.57 b |
| Increase root length | 32.07 | *** | 16.19 ± 0.38 a | 19.91 ± 0.53 b |
| Initial plant height | 3.09 | n.s | 8.50 ± 0.20 a | 8.04 ± 0.17 a |
| Final plant height | 13.14 | ** | 33.51 ± 0.93 a | 38.11 ± 0.86 b |
| Increase plant height | 20.16 | *** | 25.00 ± 0.81 a | 30.06 ± 0.79 b |
| Initial number of branches | 0.33 | n.s | 3.69 ± 0.22 a | 3.50 ± 0.24 a |
| Final number of branches | 20.54 | *** | 28.38 ± 2.40 a | 42.75 ± 2.1 b |
| Increase of branches | 23.80 | *** | 24.69 ± 2.27 a | 39.25 ± 1.93 b |
| Initial number of secondary roots | 0.40 | n.s | 3.44 ± 0.20 a | 3.25 ± 0.21 a |
| Final number of secondary roots | 27.20 | *** | 7.13 ± 0.49 a | 12.69 ± 1.06 b |
| Increase of secondary roots | 30.87 | *** | 3.69 ± 0.47 a | 9.44 ± 0.99 b |
| Initial fresh plant weight | 0.00 | n.s | 1.94 ± 0.23 a | 1.87 ± 0.19 a |
| Final fresh plant weight | 9.24 | ** | 2.37 ± 0.20 a | 3.09 ± 0.20 b |
| Increase in fresh plant weight | 38.69 | *** | 0.34 ± 0.06 a | 1.21 ± 0.13 b |
| Final shoot fresh weight | 6.57 | * | 1.94 ± 0.17 a | 2.59 ± 0.19 b |
| Final shoot root weight | 18.60 | *** | 0.30 ± 0.03 a | 0.51 ± 0.04 b |
| Increase in fresh weight of plant | 38.69 | *** | 0.34 ± 0.06 a | 1.34 ± 0.13 b |
| Shoot dry weight | 6.46 | * | 0.66 ± 0.06 a | 1.21 ± 0.15 b |
| Root dry weight | 8.86 | ** | 0.16 ± 0.02 a | 0.35 ± 0.02 b |
| Plant dry weight | 7.77 | ** | 0.68 ± 0.07 a | 1.56 ± 0.16 b |
| Mycorrhizal dependency (%) | - | - | - | 56.18 |
| Colonised root length (%) | - | - | - | 100 |
| Mycorrhizal colonisation (%) | - | - | - | 70.00 ± 3.95 |
| Arbuscules (%) | - | - | - | 23.00 ± 2.20 |
| Spores (%) | - | - | - | 15.38 ± 2.60 |
| Hyphae (%) | - | - | - | 64.63 ± 5.62 |
Table 1: F and p values from one-way Anova of growth response of J. brevifolia sixth months after AMF inoculation. * p<0.05; ** p< 0.01; *** p< 0,001; n.s = not significant; a = development of plants at the time of harvest; b = root system of plants at the time of harvest.
Effect of native inoculum on plant growth
After six months of treatment, the effect of inoculation on the growth of J. brevifolia plants was assessed (Figure 2). Mycorrhizal treatment applied had significant effects on the growth and biomass production of J. brevifolia plants (Table 1 and Figure 3). Mycorrhizal dependency was estimated at 56.18% (Table 1).
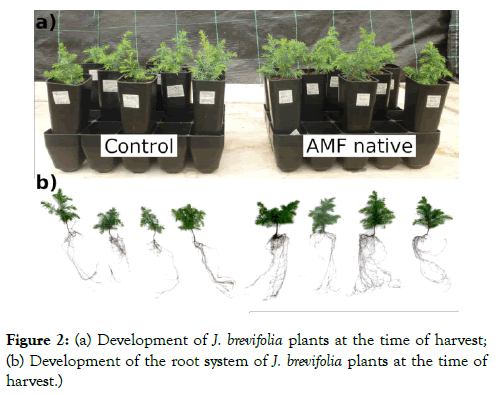
Figure 2: (a) Development of J. brevifolia plants at the time of harvest; (b) Development of the root system of J. brevifolia plants at the time of harvest.)

