Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- CiteFactor
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 15, Issue 5
Pharmacokinetic/Pharmacodynamic Model for Aptensio XR® (an Extended-Release Methylphenidate Formulation) in 6-12 year-old Children
Andre J Jackson1* and Inder Chaudhary22Imbrium Therapeutics, 201 Tresse Blvd, Stanford, CT 06901, USA
Received: 05-Oct-2023, Manuscript No. JBB-23-23354; Editor assigned: 09-Oct-2023, Pre QC No. JBB-23-23354 (PQ); Reviewed: 23-Oct-2023, QC No. JBB-23-23354; Revised: 30-Oct-2023, Manuscript No. JBB-23-23354 (R); Published: 06-Nov-2023, DOI: 10.35248/0975-0851.22.15.539
Abstract
Aptensio XR® is an Extended-Release (ER) Methylphenidate (MPH) capsule drug product, approved for use in patients 6 years and older. Absorption of Aptensio XR® in children has been characterized by a fast first-order release and a delayed, slow first-order release. The current study investigated a Pharmacokinetic (PK) and Pharmacodynamic (PD) model for children 6-12 years-old (N=15). Determination of the PK parameters’ accuracy for pAUC (partial area under the curve) for times 0-3, 3-7, and 7-12 hours), Cmax (maximum concentration), and AUC0-T (area from time 0 to time T), for the 6-12 year-olds was calculated. All had bias less than 15% from the true observed values except for pAUC0-3 and Cmax. This study also compared the current PD parameters with those previously reported from a meta- analysis (without children’s PK data) for MPH in children and adults for SKAMP scores (Swanson, Kotkin, Atkins, M-Flynn, and Pelham rating scale) and with those from this study for children ages 6-12. An indirect response model described the SKAMP composite scores corrected for placebo. The results of this study support the use of adult PK data to predict the SKAMP scores in children 6-12 years old for Aptensio XR®.
Keywords
Aptensio; Phamacokinetics; Pharmacodynamics; Methylphenidate; Partial-Area-Under-the-Curve; SKAMP scores
Introduction
A basic tenet in the determination of Bioequivalence (BE) of generic drug products has been that BE studies of drug products for pediatric patients should generally be conducted in adults for ethical reasons. It is apparent though not frequently directly tested that a generic drug product found to be bioequivalent in adults will perform comparably in children. This paradigm has seemingly worked well since passage of the Drug Price Competition and Patent Term Restoration Act of 1984 [1]. A recent publication has developed a database of BE and relative Bioavailability (BA) studies conducted in pediatric populations to identify risk factors associated with certain drug substances or products that may lead to failed BE or different Pharmacokinetic (PK) parameters in relative BA studies in pediatrics compared to studies in adults [2]. The paper investigated the following causes:
• Age-related absorption effects
• Age-related distribution effects
• Age-related metabolism and clearance
• Drug substance and formulation effects
• Age-related disease progression
• Other disease-related effects.
The paper acknowledges that Inter-Individual Variation (IIV) and Intra-Occasion Variation (IOV) in PK parameters are higher in pediatric populations. In their discussion the authors state: “Further work is warranted to also compare the magnitude of differences observed for the BE or relative BA data identified from the pediatric population with similar data from the adult populations to fully evaluate the limitations of using adult data to predict the PK in pediatric populations.”
Due to these concerns, including ethical issues related to children’s drug therapy and the need for development of suitable dosage forms for children, legislation was passed targeting children’s issues in the development and approval of new drugs. These landmark legislative actions were the Best Pharmaceuticals for Children Act (BPCA) in 2002, amended in 2007, and the Pediatric Research Equity Act (PREA) in 2003, amended in 2007 [3,4]. Since passage of this legislation, significant progress has been made in the number, timeliness, and successful completion of studies of new NDA drugs for pediatric populations. This legislation also emphasized an assessment of the pediatric programs used in drug development, as well as suggestions for improving pediatric research. Despite these legislative steps, there has been no change or discussions related to the approval of generic (ANDA) versions of older NDA drugs with the accepted paradigm being that adults would generally be used as subjects for such BE studies. The major ethical concern here is that one can’t give a child volunteer a drug they don’t need or aren’t likely to need [5].
paper related to MPH has established that efficacy is correlated with drug release from the formulation [6]. A meta-analysis on data from children who had been variously dosed with MPH formulations (Concerta®, Ritalin LA®, Metadate®, and IR methylphenidate) and had SKAMP scores (composite score from Swanson, Kotin, Agler, M-Flynn and Pelham rating scale) and PERMP (Permanent Product Measure of Performance) was published in 2012 [7]. The objective of that paper was to provide PD models that could be used to predict mean and individual effect-time profiles in children based upon adult PK data. Despite the rigorous science in the paper, the following limitations were noted by the authors:
• There was not a large data base.
