Indexed In
- Open J Gate
- JournalTOCs
- The Global Impact Factor (GIF)
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 11, Issue 1
Pharmacovigilance: A Meta-Analysis on ADRS of Past and Recent Tragedy Occurred in Gambia
Junaid Tantray*, Mohd Zaid, Sourabh Kosey, Akhilesh Patel, Ashish K Sharma, Rajesh Sharma, Deepak Nathiya, R.P Singh and Nandini KushwahaReceived: 07-Dec-2022, Manuscript No. JP-23-19114; Editor assigned: 12-Dec-2022, Pre QC No. JP-23-19114 (PQ); Reviewed: 30-Dec-2022, QC No. JP-23-19114; Revised: 09-Jan-2023, Manuscript No. JP-23-19114 (R); Published: 17-Jan-2023, DOI: 10.35248/2329-6887.23.11.409
Abstract
Pharmacovigilance is a crucial component of clinical research and is studied in depth. They are the stage of pharmaceutical research that can relate to the detection, assessment, comprehension, and avoidance of hazardous effects, with an emphasis on both the long-term and short-term impacts of medication. The company initially produced common medications including paracetamol and riboflavin 5'-phosphate. Recently the tragedy has taken place in Gambia that has killed 66 innocent children’s by serving the cough syrup to child and the company from this has been made is Maiden pharmaceutical India. The cough syrup name that has been made is Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup and MaGrip N Cold Syrup. In this the increase number of diethyl glycol and ethyl glycol in the solution that can cause renal and neurological damage. In past time many tragedies happened in world that is listed as: Tuskegee Syphilis study, Guatemala syphilis experiment, Thalidomide Tragedy, Clioquinol Tragedy, and Tragedy of Accutane. They have lack of concern for maintaining purity standards the vital roles performed by healthcare professionals, the pharmaceutical industry, the media, and WHO programmes. Regulatory relies on reporting of unfavorable drug reactions to ensure that medications are acceptable safe. Unfortunately, underreporting plagues all reporting systems. So, in India there is lack of regulatory rely are there which needs to be improved in future and more reporting programmes should be conducted to increase reporting of side effects.
Keywords
Adverse Drug Reaction; Spontaneous Reporting System; Adverse Event; Uppsala Monitoring Center; Diethylene Glycol
INTRODUCTION
Pharmacovigilance is a critical and essential component of clinical research. Throughout the lifecycle of a product, post- marketing Pharmacovigilance and clinical trial safety are both essential. "Defined as the pharmacological science dealing to the identification, assessment, understanding, and prevention of adverse effects, particularly long-term and short-term adverse effects of medicines," is how Pharmacovigilance is described. In India, Pharmacovigilance is still in its infancy and there is very little understanding of the field.
While Pharmacovigilance has made significant strides in western nations, India has not made as much progress. It is crucial to comprehend the significance of Pharmacovigilance and how it affects the product's life cycle [1]. This will make it possible to incorporate effective Pharmacovigilance practises into the systems and practises tobolster clinical trial safety, post-marketing surveillance, and regulatory compliance.
In reality, Pharmacovigilance has been practised in India from 1998. When India made the decision to become a member of the Uppsala Centre for Adverse Event Monitoring the regulatory authorities, the media, and consumers have all recognized the significance of Pharmacovigilance as the benefits and risks of medications have come to light [2]. "Any unfavourable medical occurrence that may occur during treatment with a medicine but does not necessarily have a relationship with its use is described as an adverse event," according to the definition provided by the FDA. Any harmful, unplanned, and unwanted impact of a medicine that happens at a dose utilised in humans for prophylaxis, diagnosis, therapy, or alteration of physiological function is referred to as an adverse drug reaction. An essential approach for acquiring the data is the spontaneous reporting of adverse medication reactions and adverse occurrences early detection safety information. Many Indian corporations have been boosting their (R and D) spending in recent years, which has improved their ability to create and commercialise new pharmaceutical products. A center for clinical research activity is developing in India as a result of its big population, high enrolment rate, and affordable costs [3]. The time between a drug's initial release on the market in the United States, Europe, Japan, or elsewhere in the world and its ensuing availability in India has also significantly decreased. As a result, neither the time of their commercialization in India nor the long term safety data are available for such pharmaceuticals. The fact that all of the high profile drugs that had lately been pulled from sale were still available in India. When determining the benefit-risk balance of a medicine, the Indian regulatory agencies cannot rely on the knowledge of other markets. The main concerns in ensuring the safety of pharmacological therapy are managing and reducing the side effects of medications. Medication analyzers are unable to change side effects since they are inherent characteristics of the drug ingredient. Drug analyzers are heavily involved in ensuring the quality of both bulk medication components and drug formulations, which is strongly tied to the safety problem. Identity, strength, and purity are the three key components of medication quality. Purity is particularly crucial in the case of bulk drug materials since it allows for the detection (by structural elucidation) and quantification of impurities and degradation products, reducing the likelihood that they may contribute to the side effect profile of the therapeutic materials. It has long been the practise to carefully examine instances that were reported spontaneously in order to identify and quantify adverse medication responses. An essential component of the examination of individual case reports was the determination of causality (imputation). Analysis of aggregated instances and disproportionality studies in databases of spontaneous reports were added to this. These have led to the identification of several novel adverse responses, changing medication information, in the lack of more specialized information sources. Many medications have been taken off the market as a result of it, but its application to risk quantification is still dubious. Recent access to databases of population-wide claims or electronic health records has proven the predominance of spontaneous reporting in the formation of hypotheses for major adverse medication reactions, particularly those that end in hospital admission or death. In these situations, the incidents are recognizable at the population level and may be carefully quantified with the use of contemporary Pharmacoepidemiology technologies to produce tailored benefit-risk evaluations. Despite its inherent drawbacks, spontaneous reporting continues to be crucial in the development of signals and alerts for medication safety. Additional systematic and quantitative tools, such claims databases for responses that result in hospital admissions, should be looked for in order to enhance signals and analyze them [4].