Figure 3: Shoot growth of the inoculated plants compared to the control plants.
Increase in shoot height differed significantly between the two treatment (One-way Anova: F1,31 = 4.2, P<0.05) (Table 1). Inoculation with native inoculum resulted in an increment of about 14% in the shoot growth of the inoculated plants compared to the control plants (Figure 3a).
The root length varied significantly between the two treatments The roots of the inoculated plants were significantly longer than those of the controls (One-way Anova: F1,31 = 21.25, P<0.001) (Table 1, Figure 3b), which was reflected in a higher increment of the root growth of the inoculated plants (One-way Anova: F1,31 = 32.07, P<0.001) compared to the control plants (Figure 3c). Significant differences were also observed between the two treatments in the final height of the plant (One-way Anova: F1,31 = 13.14, P<0.01), as well as in the increment of plant height (One-way Anova: F1,31 = 20.16, P<0.001) (Table 1). The height of the inoculated plants was 17% higher than the control plants (Figures 3d and 3e respectively).
The application of AMF native inoculum also influenced the final number of branches (One-way Anova: F1,31 = 20.54, p<0.001) and consequently its increament (One-way Anova: F1,31 = 23.80, P<0.001) (Table 1). The number of shoots was higher in inoculated plants than in control plants (Figure 3f), causing an increment of 37% in the inoculated plants in relation to control plants (Figure 3g).
The number of secondary roots also varied significantly between the treatments (One-way Anova: F1,31 = 27.20, P<0.001) (Table 1). Inoculated plants developed more secondary roots than controls (Figure 3h). Increment of secondary roots also differed significantly between the two treatments (One-way Anova: F1,31 = 30.87, P<0.001) (Table 1). The native inoculum resulted in an increment of 60% of secondary roots compared with controls (Figure 3i).
Biomass differed between the two treatments (Table 1). Inoculated plants had higher shoot (One-way Anova: F1,31 = 6.57, P<0.05) and root (One-way Anova: F1,31 = 18.60, P<0.001) fresh weights than control plants (Figures 3j and 3k respectively) and final fresh weight (One-way Anova: F1,31 = 9.24, P<0.01) of the inoculated plants (Table 1) was higher than the control (Figure 3l), resulting in an increase of 70% in the fresh weight (One-way Anova: F1,31 = 38.69, P<0.001) (Figure 3m). A similar pattern was obtained in relation to the dry weight of the plant, i.e., both shoot (One-way Anova: F1,31 = 6.46, P<0.05) and root (One-way Anova: F1,31 = 8.86, P<0.01) dry weights vary significantly between the two treatments (Table 1). Inoculated plants showed the highest shoot and roots dry weights (Figures 3n and 3o respectively). Consequently, the total dry weight (One-way Anova: F1,31 = 7.77, P<0.01) of the inoculated plants was higher than in the control plants (Figure 3p).
Discussion
Previously studies have recommend the use of native fungi as an effective strategy for efficient mycorrhizal inoculation in natural ecosystems [21,32,39,30,45]. The positive effect of AMF native inoculation was not only due to the direct effect of AMF inoculation, i.e., in this study, an increase of 60% in the root system of J. brevifolia inoculated plants resulting in higher root system robustness, but also, as shown for carob, due to the precolonisation by a well-adapted AMF community specific to the plant host, which promote the tolerance of inoculated plants to environmental stresses [21]. Similarly, Barea et al. [46] concluded that the use of native AMF consortia has the maximum effect in the restoration of degraded lands of the Mediterranean. Manaut et al. [21] demonstrated that native AMF consortia inoculation of Ceratonia siliqua L. seedlings more than doubled seedling survival and significantly improved seedling height and collar diameter.