• Plasma concentrations were only from adults with clinical data from pediatrics.
• Interindividual variability of the MPH concentrations could not be concluded and was based solely on the variability of ADHD (attention- deficit/hyperactivity disorder) scores.
• Due to the lack of pediatric PK data, the developed PD model did not allow for estimation of the true parameters of the pediatric PK-PD relationship.
The authors concluded: “These models can be used to predict mean and individual changes in clinical measures during treatment in the pediatric population with any MPH extended-release formulation with a known concentration-time profile in adults.” This supports the policy of using adult PK to predict children’s PD for generic drugs and specifically those with complex absorption (e.g., MPH).
The objectives of the current research based upon the authors’ conclusions are:
• To determine how well a PK/PD sequential model for individual 6-12 year-old children describes Aptensio XR® in subjects when using the individual 6-12 year-old children’s plasma data.
• To estimate the “true” parameters of the pediatric PK-PD relationship by using pediatric PK individual data.
• To determine the ability of the published children’s PK model (8) to assess the additional current pAUC metrics (i.e., pAUC0- 3, pAUC3-7, pAUC7-12 hours) currently recommended by FDA for approval of MPH generic products.
Materials and Methods
The current manuscript is based upon work done in recent publications [8,9]. Since the methods used here are the same (with a few exceptions), only those methods that are different or new will be presented. Otherwise, they will be referenced from the previous publications.
Patients
The patients were described in a prior publication [8]. Concentrationtime data from the clinical PK trial and the PD trial were analyzed. The trials were conducted in children diagnosed with ADHD. All studies in children were conducted under fed conditions in which the children were administered a standard breakfast (e.g., toast, jam, cereal with 2% milk, and orange juice) prior to being administered a single dose as sprinkles over applesauce, as described in the drug labeling. The current studies are summarized in Table 1.
| Reference study number | Subject age | PK | PD | PD clinical endpoint |
|---|---|---|---|---|
| RP-BP-EF-011 | Children N=20, 6-12 yr. Old | Optimal titrated doses (15, 20, 30, or 40 mg/day) PD samples: 0, 1.5, 3, 5, 7, 10, and 12 hours post dose No PK samples | Yes | Double-blind placebo SKAMP primary endpoint; Subjects titrated -Final study day 7* |
| 022-011 | Children N=15, 6-12 yr. old | Optimal titrated doses (15, 20, 30, and 40 mg/day) PK Samples: 0,1, 2, 3, 4.5, 6, 7.5, 9, 10.5, and 12 hours post dose No PD samples | No | - |
Note: *During visit 7, subjects underwent specific assessments of attention and behavior objective individualized math tests at specific time points to evaluate the onset and duration effects of Aptensio XR®. At the end of visit 7, the subjects were dispensed to alternate double-blind treatment with dosing beginning the following morning. The second analog classroom date was held 1 week later at visit 8 after subjects had completed a week of daily morning dosing of the alternate treatment.
Table 1: PK and PD Study Details for Aptensio XR® in Children 6-12 yr. old.
Analog classroom study RP-BP-EF001
The study was a randomized, double-blind, placebo-controlled, crossover design comparing Aptensio XR® to placebo in a laboratory school setting beginning with an open-label dose optimization phase, followed by evaluation of the time of onset, duration of efficacy, tolerability and safety of Aptensio XR® (15, 20, 30, or 40 mg) in a double-blind phase.