Pharmacovigilance is all about gathering Adverse Drug Reaction (ADR) reports that will make it easier to identify new signs of adverse reactions at the right moment. An appropriate number of reports having the correct sort of information to enable an accurate causation evaluation are sufficient rather than demanding the reporting of all ADRs. If all possible ADRs were revealed, it would be rather inconvenient only from a practical standpoint. No national reporting system is set up to handle such a volume of data. Given that each report must be considered independently in order to allow for an appropriate causality assessment and also to offer the reporting party proper feedback, it would be difficult to discover the metaphorical needles in the enormous haystack. Finney has previously mentioned how important it is to specify precisely which adverse occurrences have to be reported.
Methodology
Tragedies of pharmacovigilence
The first recorded instance of Pharmacovigilance occurred 169 years ago, on January 29, 1848, when Hannah Greener, a little child from the north of England, passed away following the administration of chloroform anaesthesia prior to the excision of an infected toenail. Chloroform was a potent and safe anaesthetic that Sir James Simpson had discovered and introduced into therapeutic use. To comprehend what happened to Hannah, the causes of her death were looked into, but it was hard to pinpoint the exact cause of her death. She most likely succumbed to pulmonary aspiration or a fatal arrhythmia [5].
The Lancet Journal organized a commission to address this issue in response to additional fatalities and concerns about the safety of anaesthesia voiced by professionals and the general public. The commission urged English physicians, especially those practising in colonies, to report cases of anaesthesia-related fatalities. In 1893, The Lancet reported the findings [6].
On June 30, 1906, the US Federal Food and Drug Act were adopted, establishing the need that pharmaceuticals be pure and free of any contamination. Additionally, this body prohibited bogus pharmacological therapeutic indications in 1911 [7]. Due to the use of sulphanilamide elixir, which used diethyl glycol as the solvent, 107 people died in the USA in 1937. The manufacturing businesses were not aware of the solvent's toxicity at the time, which was thought to be the cause of the deaths [8]. As a result, in 1938, the Federal Food, Drug, and Cosmetic Act were created with the intention of modernizing the public health system. In fact, the new approach recognized that proving a drug's safety prior to its permission for sale and introduced On June 30, 1906, the US Federal Food and Drug Act was adopted, establishing the need that pharmaceuticals be pure and free of any contamination. Additionally, this body prohibited bogus pharmacological therapeutic indications in 1911. Due to the use of sulfanilamide elixir, which used diethyl glycol as the solvent, 107 people died in the USA in 1937. The manufacturing businesses were not aware of the solvent's toxicity at the time, which was thought to be the cause of the deaths. As a result, in 1938, the Federal Food, Drug, and Cosmetic Act were created with the intention of modernising the public health system. In fact, the new approach recognized that proving a drug's safety prior to its permission for sale and introduced the potential for doing manufacturing checks [9]. Acetylsalicylic Acid (ASA) was thought to be a potential cause of melena by Douthwaite in 1938. Different results emerged from the investigation on the gastrointestinal toxicity of ASA. ASA is currently contraindicated in people with gastrointestinal ulcers because it was demonstrated in 1955 that it can cause gastrointestinal disorders [10].