The success of habitat restoration strategies strongly depends on the quality of the seedlings, which is fundamentally dependent on seedling growth under nursery conditions as well as their transplantation into field conditions [47,48]. These results show that inoculation with Azorean native AMF stimulated the growth of J. brevifolia plants under nursery conditions, demonstrating high mycorrhizal dependency of J. brevifolia. These results indicate that it may be beneficial to inoculate with suitable AMF in the nursery because most substrates used in the Azores often are sterilised to reduce or eliminate certain pests and diseases. Doing so also destroys any beneficial microorganisms such as AMF [49].
Some forest species may require AMF for optimum establishment and growth. Thus, inoculation at the early nursery stages of plant development can potentially benefit successful establishment and growth of these seedlings after outplanting [50-52].
Although the commercial native inoculum used in this study is composed of AMF species isolated from different islands of Azores archipelago, they all originated from long term organic farms. Moreover, all component fungi were previously detected in the rhizosphere of different endemic plants [53] including in the rhizosphere of J. brevifolia [54]. It is likely that these are physiologically and genetically adapted to the environmental stress conditions of the target areas.
The AMF richness in AMF inocula is considered to improve inocula effectiveness. Many commercial mycorrhizae inoculum comprise either a single or a limited number of AMF species. Thus synergistic interactions among the AMF species of the native inoculum could be responsible for the promotion of J. brevifolia plants growth [55]. Hoeksema et al. [56] in a metastudies showed that plant response was substantially lower when plants were inoculated with single AMF species, compared with inoculations with multiple AMF species. Different AMF species have different hyphal growth patterns, anastomoses and branching frequencies, and these differences possibly reflect different strategies and the occupation of different niches within the roots and rhizosphere [57]. Adding, many species that are common in commercial inoculum are considered as early successional species such as Rhizophagus intraradices, Funneliformis mosseae and Rhizophagus aggregatus given their capacity to proliferate with disturbance [58], while species that are sensitive to disturbance, i.e, Acaulospora, Cetraspora and Gigaspora are typically absent in commercial inocula [39]. Because, AMF composition changes during succession, some commercial inoculum composed of early successional species have been shown to reduce growth and establishment of late successional plants [59,60]. In this study, J. brevifolia plants were inoculated by a commercial native inoculum composed of either early or late successional species. For this reason, we suggested the incorporation of native and late successional AMF species for restoring native plant communities.
Conclusion
To sum, this work highlights the importance of applying AMF native inoculum in the early stages of juniper establishment, to overcome the stress of transplantation, and enhance survival and establishment in the forest. To be efficient in restoration programs the commercial inoculum should be composed of AMF species from both early and late successional stages to improve the growth and establishment of late successional plants as the major of endemic plants including J. brevifolia. This is particularly important in the case of endemic species, given the difficulty of propagation of these species. Providing optimal symbiont establishment would be expected to facilitate the recovery of rare and ecologicaly important species such as J. brevifolia, and the restoration of their habitats. Further studies are needed to improve our knowledge of how best to apply and use these beneficial organisms to successfully incorporate them into restoration strategies and other sustainable commercial cropping systems.
Acknowledgement
The authors would like to thank to the Regional Directorate for Forest Resources for provide the J. brevifolia seedling plants, and to MICOazorica company for supply the AMF native inoculum, the greenhouse and the laboratory equipments to carry out this assay.
This research was funded by Fundo Regional para a Ciênciae Tecnologia – Governo dos Açores (M3.1.a/F/059/2016).
REFERENCES
- Triantis KA, Borges PAV, Ladle RJ, Hortal J, Cardoso P, Gaspar C, et al. Extinction debt on oceanic islands. Ecography. 2010;33:285-294.
- Elias R, Dias E, Pereira F. Disturbance, regeneration and the spatial pattern of tree species in Azorean mountain forests. Community Ecol. 12:23-30.
- Borges PAV, Santos AMC, Elias RB, Gabriel R. The Azores Archipelago: Biodiversity Erosion and Conservation Biogeography. In: Encyclopedia of the World's Biomes -Earth Systems and Environmental Sciences. Reference Module in Earth Systems and Environmental Sciences, Elsevier, Amsterdam, The Netherlands. 2019; 1-18.