The total SKAMP combined score was the endpoint used in assessing drug effects in this study. SKAMP total score was obtained by summing up 13 behavior items where each item is rated on a 7-point impairment scale (0=normal to 6=maximal impairment) for a total possible combined score of 0 to 78, where higher scores signify worsening impairment. The primary efficacy endpoint was the mean of the on-treatment, post-dose SKAMP total scores (mean total score over time points: 1.0, 2.0, 3.0, 4.5, 6.0, 7.5, 9.0, 10.5 and 12.0 hours). The means of the on-treatment SKAMP total scores for Aptensio XR® and placebo were compared. The dosing and sampling sequence is presented in Table 2 for the analog classroom study.
|
Screening (Phase1) | Wash-out call | Baseline (Phase2) | Treatment period-dose optimization (Phase2) | Laboratory school visits (Phase 3) | 30-day follow-up phone call (Phase4) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Visit | 1 | No visit | 2 | 3 | 4 | 5 | 6 Practice | 7 Measurements | 8/ET* | No visit |
| Study day | Up to -28 | -7 | 0 | 7 | 14 | 21 | 28 | 35 | 42 | 72 |
Note: *Early termination visit.
Table 2: Timeline design for laboratory analog classroom study.
Analytical methods
Plasma from blood samples obtained in the study was extracted and analyzed to determine MPH concentration using a fully validated liquid chromatography method with tandem mass spectrometry (LC/MS/MS) analysis (calibration range 0.05 ng/mL-25 ng/mL). Details were presented in a previous publication [10]. The d-threo- enantiomer of MPH is ten times more potent than the l-form and accounts for approximately 95% of observed total MPH plasma concentrations.
Data analysis
Base model: A physiological PK model was previously developed for adults and children for intravenous MPH formulations [11]. Subsequent model development investigated its application to the oral ER formulations Meta-date CD® and Ritalin LA® in adults [12].
The semi-physiological model used in the present study applied the parameters from the prior publications cited immediately above, but on the NONMEM platform based upon the population kinetics of the oral ER MPH drug product Concerta® [13]. The model contains covariates for weight and sex which are important factors for describing drug absorption, drug distribution and elimination. Therefore, there was no need to further investigate additional covariates.
Final model: The final model for the 6-12 year-olds was different from that used in the previous publication [8]. The final children’s model (Figure 1) incorporated a fast drug release and a delayed drug release with the early drug release characterized by an early lag period which was unusual. Only one subject had a measurable level at 0.5 hours, while the other subjects had their first measurable level at 1 hour or later. All subjects had the formulation-related, later lag time. Covariates for the children’s model were weight and sex, the same as used in the previous publication [8].
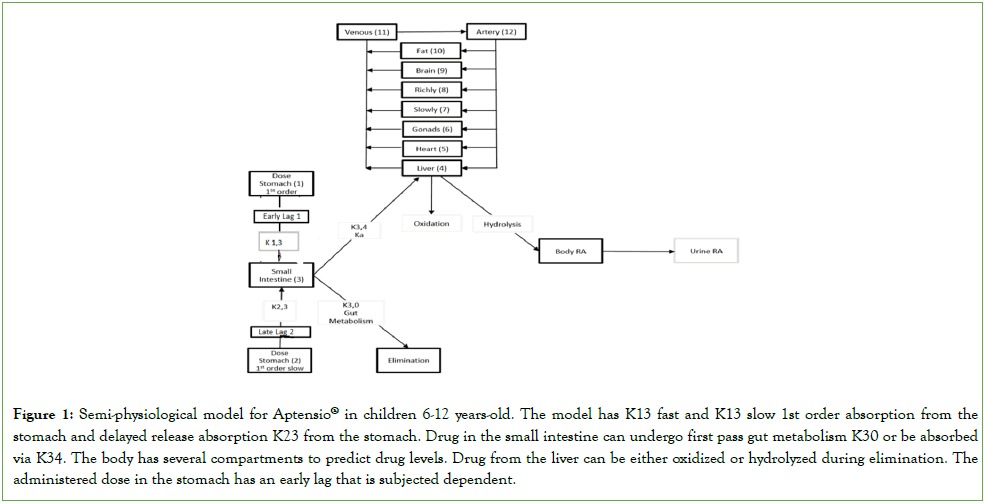
Figure 1: Semi-physiological model for Aptensio® in children 6-12 years-old. The model has K13 fast and K13 slow 1st order absorption from the stomach and delayed release absorption K23 from the stomach. Drug in the small intestine can undergo first pass gut metabolism K30 or be absorbed via K34. The body has several compartments to predict drug levels. Drug from the liver can be either oxidized or hydrolyzed during elimination. he administered dose in the stomach has an early lag that is subjected dependent.