A significant development in European Pharmacovigilance occurred in 1961 as a result of the tragedy of thalidomide. Australian physician Dr. McBride made a relationship between thalidomide and congenital malformations in infants in a letter to the editor of the Lancet Journal. In fact, he found that thalidomide use during pregnancy raised the incidence of congenital abnormalities in newborns (1.5%) by up to 20% [11]. At the same time, Dr. Lenz made a correlation between deformities and thalidomide during a pediatric convention in Germany, and his theory was published in a German journal (Welt am Sonnatag) [12]. Retrospective research conducted in 1973 revealed a connection between thalidomide consumption during pregnancy and congenital abnormalities in infants [13]. In USA, Dr. Kelsey expressed severe concerns regarding the safety of thalidomide during pregnancy, which prevented the thalidomide catastrophe from being seen. The thalidomide catastrophe brought to light a number of difficulties and significant challenges, including the validity of animal testing, the conduct of the industrial business, and the significance of medication monitoring following their introduction. Since the spontaneous reporting of adverse drug reactions became systematic, coordinated, and controlled as a result of this tragedy, the Pharmacovigilance system has undergone significant development. This letter already had every component required to spark spontaneous reporting and prove a causal link between the adverse event and the medication [14]. The "Yellow card" (YC) was established in the UK in 1964. YC is a particular format to assemble a spontaneous drug toxicity report [15]. The amendment requiring safety and effectiveness data of pharmaceuticals prior to premarketing submission was enacted in the United States in 1962. This change requires that the teratogenicity test results from three separate animals be included in the safety data. Thalidomide's devastation in Europe in 1965 sparked the creation of a European legal framework with the EC Directive 65/65 [16]. Boston Collaborative Drug Surveillance Program began as a pilot project in 1966. It played a crucial part in the development and use of techniques in drug epidemiology and was the first organization to perform epidemiologic studies to estimate potential adverse effects of medications using inhospital monitoring [17]. The WHO Program for International Drug Monitoring was established in 1968, and ten people took part in this programme (Australia, UK, USA, Germany, Canada, Ireland, Sweden, Denmark, New Zealand, and Netherlands). In 1975, Italy took part in this programme [18]. Between 1968 and 1982, numerous studies of documented negative medication responses were carried out [19]. The International Society of Pharmacovigilance (ISOP) was founded in 1992 and grew out of the European Society of Pharmacovigilance (ESoP). This society's objectives were to advance Pharmacovigilance and all other areas of safe and appropriate medication use [20]. The European Medicines Agency (EMA) was founded in 1995 [21]. EudraVigilance received funding in 2001. It serves as the official European database for monitoring and analysing data on alleged adverse drug reactions to medications approved for sale or being researched in Europe medical research.
Thalidomide tragedy
The "largest man-made medical disaster ever" occurred in 46 nations in the late 1950s and early 1960s as a result of pregnant or postpartum women using thalidomide. More than 10,000 children were born with a variety of severe abnormalities, including Phocomelia, as well as thousands of miscarriages [22-23].
Thalidomide was initially developed as a sedative in 1953 and later marketed as Contergan by the German pharmaceutical company Chemie Grünenthal as a treatment for anxiety, insomnia, tension, and morning sickness [23-24]. It was released without having been put to the test on expectant mothers as a sedative and morning sickness treatment [25]. Despite being previously thought to be safe during pregnancy, concerns about birth abnormalities were found in 1961, and the drug were subsequently banned Europe during the year [26-27].
Development of tragedies
Swiss pharmaceutical company Ciba created thalidomide as a tranquillizer for the first time in 1953. When Ciba discontinued the product in 1954, Chemie Grünenthal, a German pharmaceutical business, bought it. After World War II, Hermann Wirtz, Sr., a Nazi Party member, founded the business as a division of the family's Mäurer and Wirtz business. The initial goal of the company was to create antibiotics that the market had a desperate need for. Due to his prior work on an anti-typhus vaccine for Nazi Germany, Wirtz chose scientist Heinrich Mückter to lead the development programme. Mückter had managed to avoid war crimes charges for his tests on detainees of Nazi concentration camps [28]. He hired Martin Staemmler, a physician and prominent Nazi eugenicistOtto Ambros, a chemist and Nazi war criminal, Heinz Baumkötter, the chief medical officer of the Sachsenhausen concentration camp, and the head of pathology for the programme. Ambros served as a board member when Contergan was sold and served as the chairman of Grünenthal's advisory group throughout the development of thalidomide [29].
Thalidomide is a medicine used to treat a variety of cancers, including multiple myeloma, graft-versus-host disease, and a variety of skin disorders, including leprosy-related skin problems. It is marketed under the trade names Contergan and Thalomide among others. Although it has been used to treat a number of HIV-related diseases, doing so is linked to higher levels of the virus. It is consumed orally dizziness, rash, and tiredness are typical adverse reactions. Blood clots, peripheral neuropathy, and tumor lyses’ syndrome are among the serious adverse effects [30-31]. Use during pregnancy may cause harm to the fetus, including limb deformity. If a partner might become pregnant while a man is taking the medicine, contraception is crucial. It is an immunomodulatory drug that functions in a variety of ways, including activating T cells while reducing the generation of TNF-West Germany saw the introduction of thalidomide in 1957, when it was sold without a prescription [32-33]. When thalidomide was originally made available, it was marketed as a treatment for anxiety, restlessness, tension, and morning sickness. Although it was first believed to be safe during pregnancy, concerns about birth malformations developed before the drug was taken off the market in Europe in 1961. It is estimated that use during pregnancy affected 10,000 children in total, of whom 40% passed away shortly after birth. The survivors suffered issues with their limbs, eyes, urinary tracts, and hearts. Frances Kelsey, a reviewer at the FDA, stopped it from entering the US market at first [34]. The thalidomide-related birth problems prompted the usage as a cancer treatment was authorized in the United States in 1998. It is listed in Figure 1 as one of the Essential Medicines by the World Health Organization [35]. It is accessible as a generic drug.