- Dias E, Elias RB. Cadernos de Botanica no 5. Ecologia das Florestas de Juniperus dos Acores. Herbario da Universidade dos Acores. Angra do Heroismo. 2008;1-18
- Elias RB, Martins V. Anthropogenic succession on Juniperus brevifolia forests in Terceira Island (Azores). Proceedings of the 5th international symposium on the fauna and flora of Atlantic Islands. Occasional publication of the Irish Biogeographical Society. 2006;9:111-120.
- Thomas P. Juniperus brevifolia (Errata version published in 2018). The IUCN Red List of Threatened Species. 2011.
- Silva L, Martins M, Moura M, Maciel GB. Azorean vascular flora: priorities in conservation. Amigos dos Acores and CCPA, Ponta Delgada, IUCN. 2009:1-122.
- Martins J, Moreira O, Silva L, Moura M. Propagation of the endangered Azorean endemic tree, Picconia azorica, by air layering. Archipelago, Life and Marine Sciences. 2011;28:39-46.
- Terzopoulou S, Rigal F, Whittaker RJ. Drivers of extinction: The case of Azorean beetles. Biol Lett. 2015;11:20150273.
- Brundrett MC, Tedersoo L. Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol 018;220(4):1108-1115.
- Lekberg Y, Koide RT (2005) Is plant performance limited by abundance of arbuscular mycorrhizal fungi? A meta-analysis of studies published between 1988 and 2003. New Phytol 168:189–204.
- Ruiz-Lozano JM, Aroca R, Zamarreño ÁM. Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant Cell Environ. 2016;9:441–452.
- Jaizme-Vega M del C, Rodríguez-Romero AS, Barroso Núñez LA. Effect of the combined inoculation of arbuscular mycorrhizal fungi and plant growth-promoting rhizobacteria on papaya (Carica papaya L.) infected with the root-knot nematode Meloidogyne incognita. Fruits. 2006;61:151–162.
- Fiorilli V, Catoni M, Francia D, Cardinale F, Lanfranco L. The arbuscular mycorrhizal symbiosis reduces disease severity in tomato plants infected by Botrytis cinerea. J Plant Pathol. 2011;237–242.
- Babu AG, Reddy MS. Diversity of Arbuscular Mycorrhizal Fungi Associated with Plants Growing in Fly Ash Pond and Their Potential Role in Ecological Restoration. Curr Microbiol. 2011;63:273–280.
- Pouyu-Rojas E, Siqueira JO. Arbuscular mycorrhizal and soil fertilization on post-transplant development of outplants of seven forest species. Pesqui Agropecuária Bras. 2000;35:103–114.
- Habte M, Miyasaka SC, Matsuyama DT. Arbuscular mycorrhizal fungi improve early forest-tree establishment. In: Horst WJ, Schenk MK, Bürkert A, et al. (eds) Plant Nutrition: Food security and sustainability of agro-ecosystems through basic and applied research. Springer Netherland. 2001;644–645
- Ouahmane L, Hafidi M, Thioulouse J. Improvement of Cupressus atlantica Gaussen growth by inoculation with native arbuscular mycorrhizal fungi. J Appl Microbiol. 2007;103:683–690.
- Kapulnik Y, Tsror L, Zipori I. Effect of AMF application on growth, productivity and susceptibility to Verticillium wilt of olives grown under desert conditions. Symbiosis. 2010;52:103–111.
- Karthikeyan A, Krishnakumar N. Reforestation of Bauxite mine spoils with Eucalyptus tereticornis Sm. seedlings inoculated with Arbuscular mycorrhizal fungi. Ann For Res. 2012;55:207-216–216.
- Manaut N, Sanguin H, Ouahmane L. Potentialities of ecological engineering strategy based on native arbuscular mycorrhizal community for improving afforestation programs with carob trees in degraded environments. Ecol Eng. 2015;79:113–119.
- Neuenkamp L, Moora M, Öpik M. The role of plant mycorrhizal type and status in modulating the relationship between plant and arbuscular mycorrhizal fungal communities. New Phytol. 2018;220:1236–1247.