Pharmacokinetic analysis
For the semi-physiological model, a first-order conditional method with interaction was employed for parameter estimation using Advan 13 and Trans 4. The individual concentration-versus-time d- and l- MPH data for the ER model for Aptensio XR® were simultaneously analyzed. The extent of shrinkage of empiric Bayesian parameter estimates obtained from NONMEM was determined for the PK parameters. Subject body weight was a covariate for all parameters. Scaled fractional tissue volumes and blood flows for organs had sex and weight as covariates (e.g., cardiac output=15.87*wt**0.75; male flow=0.038 cardiac output; female flow=0.047*cardiac output).
Pharmacostatistical model-children
An additive residual error model, a proportional residual error model, and a combination of the two were tested for d-MPH. The theta values for all weights were fixed. The standard deviation of the residual error (W) was obtained from the square root of the variance resulting [14]:

Pharmacodynamic model
The SKAMP endpoint selected for this estimation was used in previous FDA approvals of Attention Deficit Hyperactivity Disorder (ADHD) drug products [15]. The SKAMP PD model developed by Kimko, et al. was used for estimating total and baseline-corrected SKAMP scores using the previously described PK model [7]. Methylphenidate studies in healthy adults were used to develop the placebo-effect and Emax drug-effect model. All initial model parameter estimates used in the PD estimation were obtained from this reference.
The estimated SKAMP composite score was modeled as the difference between placebo and drug effects. The model structure is described below. The rate of change of the simulated response over time following placebo was described using an indirect response model:
dR/dt = Kin −α (t )*Kout *R ………………… (2)
Where biomarker quantity R is being produced with the constant rate Kin which represents the zero-order constant for response production and Kout defines the first-order rate constant for loss of response. The time-dependent elimination rate was assumed to be controlled by a periodic piecewise constant time-dependent coefficient α (t). Coefficients and number of switches for α (t) were estimated by Kimko, et al. [7]. It was assumed that α (t)=1 at night and has different values during the day between waking and going to bed.
For the current analog study, SKAMP composite scores were estimated for post-dose sampling times of 0, 1.5, 3, 5, 7, 10, and 12 hours.
The final MPH response model was an Emax model having acute tolerance on EC50 described as:
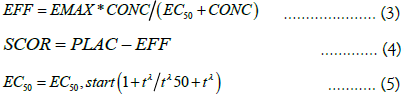
Where t is time from the morning dose and λ controls the steepness in the relationship. Tλ50 is the time that EC50 occurs.
Estimation of parameter standard errors and confidence intervals
As a consequence of rounding errors, the NONMEM covariance step was not successful, which resulted in an inability to calculate asymptotic standard errors. NONMEM with the UNCOND option was also unsuccessful. However, the models ran successfully when the covariance was not implemented. Therefore, the standard errors for the parameter estimates were determined by bootstrapping (i.e., generating pseudo-samples using the same distribution as for the original samples). 1000 bootstrap replicates were generated. Runs that did not have at least three significant figures and minimized successfully were discarded. The 90% confidence intervals were calculated based upon the standard error values for each parameter.
Determination of pAUC bioequivalence parameters
The Aptensio XR® experimental mean parameter-derived data for pAUC0-3, pAUC3-7, and pAUC7-12 hours, Cmax, and AUC0-T (area to time T) was compared to the mean values predicted by the semiphysiological model after 1000 simulations of the N=15 subjects. This allowed a comparison of the semi-physiological model parameter estimation accuracy (measured as percent bias) relative to observed parameter values. The other general BE parameters of Cmax (peak concentration), AUC0-T were also evaluated.
Results
Parameter estimation for d-methylphenidate-Aptensio XR®
The parameter estimation process for Aptensio XR® converged successfully using the first-order conditional estimation method with interaction with three significant figures. The final parameter estimates are shown in Table 3 for Aptensio XR® in children with the corresponding estimates of standard error and 90% CIs. Standard error was estimated by bootstrapping. The population PK parameters were estimated with minimal shrinkage except for F1, K30 and Alagfast.