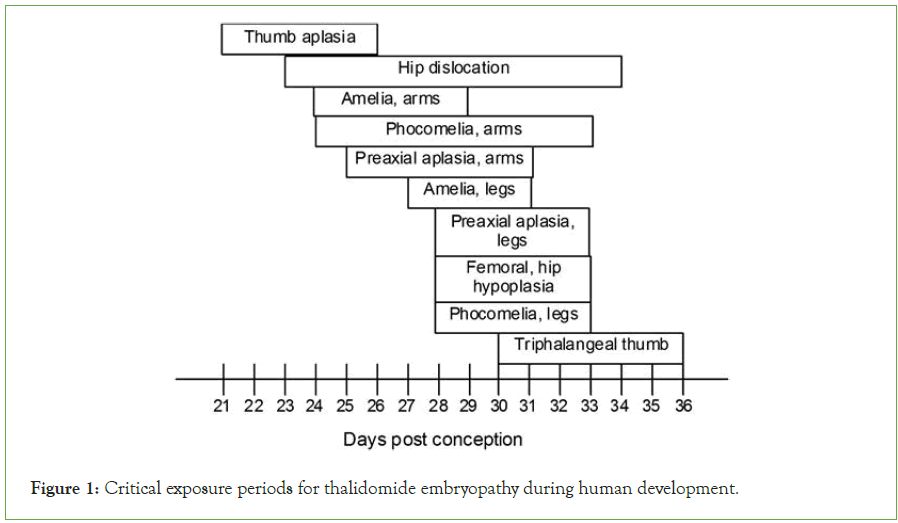
Figure 1: Critical exposure periods for thalidomide embryopathy during human development.
Clioquinol tragedy
Iodochlorhydroxyquin, often known as clioquinol, is an antifungal and antiprotozoal medication. In excessive amounts, it is neurotoxic. It belongs to a class of medications called hydroxyquinolines that block specific DNA replication-related enzymes. It has been discovered that the medications are effective against both viral and protozoa infections [36-37].
In 1934, clioquinol was created as a topical antiseptic and promoted as an oral intestinal amebicide. It was used to treat a variety of gastrointestinal conditions, such as lambliasis, shigellosis, chronic nonspecific diarrhoea, and traveler's diarrhea. It was taken off the market as an oral medication in the early 1970s due to its link to Subacute Myelo-Optic Neuropathy (SMON), a condition characterized by sensory and motor dysfunction in the lower limbs as well as visual abnormalities. Abdominal pain and/or diarrhea were frequently present together with painful dysesthesia within a few days or weeks. The spinal cord, optic nerve, and peripheral nerves' lateral posterior funiculi have all undergone symmetrical demyelinization, which is the cause of these symptoms [38]. SMON was widely used in Japan where affected allegedly were 10,000 persons.
The incidence of the condition as well as the dosage (between 300 mg/day and 3.5 g/day) and length of treatment before the development of symptoms varied greatly. The link between clioquinol and SMON, which was incredibly uncommon outside of Japan, is still up for debate [39]. Although the cause of the neurological adverse effect in Japanese patients is uncertain, concurrent vitamin B insufficiency has been linked to it [40-41].
Circumstantial evidence suggests that clioquinol caused SMON, although there are still concerns supported by highly reliable data [42].
These are further confirmed by the fact that, despite the significant foreign sales of clioquinol, SMON was largely unknown outside of Japan or before 1955 and vanished before the medicine was taken off the market.
Although clioquinol is now sold as a topical preparation for the treatment of skin infections, its capacity to function as a copper and zinc chelator has led several researchers to speculate about its potential application in the management of Alzheimer's disease [43]. As a result, Prana Biotechnology reintroduced it for this indication on the basis of the metals hypothesis of Alzheimer's disease, and a novel quinoline derivative (PBT2) as a prospective disease-modifying medication has since been introduced [44-45].
In order to establish a connection between the drug's harmful effects and plasma and tissue clioquinol levels, Japanese researchers developed the first means for doing so in the 1970s. High Performance Liquid Chromatography (HPLC) with Ultraviolet (UV) detection was used to separate the samples, but it lacked sensitivity and specificity, necessitating laborious extraction techniques [46-48].
Two different teams created even more intricate Gas Chromatographic (GC) techniques with electron capture detection following acetylation: Jack and Riess, who used them to examine the levels of clioquinol in human plasma and urine, and Chen et al., who identified conjugate metabolites in the bodily fluids of various animals [49-50]. With a sensitivity of 50 ng/mL, solvent extraction was used in both techniques. Following benzene extraction and clioquinol conversion into pentafluorobenzyl ether, a highly sensitive Gas Chromatographic-Mass Spectrometric (GCMS) technique was created [51].
Tragedy of accutane
A vitamin A formulation called Accutane was created in 1979 and is used to treat acne. Accutane was approved for the treatment of severe acne [43]. Roche Pharmaceuticals was the company that produced Accutane. In 1982, the US FDA gave its approval. It was the only option for treating acne when there was no other alternative therapy. Later, it was shown that the medicine was linked to cases of congenital abnormalities, miscarriage, depression, crohan's disease, hepatic damage, and ulcerative colitis. FDA prohibited it in 2009. Although Accutane is no longer produced, its generic equivalent, Isotretinoin, is still available on the market. In response to Accutane's side effects, the FDA had proposed the "iPLEDGE" Program in 2006. As part of this effort, doctors, chemists, and Consumers must adhere to specific rules for medicine safety drug not to be taken if allergic reaction pregnancy history, cardiac condition, bone disorder, hepatic disorder, and intestinal disease are all contraindications to isotretinoin [52]. If used during pregnancy, a fatal flaw may develop. Pregnancy is not advised when using the drug. The iPLEDGE Program's first requirement is a pregnancy test.
