Indexed In
- RefSeek
- Hamdard University
- EBSCO A-Z
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Case Report - (2024) Volume 9, Issue 2
Plexiform Neurofibroma Transformation to a Malignant Peripheral Nerve Sheath Tumour: A Case Report in a Patient with Neurofibromatosis-1
Tebogo Linky Medupe*Received: 17-May-2024, Manuscript No. JTRR-24-25708; Editor assigned: 20-May-2024, Pre QC No. JTRR-24-25708 (PQ); Reviewed: 03-Jun-2024, QC No. JTRR-24-25708; Revised: 10-Jun-2024, Manuscript No. JTRR-24-25708 (R); Published: 18-Jun-2024, DOI: 10.35248/2684-1614.24.9.224
Abstract
One of the most prevalent dominantly inherited genetic disorders is neurofibromatosis-1. The majority of epidemiological research has indicated that at least one in three thousand people experience this globally. Neurofibromatosis-1 people are more likely to develop cancer, and the illness affects several systems, with primary manifestations occurring in the cutaneous, neurologic, and orthopaedic sites. These conditions can cause severe morbidity or fatality. The neurocutaneous-skeletal Neurofibromatosis-1 (NF1) syndrome can only be caused by a mutation in the NF1 gene; nevertheless, the pathogenesis of the disease's various manifestations in various organ systems appears to be becoming more complex. The formation, severity, and prognosis of a wide range of distinct clinical phenotypes appear to be caused by interactions between many cell types, cell signaling networks, and the extracellular matrix. A "second hit" that causes the NF1 gene to become bi-allelic inactivated appears to be critical for the development of several symptoms, including neurofibroma, café-au-lait macules, and glomus tumors. We present a rare example of a plexiform neurofibroma undergoing malignant transformation from our database of cases. In order to increase public knowledge of the condition and genetic testing, a review of the disease is required.
Keywords
Neurofibromatosis-1, Plexiform neurofibroma
Introduction
Neurofibromatosis type-1 is a frequently occurring syndrome predisposing to tumors, with an estimated prevalence of 1 in 3000 individuals. It is a multisystem illness that exhibits a broad spectrum of clinical symptoms along with elevated morbidity and mortality. Typical characteristic features of cutaneous manifestations include Lisch nodules, neurofibromas, intertriginous freckling, and café-au-lait macules [1-3]. In particular, neurofibromas are the most often observed cutaneous lesions. These are benign lesions that originated from neurological lesions. When connected to Neurofibromatosis type-1, the plexiform neurofibroma has the potential to develop into a malignant tumor, in contrast to isolated neurofibromas that are not connected to neurofibromatosis type-1 [4].
Over the past ten years, the treatment of tumors linked to NF-1 has changed, with substantial research concentrated on symptom reduction and signaling dysregulation suppression. Until recently, the recommended course of treatment for symptomatic tumors was surgical resection.
The case report serves to highlight the significance of genetic testing by reflecting and highlighting the neurofibromatosis-1 and its related cutaneous manifestation.
Case Presentation
A male child aged ten is diagnosed with neurofibromatosis-1. He had a neurofibroma removed from his face when he first showed. The patient was referred by a nearby facility; unfortunately, the report regarding the excision was lost. He complained of a mass on his face months after the surgery, and the CT scan revealed involvement of the orbit, maxillary sinus, and anterior skull base. The mass was excised in the operating room, and the specimen was sent for histological analysis. There was no mention of any past radiation history.
The specimen was sent in pieces to the histology lab; the biggest piece was 70 × 55 × 50 mm in size. Tumor was represented by each fragment. In the majority of these fragments, a fibrous capsule was observed, and upon sectioning the tumor, it revealed a firm, tan colour with areas of mixed necrosis and hemorrhage. There were isolated observations of bone tissue connected to certain tumor tissue. When the material was grossed, the tumor was observed near the painted margins.