| Parameter | Units | Estimate | %RSE | 90% Confidence interval | % Shrinkage |
|---|---|---|---|---|---|
| K13 | h-1 | 4.37 | 0.56 | (2.67 5.00) | - |
| K23 | h-1 | 0.71 | 0.76 | (0.53 1.01) | - |
| K30 | h-1 | 2.57 | 1.03 | (1.63 4.05) | - |
| K34 | h-1 | 0.06 | 3.80 | (0.01 0.13) | - |
| V11 | L | 3.14 | 4.52 | (1.04 7.36) | - |
| LagF | hrs | 1.38 | 0.32 | ((1.20 1.57) | - |
| LagS | hrs | 11.11 | 0.32 | (10.0 13.4) | - |
| Vmax | mg/hr/kg 0.75 | 37.30 | 2.85 | (21.7 60.0) | - |
| Km | mg/L | 13.80 | 2.49 | (10.0 19.3) | - |
| F1 | - | 0.59 | 0.38 | (0.49 0.71) | - |
| F2 | - | 0.41 | 0.55 | (0.29 0.51) | - |
| ω2 K13 Fixed | - | 0.20 | - | - | - |
| ω2 K23 | - | 0.20 | 2.59 | (0.02 0.47) | 22 |
| Ω2 F1 | - | 0.22 | 2.19 | (0.01 0.48) | 58 |
| ω2 K30 | - | 0.26 | 1.64 | (0.05 0.46) | 39 |
| ω2 K34 | - | 0.26 | 1.64 | (0.05 0.50) | 20 |
| ω2 V11 | - | 0.16 | 2.13 | (0.04 0.35) | 29 |
| ω2 AlagF | - | 0.16 | 2.88 | (0.04 0.43) | 89 |
| ω2 AlagS Fixed | - | 0.17 | 0.01 | - | - |
| ω2 SD,Res add Fixed | - | 0.003 | - | - | - |
Table 3: Nonmem parameter estimates for aptensio XR® for children 6-12 years old.
The estimated PD parameters are presented in Table 4 with their respective relative standard errors, per cent shrinkage, and 90% confidence intervals. Shrinkage was high only for the ω2BASE value.
| Parameter | Description | Estimate | Omega | % RSE | %Shrinkage | 90% confidence interval |
|---|---|---|---|---|---|---|
| B0 | Value of B at a steady-state with α(t)=1 | 54.5 Fix | - | - | - | - |
| α1 | Coefficients morning after waking to describe time-dependent elimination | 6.0 Fix | - | - | - | - |
| α2 | Coefficients during school day to describe time-dependent elimination | 1.18 | - | 9.5 | - | (0.96 1.40) |
| Kout (1/hr) | Elimination rate constant | 0.023 | - | 15.4 | - | (0.016 0.029) |
| Emax | Maximum drug effect | 21.9 | - | 0.006 | - | (20.99 22.81) |
| EC50 (ng/ml) start | Concentration that corresponds to half the maximum effect | 1.50 Fix | - | - | - | - |
| ϒ tol | Steepness parameter of the tolerance model | 4.23 Fix | - | - | - | - |
| t50 h | Time at which tolerance effect reached | 5 Fix | - | - | - | - |
| t1 h | End of early morning | 0.54 Fix | - | - | - | - |
| t2 h | t1 + length of school day | 6.71 Fix | - | - | - | - |
| t3 h | Start of school day 0.1 h predose | 23.9 Fix | - | - | - | - |
| ω2BASE | Variance of α1 | - | 0.01 Fix | - | 74 | - |
| ω2 α1 | Variance of α1 | - | 0.15 Fix | - | 32 | - |
| ω2α2 | Variance of α2 | - | 0.02 Fix | - | 34 | - |
| δ2 | Variance of residual error | 0.475 | 11.4 | - | (0.36 0.58) |
Table 4: Parameter estimates for the SKAMP model in children 6-12 yrs old.
Model evaluation for d-methylphenidate- aptensio XR®
PK: The goodness-of-fit plots for Aptensio XR® concentrations in children (Figure 2) showed reasonable correlation between predicted and observed data for population versus individual predictions over time. The plot of the Conditionally-Weighted Residuals (CWRES) also showed a reasonable uniform distribution between 0 and 24 hours.