Isotretinoin was first administered to patients with severe acne in 1979. The majority of these patients responded by having their acne symptoms dramatically and permanently clear up, earning the drug the brand name Accutane®, also known as Roaccutane® in some parts of the world. It is a vitamin A derivative (13-cis-retinoic acid) that is taken orally with a meal that has a sufficient amount of fat, typically for 15-20 weeks (3.5-4.5 months), however it is occasionally recommended at lesser dosages for up to 6 months or longer. Isotretinoin was initially advised for those with severe acne who did not react to other treatments [3], but in the last 25 years, it has become more popular and is now more routinely taken for acne that is not as severe. Because isotretinoin affects every human system and has potential for life-long negative effects, this approach is debatable.
Results
Tuskegee syphilis study (1932-1972)
Tuskegee in Alabama, a syphilis investigation started in 1932 and was completed in 1972. This 40-year biomedical research investigation was conducted over a longer period. This study used unethical procedures and experiments [53]. The specifics of the experiment were kept from the victims. The study's goal was to look into how syphilis spreads among untreated individuals. Asymptomatic disease stage was present during the investigation. Despite the availability of the treatment and facilities, neither the individuals recruited nor the disease was informed [54]. Penicillin was a medication that was sold on the market for treating [55]. The tests were stopped in 1972 as a result of media disclosure to the public. The National Research Act of 1974 established the National Commission for the Protection of Human Tuskegee in Alabama; a syphilis investigation started in 1932 and was completed in 1972. This 40-year biomedical research investigation was conducted over a longer period. This study used unethical procedures and experiments [56]. The specifics of the experiment were kept from the victims. The study's goal was to look into how syphilis spreads among untreated individuals. Asymptomatic disease stage was present during the investigation. Despite the availability of the treatment and facilities, neither the individuals recruited nor the diseases were informed. Penicillin was a medication that was sold on the market for treating. The tests were stopped in 1972 as a result of media disclosure to the public. The National Commission for the Protection of Humans was established by the National Research Act of 1974.
Recent tragedy of gambia
Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup, and Magrip N Cold Syrup are the four cough syrups reportedly responsible for the fatalities. It is "beyond heart-breaking for their families" that children have died as a result of the goods, and laboratory testing found that each of the four cough syrups Unsafe concentrations of ethylene and diethylene glycol contaminants. There were "unacceptable" levels of diethylene glycol and ethylene glycol in the cough syrups that were connected to the deaths of the children in the African country. Diethylene glycol is a liquid that has a sweetish taste and is virtually colourless, odourless, and hygroscopic. A derivative of ethylene glycol, diethylene glycol has similar effects on the body when consumed. The substance is harmful to people when consumed and be deadly. Diethylene glycol in excess might result in gastrointestinal issues such nausea, vomiting, pain in the abdomen, and diarrhea. Early neurological signs include altered mental state, central nervous system depression, coma, and moderate hypotension may appear in certain people. The substance causes renal insufficiency and failure and may potentially result in coma and death. It is used in antifreeze, braking fluids, cosmetics, and lubricants. The first time those deaths have been connected to cough syrups containing diethylene glycol. In Bangladesh, between 1990 and 1992, 339 kids who had been fed diethylene glycol-tainted paracetamol (acetaminophen) syrup developed kidney failure and most of them passed away. According to the Centers for Disease Control and Prevention (CDC), reports of "13 cases of unexplained acute renal failure among children from a hospital in Lagos state" were given to the Nigerian Federal Ministry of Health in 2009. The Nigerian authorities revealed that the teething medication these patients had been subjected to had been tainted with Diethylene glycol. In Diethylene glycol poisoning claimed the lives of over a hundred persons in Panama in 2006. Due to the presence of a "poisonous ingredient" in a cough syrup produced by Digital Vision in 2020; nine children had perished in Jammu. Diethylene glycol was later identified as the "poisonous chemical. “The sheer number of deaths calls for prompt and effective action against the offenders. However, the World Health Organization chief's medical product alert on these syrups is a loud red warning siren that the pharmaceutical sector and drug authorities would be wise to heed as shown below in the newspaper cutting as (Figure 2). Following the death of 66 children in Gambia, the World Health Organization (WHO) has raised an alert over four fever, cold, and cough syrups made by an Indian company, urging people to not use them.

Figure 2: World Health Organization chief's medical product alert.
The threat of contamination highlights a broader issue with product sourcing and production, a supply chain that can mean the difference between life and death, particularly when it comes to medications. Early examinations indicate that the hazardous DEG is present in this case di-ethylene glycol. Industry insiders speculate that this may be due to suspected adulteration of the commonly used solvent (propylene glycol) with a less expensive solvent Di- Ethylene Glycol (DEG). These medications were taken off the market and recalled from homes in the Gambia. Investigation finds that these were imported from an Indian company privately rather than through a public tender. There is no Indian importer. These were offered for sale at small-town pharmacies, hospitals, and private clinics. Diethylene glycol and ethylene glycol are the primary pollutants, which is an issue. Medical professionals have been asked not to prescribe them by the MCA. There isn't a lab there to check the quality of the medications. Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup, and Magrip N Cold Syrup are the four syrups produced by Maiden Pharmaceuticals, a company located in Haryana. It has been determined through laboratory examination of samples of each of the four items that they are contaminated with Diethylene glycol and ethylene glycol in unacceptable quantities. These four items have so far only been found in the Gambia, but the WHO warned that they may have travelled through unofficial markets to other nations or areas. All product batches "should be regarded dangerous" up until they are examined by the relevant national regulatory agencies, it stated as it was publicly published in India’s leading newspaper The Indian Express as shown below (Figure 3).