The heavily sampled tumor tissue underwent microscopic analysis, which revealed a highly malignant tumor grouped in nodules with a plexiform growth pattern. Composed of spindle to ovoid cells, areas of fibrosarcoma-like fascicular growth pattern with perivascular accentuation and alternating hyper and hypocellularity were seen (Figures 1 and 2).The cells cytoplasm was eosinophilic, and their nuclei were pleomorphic and hyperchromatic.

Figure 1: Area represents fibrosarcoma-like fascicular growth pattern.
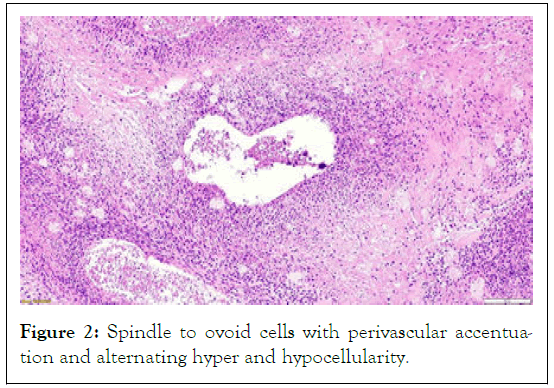
Figure 2: Spindle to ovoid cells with perivascular accentuation and alternating hyper and hypocellularity.
Thirty-eight mitotically active cells were counted for every 10 high power fields, indicating brisk mitosis (Figure 3). There was extensive tumor necrosis and isolated myxoid regions.
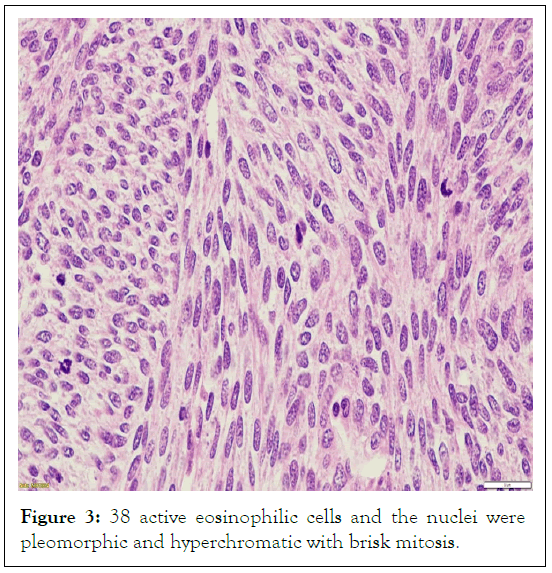
Figure 3: 38 active eosinophilic cells and the nuclei were pleomorphic and hyperchromatic with brisk mitosis.
There was a pre-existing, peripherally placed area of hypocellularity near a fibrous capsule (Figure 4). It was made up of cytologically bland spindle cells with thin, wavy nuclei that morphologically resembled plexiform neurofibroma. There was a recognized part of the face's cortical bone, but the tumor had not penetrated it.
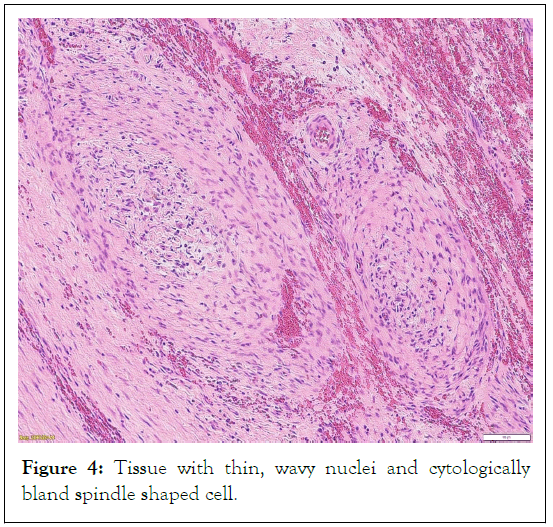
Figure 4: Tissue with thin, wavy nuclei and cytologically bland spindle shaped cell.
Heterologous elements such as rhabdomyosarcomatous areas, or epithelioid areas were not seen. In hotspot locations, the S100 and SOX 10 immunoperoxidase stains were found to be localized and patchy, while in the Ki 67 immunoperoxidase stain demonstrates a high proliferative index (Figures 5-7).