- Rillig MC, Mummey DL. Mycorrhizas and soil structure. New Phytol. 2006;171:41–53.
- van der Heijden MG, Klironomos JN, Ursic M (1998) Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 396:912–927
- Vogelsang KM, Reynolds HL, Bever JD. Mycorrhizal fungal identity and richness determine the diversity and productivity of a tallgrass prairie system. New Phytol. 2006;172:554–562.
- Lin G, McCormack ML, Guo D. Arbuscular mycorrhizal fungal effects on plant competition and community structure. J Ecol. 2015;103:1224–1232.
- Kardol P, Cornips NJ, Kempen MML van. Microbe-Mediated Plant–Soil Feedback Causes Historical Contingency Effects in Plant Community Assembly. Ecol Monogr. 2007;77:147–162.
- Bauer JT, Mack KML, Bever JD. Plant-soil feedbacks as drivers of succession: evidence from remnant and restored tallgrass prairies. Ecosphere. 2015;6: 1-158.
- Bongard C. A review of the influence of root-associating fungi and root exudates on the success of invasive plants. Neo Biota. 2012;14:21–45.
- Ferrol N, Calvente R, Cano C. Analyzing arbuscular mycorrhizal fungal diversity in shrub-associated resource islands from a desertification-threatened semiarid Mediterranean ecosystem. Appl Soil Ecol. 2004;25:123–133.
- Huante P, Ceccon E, Orozco-Segovia A, Sánchez-Coronado MS, Acosta I, Rincón E. The role of arbuscular mycorrhizal fungi on the early-stage restoration of seasonally dry tropical forest in Chamela, Mexico. Rev Árvore. 2012;36:279–289.
- Asmelash F, Bekele T, Birhane E. The Potential Role of Arbuscular Mycorrhizal Fungi in the Restoration of Degraded Lands. Front Microbiol. 2016;7:1095.
- Smith SE, Facelli E, Pope S, Andrew Smith F. Plant performance in stressful environments: interpreting new and established knowledge of the roles of arbuscular mycorrhizas. Plant Soil. 2010;326:3–20.
- Velázquez MS, Cabello MN, Barrera M. Composition and structure of arbuscular-mycorrhizal communities in El Palmar National Park, Argentina. Mycologia. 2013;105:509–520.
- Mariotte P, Meugnier C, Johnson D, Thébault A, Spiegelberger T, Buttler A. Arbuscular mycorrhizal fungi reduce the differences in competitiveness between dominant and subordinate plant species. Mycorrhiza. 2013;23:267–277.
- Friede M, Unger S, Hellmann C, Beyschlag W. Conditions Promoting Mycorrhizal Parasitism Are of Minor Importance for Competitive Interactions in Two Differentially Mycotrophic Species. Front Plant Sci. 2016;7:1-10
- Klironomos JN. Variation in Plant Response to Native and Exotic Arbuscular Mycorrhizal Fungi. Ecology. 2003;84:2292–2301.
- Graham LLB, Turjaman M, Page SE. Shorea balangeran and Dyera polyphylla (syn. Dyera lowii) as tropical peat swamp forest restoration transplant species: effects of mycorrhizae and level of disturbance. Wetl Ecol Manag. 2013;21:307–321.
- Koziol L, Schultz PA, House GL, Bauer JT, Middleton El, Bever JD. The Plant Microbiome and Native Plant Restoration: The Example of Native Mycorrhizal Fungi. BioScience. 2018;68:996-1006.
- Sieverding E. Vesicular-Arbuscular Mycorrhiza Management in Tropical Agrosystems. GTZSchriftenreihe No. 224. Hartmut Bremer Verlag, Friedland, Germany. 1991.
- Melo CD, Walker C, Krüger C, Borges PAV, Luna S, Mendonça D, et al. Environmental factors driving arbuscular mycorrhizal fungal communities associated with endemic woody plant Picconia azorica on native forest of Azores. Ann Microbiol. 2019;69:1309–1327.
- McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA. A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol. 1990;115:495–501.
- Minitab I. Minitab: release 13 for Windows. Minitab Inc., State College, PA. 2000.