Figure 3: All product batches "should be regarded dangerous" up until they are examined by the relevant national regulatory agencies.
Discussion and Conclusion
In the Asian Region of the WHO, Pharmacovigilance is gaining momentum about 50 years after the thalidomide incident. India has just become a full participant of the WHO programme for global drug monitoring. The recent tragedy in Gambia that saw 66 children killed by cough syrup manufactured by an Indian company has caused another catastrophe from which many factors need to be taken into consideration.. In order to save many lives, several initiatives and actions have to be taken. The core of Pharmacovigilance is the identification of signs of adverse drug responses based on patient experiences with the medications as reported by physicians and pharmacists. Meyboom described a signal as a collection of information that supports a theory pertinent to the reasonable and secure use of a medicine in people. The main goal of Pharmacovigilance is to support this theory and establish its validity. As previously mentioned the data on which the signal is based come from the everyday interactions between doctors and pharmacists and have shown up in certain patients. Therefore, it is crucial that suitable means be made available to aid in the gathering of these experiences and observations. Several of these techniques have been developed in the recent decades.
The so-called Spontaneous Reporting System (SRS) is the approach that is most frequently employed. For the detection of uncommon and severe medication adverse events, an SRS is particularly useful. A SRS is applied to all medications during the course of their lifespan. It asks doctors, nurses, and increasingly patients to contact a Pharmacovigilance center with any observations or information. These reports must include enough details to enable a precise evaluation and a solid assessment of the causality—the relationship—between the suspected ADR and the medicine in issue. Pharmacovigilance also has access to a number of additional sources besides to the SRS. It can utilise the information gathered by the different (pharmaco) epidemiological research techniques; information that could support preexisting concerns. This thesis will also go into further detail on intensive monitoring techniques like prescription event monitoring, which have long been used successfully in the UK and New Zealand. Publication of case reports is another significant source of data that can help identify signals or bolster existing ones. Furthermore, there are the analytical techniques for signal identification in large ADR datasets, which have been the subject of several studies throughout the world. This method should not be viewed as a cure-all for the clinical examination of cases, but rather as an extra source of information. The improvement of these aspects of pharmacovigilance is a major focus of this thesis, which should result in better and more reporting on alleged adverse drug occurrences.
More work is being done to guarantee that resource-poor nations, which account for approximately 90% of the world's illness burdens, have access to efficient medications. Governments, the World Health Organization, other patients advocacy organizations are putting more pressure on medication corporations to lower access costs and legal hurdles. Although these initiatives are obviously important and commendable, they are not being complemented by the creation or expansion of mechanisms for assessing the safety of pharmaceuticals. The occurrence, pattern, and intensity of adverse responses may differ noticeably across poor and developed nations due to local genetic and environmental variables, despite the fact that many medications have been widely used and investigated in industrialized countries (informing global practice).
Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup, and Magrip N Cold Syrup are the four syrups produced by Maiden Pharmaceuticals, a company located in Haryana. It has been determined through laboratory examination of samples of each of the four items that they are contaminated with diethylene glycol and ethylene glycol in unacceptable quantities. These four items have so far only been found in the Gambia, but the WHO warned that they may have travelled through unofficial markets to other nations or areas. All product batches "should be regarded dangerous" up until they are examined by the relevant national regulatory agencies, it stated. Sources claim that the Central Drugs Standard Control Organisation (CDSCO), India's top drug regulatory body, has already started an investigation into the situation after being made aware of it on September 29. The Haryana state regulatory body verified that the company indeed produce and ship the syrups to Gambia. The product has only been sold to the Gambia thus far by the firm.
According to sources, diethylene glycol or ethylene glycol contamination was discovered in four of the 23 samples analysed by the WHO. However, the internal government agency has not given India any information on the death's causative relationship or any documentation demonstrating that the syrups were to blame for the fatalities. Diethylene glycol and ethylene glycol have severe side effects that can include nausea, vomiting, diarrhoea, altered mental status, headaches, and acute renal damage that may be fatal. The WHO notice warned that the linked inferior items were dangerous and that using them, particularly on children, might lead to fatalities or serious injuries. Additionally, it recommended that nations step up their supply chain monitoring in order to find and eliminate any subpar goods. It was also important to demand for the monitoring of unofficial or unregulated marketplaces. Please do not utilise these inferior items if you have them. 'You are advised to seek immediate medical advice from a qualified healthcare professional and report the incident to the National Regulatory Authority or National Pharmacovigilance Center if you or someone you know has used these products or suffered any adverse reaction/event after use,' the WHO alert advised.