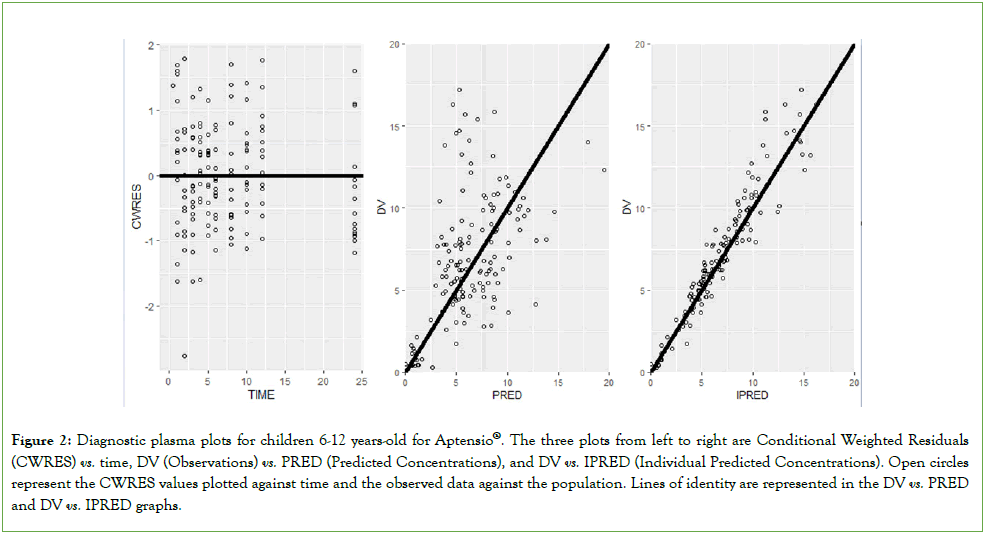
Figure 2: Diagnostic plasma plots for children 6-12 years-old for Aptensio®. The three plots from left to right are Conditional Weighted Residuals (CWRES) vs. time, DV (Observations) vs. PRED (Predicted Concentrations), and DV vs. IPRED (Individual Predicted Concentrations). Open circles represent the CWRES values plotted against time and the observed data against the population. Lines of identity are represented in the DV vs. PRED and DV vs. IPRED graphs.
PD: Goodness-of-fit plots for the SKAMP scores are presented in Figure 3. The CWRES vs. time plot was well distributed around the line of identity indicating minimal bias. In addition, the dependent variable (dv) vs population and dv vs individual predictions (ipred) diagnostics were acceptable and evenly distributed around the identity line.
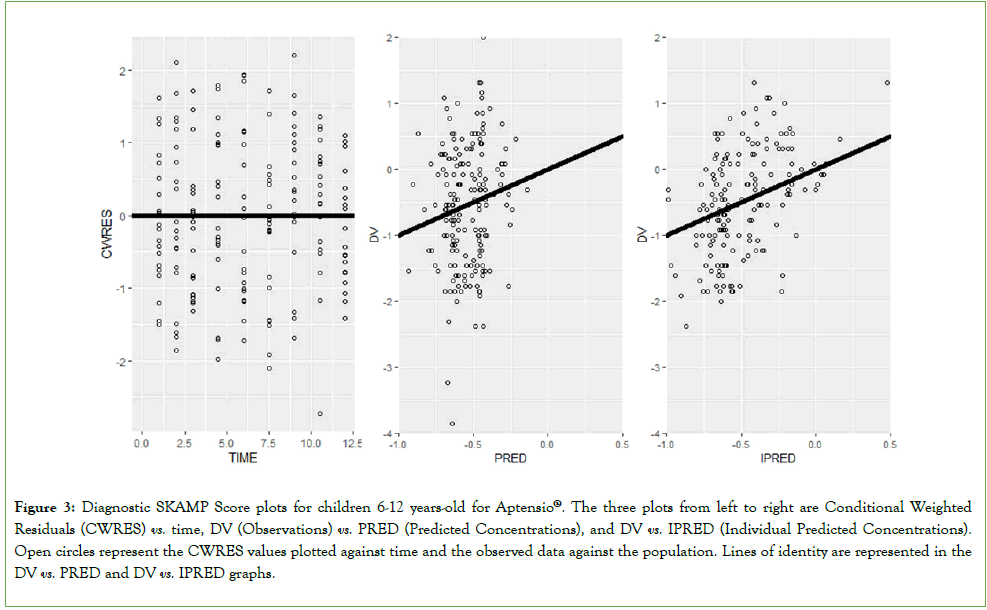
Figure 3: Diagnostic SKAMP Score plots for children 6-12 years-old for Aptensio®. The three plots from left to right are Conditional Weighted Residuals (CWRES) vs. time, DV (Observations) vs. PRED (Predicted Concentrations), and DV vs. IPRED (Individual Predicted Concentrations).