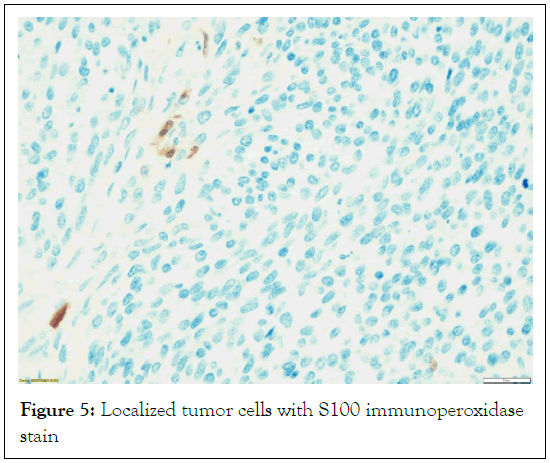
Figure 5: Localized tumor cells with S100 immunoperoxidase stain
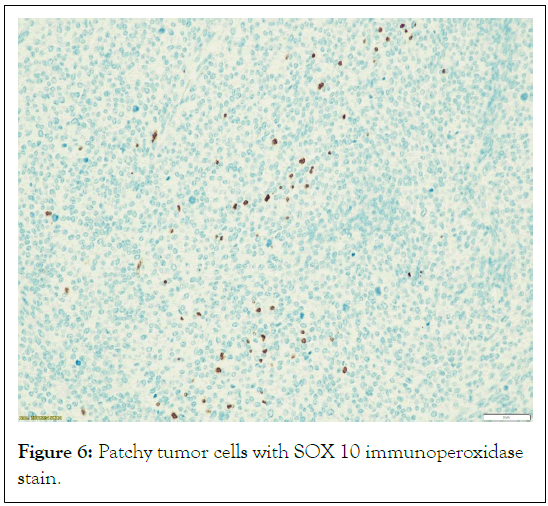
Figure 6: Patchy tumor cells with SOX 10 immunoperoxidase stain.
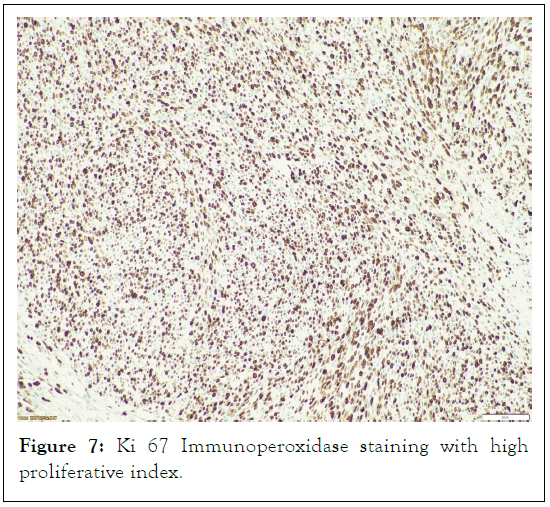
Figure 7: Ki 67 Immunoperoxidase staining with high proliferative index.
Results and Discussion
Different sized plexiform neurofibromas typically arise along a lengthy nerve trunk or in any area where there is a greater accumulation of adipose tissue. The surrounding tissue may swell and thicken as a result of the affected nerve and other nearby tissue components may be compressed as a result of this. One possible source of plexiform neurofibroma is a cranial nerve. The trigeminal nerve is the most common cranial nerve to be documented with neurofibromas. As the lesion spreads into the eyelid, it tends to infiltrate into the orbit, resulting in considerable proptosis and deformity of the face. A disorder known as elephantiasis neuromata involves plexiform neurofibromas across the extremities. The lesion appears hyper pigmented loose, superfluous skin that may impede the limb's ability to move. It follows the axis of a lower limb used for nerve tracking. Plexiform neurofibroma can potentially affect the gastrointestinal tract or urogenital area, as well as deep tissue or internal organs without showing signs of superficial expansion. NF-1 has a high correlation with plexiform neurofibromas. Nonetheless, there have been a few documented examples of solitary plexiform neurofibromas in the tongue, orbit, hand, tip of the nose, and oropharynx. The therapy is surgical resection, however this can involve several nerve branches, making it challenging. There is a substantial recurrence incidence associated with invasive plexiform neurofibroma upon debulking and excision. Recurrence rates for complete resections are reported at 20%, although one case series found that incomplete resections had recurrence rates of up to 45% [1-3].