If any of these inferior items are found in the national jurisdictions, they must be reported. In a tweet from the WHO Twitter account, Dr. Tedros Adhanom Ghebreyesus, the organization's director general, was cited as stating, "The four drugs are cough and cold syrups made by Maiden Pharmaceuticals Limited, in India. In collaboration with the business and Indian regulatory authorities, WHO is conducting additional investigations. In 2020, Jammu and Kashmir in India reported the deaths of 17 children who had consumed another type of cough syrup tainted with the same diethylene glycol. Another example included at least three children who died in New Delhi last year after ingesting a cough syrup that contained dextromethorphan, one of the four syrups WHO have warned consumers against.
Conflict of Interest
No conflict of interest
References
- Biswas P, Biswas AK. Setting standards for proactive Pharmacovigilance in India: The way forward. Indian phar. 2007; 39(3): 124.
- Kumanan R, Sudha S, Vijayashre P, Charumath S, Gowridevi KC, Mahesh M. Imperative Approach on pharmacovigilance in Indian systems of medicines. Int J pha sci and Res. 2010;1(9):378-390.
- World Health Organization. Pharmacovigilance: ensuring the safe use of medicines. No. WHO/EDM/2004.8. World Health Org. 2004.
- Rohilla A, Singh N, Kumar V, Kumar M, Sharma AD, Kushnoor A. Pharmacovigilance: Needs and objectives. Journal of Advanced Pharmacy Education & Research Oct-Dec. 2012;2(4):12.
- Routledge P. 150 years of pharmacovigilance. Lancet. 1998;351(9110):1200-1201.
[Crossref] [Google Scholar] [PubMed]
- Fornasier G, Francescon S, Leone R, Baldo P. An historical overview over Pharmacovigilance. Int J Clin Pharm. 2018;40(4):744-747.
[Crossref] [Google Scholar] [PubMed]
- Ledón N, Lage A. Biosimilars and the real world. MEDICC Rev. 2017;19(4):9-15.
[Crossref] [Google Scholar] [PubMed]
- Levy M. The epidemiological evaluation of major upper gastrointestinal bleeding in relation to aspirin use. Epi conc cli pharm 1987. pp 100-104.
- McBride WG. Thalidomide and congenital abnormalities. Lancet. 1961;2(1358):90927-90928.
- Lenz W, Knapp K. Foetal malformations due to thalidomide. Prob Birth Def. 1977:200-206.
- Kajii T, Kida M, Takahashi K. The effect of thalidomide intake during 113 human pregnancies. Teratology. 1973;8(2):163-166.
[Crossref] [Google Scholar] [PubMed]
- Peyvandi F, Garagiola I, Mannucci PM. Post-authorization Pharmacovigilance for hemophilia in Europe and the USA: Independence and transparency are keys. Blood Rev. 2021;49: 100828.
[Crossref] [Google Scholar] [PubMed]
- Al-Worafi YM . Drug Safety in Developing Countries: Achievements and Challenges. 2020.
- Kerkhof M, Tran TN, Soriano JB, Golam S, Gibson D, Hillyer EV, et al. Healthcare resource use and costs of severe, uncontrolled eosinophilic asthma in the UK general population. Thorax. 2018;73(2):116-124.
[Crossref] [Google Scholar] [PubMed]
- Meher BR, Agrawal K, Padhy BM. The global perspective of pharmacovigilance in nuclear medicine practice. Indian J Nucl Med. 2018;33(4):269-272.
[Crossref] [Google Scholar] [PubMed]
- Agius R. Evolvement of EU regulations on innovative medicines. 2017.
- Vargesson N. Thalidomide‐induced teratogenesis: History and mechanisms. Birth Defects Res C Embryo Today. 2015;105(2):140-156.
[Crossref] [Google Scholar] [PubMed]
- Bren, Linda. Frances Oldham Kelsey: FDA medical reviewer leaves her mark on history. FDA Consum. 2001;35(2):24-29.
[Crossref] [Google Scholar] [PubMed]
- Miller MT. Thalidomide embryopathy: a model for the study of congenital incomitant horizontal strabismus. Trans Am Ophthalmol Soc. 1991;89:623-674.
[Google Scholar] [PubMed]
- Loue S, Sajatovic M. Encyclopedia of women's health. Spr Sci Bus Media. 2004.
- Sneader W. Drug discovery: a history. John Wiley Sons. 2005.
- Nicolson, Malcolm. Sounds of the body. 2001.
- Thomas K. The Unseen Survivors of Thalidomide Want to Be Heard. New York Times. 2020;7:2022.
- Williams R. The Nazis and Thalidomide: The Worst Drug Scandal of All Time. 2012.
- Wapwera JA. Extraction and Isolation of Ascorbic Acid (Vitamin C) from australian pine (casuarina equisetifolia) needles grown in jos and environs. Diss. UNI. 2021.
- Miller MT. Thalidomide embryopathy: a model for the study of congenital incomitant horizontal strabismus. Trans Am Ophthalmol Soc. 1991;89:623-674.
[Crossref] [Google Scholar] [PubMed]
- Loue S, Sajatovic M. Encyclopedia of women's health. Spr Sci Bus Media. 2004.
- Dayer MR. Old drugs for newly emerging viral disease, COVID-19: Bioinformatic Prospective. 2021; 7(1):32-41.
- World Health Organization. World Health Organization model list of essential medicines: 22nd list (2021). World Health Organization. 2021.