- Hetrick BD, Wilson GWT, Todd TC. Relationships of mycorrhizal symbiosis, rooting strategy, and phenology among tallgrass prairie forbs. Can J Bot. 1992;70:1521–1528.
- Al-Yahya’ei MN, Oehl F, Vallino M, Lumini E, Redecker D, Wiemken A. Unique arbuscular mycorrhizal fungal communities uncovered in date palm plantations and surrounding desert habitats of Southern Arabia. Mycorrhiza. 2011;21:195–209.
- Barea JM, Palenzuela J, Cornejo P, Sánchez-Castro I, Navarro-Fernández C, Lopéz-García A, et al. Ecological and functional roles of mycorrhizas in semi-arid ecosystems of Southeast Spain. J Arid Environ. 2011;75:1292–1301.
- Grossnickle SC. Why seedlings survive: influence of plant attributes. New Forest. 2012;43:711–738.
- Ley-Lopez JM, Avalos G, Chacon-Madrigal E. Seedling growth and survival of five tree species in secondary forests and adjacent pastures in the montane rain forests of Southern Costa Rica. Rev. Biol. Trop. 2016;64:1565-1583.
- Boyer LR, Feng W, Gulbis N, Hajdu K, Harrison RJ, Jeffries P, et al. The use of arbuscular mycorrhizal fungi to improve strawberry production in coir substrate. Front Plant Sci. 2016;7:1237.
- Jaizme-Vega MC, Rodríguez-Romero AS, Marín Hermoso C, Declerck S. Growth of micropropagated bananas colonized by root-organ culture produced arbuscular mycorrhizal fungi entrapped in Ca- alginate beads. Plant Soil. 2003;254:329–335
- Young T, Cameron DD, Phoenix GK. Using AMF inoculum to improve the nutritional status of Prunella vulgaris plants in green roof substrate during establishment. Urban For Urban Green. 2015;14:959–967.
- Wang J, Zhong H, Zhu L, Yuan Y, Xu H, Wang GG, et al. Arbuscular mycorrhizal fungi effectively enhances the growth of Gleditsia sinensis Lam. Seedlings under Greenhouse Conditions. Forests. 2019;10:567.
- Melo CD, Luna S, Krüger C, Christopher W, Duarte M, Henrique F, et al. Communities of arbuscular mycorrhizal fungi under Picconia azorica in native forests of Azores. Symbiosis. 2018;1–12.
- Melo CD, Luna S, Krüger C, Christopher W, Duarte M, Henrique F, et al. Arbuscular mycorrhizal fungal community composition associated with Juniperus brevifolia in native Azorean forest. Acta Oecologica. 2017;79:48–61.
- Wagg C, Jansa J, Schmid B, Van der Heijden MGA. Belowground biodiversity effects of plant symbionts support aboveground productivity. Ecol Lett. 2011;14:1001–1009.
- Hoeksema JD, Chaudhary VB, Gehring CA, Johnson NC, Karst J, Koide RT, et al. A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol Lett. 2010;13:394–407.
- Parniske M. Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nature Reviews Microbiology. 2008;6:763–775.
- Oehl F, Sieverding E, Mäder P. Impact of long-term conventional and organic farming on the diversity of arbuscular mycorrhizal fungi. Oecologia. 2004;138:574–583.
- Middleton EL, Bever JD. Inoculation with a native soil community advances succession in a grassland restoration. Restor Ecol. 2012;20:218–226.
- Cheeke TE, Zheng C, Koziol L, Gurholt CR, Bever JD. Sensitivity to AMF species is greater in late‐successional than early‐successional native or nonnative grassland plants. Ecology. 2019;100.
Citation: Drumonde-Melo C, Borges P, Freitas H, Nunes L (2020) Potential Role of Native Arbuscular Mycorrhizal Fungi (AMF) in the restoration of Laurisilva. J Plant Pathol Microbiol. 11: 503. doi: 10.35248/ 2157-7471.20.11.503
Copyright: © 2020 Drumonde-Melo C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