With a 51% overall 5-year survival rate and a significant local recurrence risk, high grade MPNST patients have a prognosis that appears to be strongly correlated with aggressive surgery, with just 14% of patients surviving after incomplete surgery [5,6].
The high grade morphology of the tumor, its local extension to the surrounding tissues within the head and neck region, and the indications of an insufficient surgical intervention all point to a low survival rate, which is exactly what our case illustrates. In order to reduce incomplete surgery with local extension and involvement of the surrounding structures, it is imperative to time the procedure perfectly. Although NF-1-related lesions will inevitably develop, early lesion treatments and genetic testing may increase the likelihood of survival.
Plexiform neurofibroma has a high propensity to convert into a Malignant Peripheral Nerve Sheath Tumor (MPNST) and is pathogenomic for NF-1.
Conclusion
In conclusion, plexiform neurofibromas are highly associated with NF-1 and have a significant risk of transforming into Malignant Peripheral Nerve Sheath Tumors (MPNSTs). These neurofibromas typically develop along major nerve trunks or in areas rich in adipose tissue, leading to swelling, thickening of surrounding tissues, and compression of adjacent structures. While cranial nerves, particularly the trigeminal nerve, are common sites for these tumors, they can also appear in various other locations, including the extremities, gastrointestinal tract, and urogenital area, often without superficial signs. Our case highlights the critical need for precise timing in surgical interventions to minimize local extension and involvement of surrounding structures, thereby improving survival outcomes. Early treatment of lesions and genetic testing may provide better prognostic outcomes for NF-1-related tumors, highlighting the importance of early detection and intervention in these patients.
References
- Sanchez LD, Bui A, Klesse LJ. Targeted therapies for the neurofibromatoses. Cancers. 2021;13(23):6032.
[Crossref] [Google Scholar] [PubMed]
- Yao R, Yu T, Xu Y, Yu L, Wang J, Wang X, Wang J, Shen Y. Clinical presentation and novel pathogenic variants among 68 Chinese neurofibromatosis 1 children. Genes. 2019;10(11):847.
[Crossref] [Google Scholar] [PubMed]
- Bergqvist C, Servy A, Valeyrie-Allanore L, Ferkal S, Combemale P, Wolkenstein P. Neurofibromatosis 1 French national guidelines based on an extensive literature review since 1966. Orphanet journal of rare diseases. 2020;15:1-23.
[Crossref] [Google Scholar] [PubMed]
- Lam HY, Rashid SA, Yuan LH, Rashid SA. A Case Series of Plexiform Neurofibroma: The Unusual Presentations and Surgical Challenges. Cureus. 2022;14(3).
[Crossref] [Google Scholar] [PubMed]
- Magro G, Broggi G, Angelico G, Puzzo L, Vecchio GM, Virzì V, Salvatorelli L, Ruggieri M. Practical approach to histological diagnosis of peripheral nerve sheath tumors: an update. Diagnostics. 2022;12(6):1463.
[Crossref] [Google Scholar] [PubMed]
- Solares I, Viñal D, Morales-Conejo M, Rodriguez-Salas N, Feliu J. Novel molecular targeted therapies for patients with neurofibromatosis type 1 with inoperable plexiform neurofibromas: A comprehensive review. ESMO open. 2021;6(4):100223.
[Crossref] [Google Scholar] [PubMed]
Citation: Medupe TL (2024) Plexiform Neurofibroma Transformation to a Malignant Peripheral Nerve Sheath Tumour: A Case Report in a Patient with Neurofibromatosis-1. J Tum Res Reports. 9.224.
Copyright: © 2024 Medupe TL. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