- Kim, James H., and Anthony R. Scialli. Thalidomide: the tragedy of birth defects and the effective treatment of disease. Toxicological sciences 122.1 (2011): 1-6.
[Crossref] [Google Scholar] [PubMed]
- Rohde W, Mikelens P, Jackson J, Blackman J, Whitcher J, Levinson W. Hydroxyquinolines inhibit ribonucleic acid-dependent deoxyribonucleic acid polymerase and inactivate Rous sarcoma virus and herpes simplex virus. Anti Age Chemo. 1976;10(2):234-240.
[Crossref] [Google Scholar] [PubMed]
- Tateishi J. Subacute myelo‐optico‐neuropathy: Clioquinol intoxication in humans and animals. Neuropathology. 2000;20:20-4.
[Crossref] [Google Scholar] [PubMed]
- Tsubaki T, Honma Y, Hoshi M. Neurological syndrome associated with clioquinol. Neurological syndrome associated with clioquinol. 1971:696-697.
[Crossref] [Google Scholar] [PubMed]
- Gilland, Olof. A neurological evaluation of purported cases of SMON in Sweden. Acta Neurol Scand Suppl. 1984;100: 165-169.
[Crossref] [Google Scholar] [PubMed]
- Helmuth, Laura. An antibiotic to treat Alzheimer's? (2000): 1273-1274.
[Crossref] [Google Scholar] [PubMed]
- Meade TW. Subacute myelo-optic neuropathy and clioquinol. An epidemiological case-history for diagnosis. J Epi Com Hea. 1975;29(3):157-169.
[Crossref] [Google Scholar] [PubMed]
- Cuajungco MP, Faget KY, Huang X, Tanzi RE, Bush AI. Metal chelation as a potential therapy for Alzheimer's disease. Ann N Y Acad Sci. 2000;920(1):292-304.
[Crossref] [Google Scholar] [PubMed]
- Huckle R. PBT-1 Prana Biotechnology. Curr Opin Investig Drugs. 2005;6(1):99-107.
[Crossref] [Google Scholar] [PubMed]
- Bush AI. Drug development based on the metals hypothesis of Alzheimer's disease. J Alzheimers Dis. 2008;15(2):223-240.
[Crossref] [Google Scholar] [PubMed]
- Hayakawa K, Kitada K, Hamaki M, Miyazaki M. High-performance liquid chromatographic determination of clioquinol and its conjugates in biological materials. J Chromatogr. 1982; 229(1): 159-165.
[Crossref] [Google Scholar] [PubMed]
- Kotaki H, Yamamura Y, Tanimura Y, Saitoh Y, Nakagawa F, Tamura Z. Intestinal absorption and metabolism of clioquinol in the rat. J Pharmacobiodyn . 1983; 6(11): 881-887.
[Crossref] [Google Scholar] [PubMed]
- Ezzedeen, F. W., S. J. Stohs, and M. Stublar. Analysis of iodochlorhydroxyquin in biological materials by high-performance liquid chromatography. J Chromatogr. 1983;276(1): 121-128.
[Crossref] [Google Scholar] [PubMed]
- Jack DB, Riess W. Pharmacokinetics of iodochlorhydroxyquin in man. Journal of Pharmaceutical Sciences. 1973;62(12):1929-1932.
[Crossref] [Google Scholar] [PubMed]
- Chen C, Samejima K, Tamura Z. A gas chromatographic determination method of 5-chloro-7-iodo-8-quinolinol and its conjugates in biological fluids. Chem Pharm Bull. 1976;24(1):97-101.
[Crossref] [Google Scholar] [PubMed]
- Matsuki, Yasuhiko, et al. Determination of chinoform in biological fluids and nervous tissues of the dog by gas chromatography-mass spectrometry. Arch Toxicol. 1987; 59(5):374-378.
[Crossref] [Google Scholar] [PubMed]
- Choi JS, Koren G, Nulman I. Pregnancy and isotretinoin therapy. CMAJ. 2013;185(5):411-413.
[Crossref] [Google Scholar] [PubMed]
- Katz RV, Russell SL, Kegeles SS, Kressin NR, Green BL, Wang MQ, et al. The Tuskegee Legacy Project: willingness of minorities to participate in biomedical research. J Health Care Poor Underserved. 2006;17(4):698.
[Crossref] [Google Scholar] [PubMed]
- Newkirk VR. II. A generation of bad blood. Atlantic. 2016.
- Crenner C. The Tuskegee Syphilis Study and the scientific concept of racial nervous resistance. Journal of the history of medicine and allied sciences. 2012;67(2):244-280.
[Crossref] [Google Scholar] [PubMed]
- United States. National Commission for the Protection of Human Subjects of Biomedical, Behavioral Research. The Belmont report: ethical principles and guidelines for the protection of human subjects of research. J Am Coll Dent. 1978;81(3):4-13.
[Crossref] [Google Scholar] [PubMed]
Citation: Tantray J, Zaid M, Kosey S, Patel A, Sharma AK, Sharma R, et al. (2023) Pharmacovigilance: A Meta-Analysis on ADRS of Past and Recent Tragedy Occurred in Gambia. J Pharmacovigil. 11:409.
Copyright: © 2023 Tantray J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