Open circles represent the CWRES values plotted against time and the observed data against the population. Lines of identity are represented in the
DV vs. PRED and DV vs. IPRED graphs.
Model qualification
PK: A visual predictive check indicated that the physiological model adequately described Aptensio XR® data in children 6-12 (Figure 4). The Aptensio XR® model qualification plot is based on 1000 simulations (N=15 per simulation). Shaded blue areas are the 95% CIs of the 5th and 95th percentiles of the simulated data. The dashed lines are the 5th, 50th, and 95th percentiles for the observed data. Observed data are the open data points.
Figure 4: Visual predictive check model plasma qualification plots for children 6-12 years of age for Aptensio® Predicted corrected visual predictive check. Blue dots=prediction corrected observations, red dashed lines=median of the corrected observations, orange shaded area=95% Confidence Interval (CI) of the median prediction, blue shaded area=95% CI of the 5th and 97.5th prediction interval.
PD: The comparable visual predictive check for the SKAMP scores is presented in Figure 5. The results were based upon 1000 simulations (N=20 per simulation).
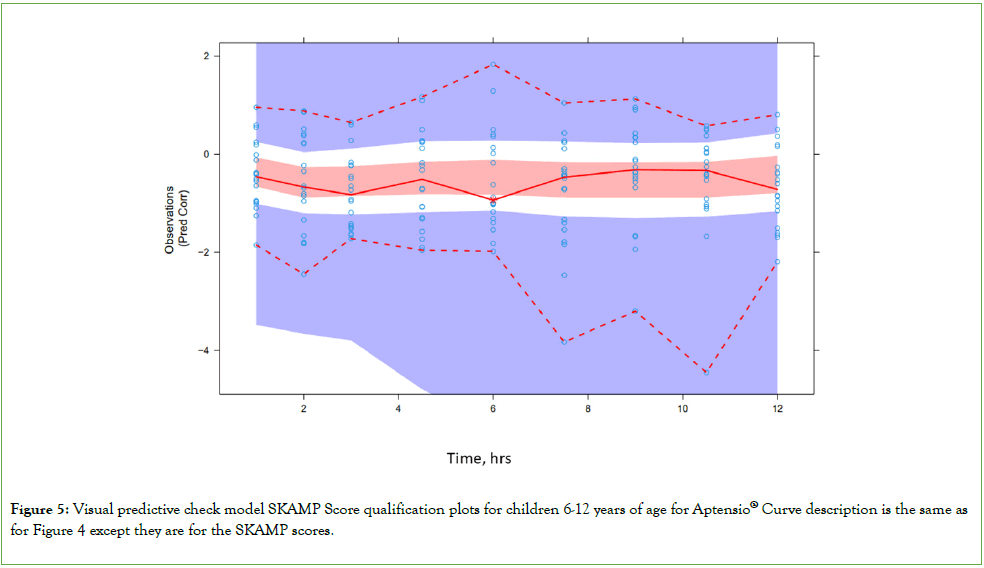
Figure 5: Visual predictive check model SKAMP Score qualification plots for children 6-12 years of age for Aptensio® Curve description is the same as for Figure 4 except they are for the SKAMP scores.
Shaded areas have the same designations as for the PK model qualification graph in Figure 4.
pAUC values precision
The 90% CIs were calculated for the observed and model- simulated (N=1000 studies) data for children for all of the relevant BE parameters (C max, pAUC0-3, pAUC3-7, pAUC7-12 AUC0-T) using best-fit parameters. The means of the simulated relevant BE parameters were then compared with the observed the observed experimental data and the percent bias calculated. Results are presented in Table 5 for children. Final bias values were all within the nominal value of 15% except for Cmax and pAUC0-3 hr [16]. A possible explanation for these two parameters being outside the 15% limit is presented as a footnote to Table 5.
| Parameter | Observed | Simulated | % Bias |
|---|---|---|---|
| pAUC0-3 hr | 6.13 | 4.82 | -21.3* |
| pAUC3-7 hr | 8.86 | 9.37 | 5.7 |
| pAUC7-12 hr | 16.16 | 17.25 | 6.1 |
| AUC0-T hr | 59.0 | 63.36 | 7.4 |
| AUC0-inf | - | - | - |
| Cmax | 11 | 16 | 17.0** |
Note: *Subjects had different simulated lag times with some with no lag time resulting in poor estimation of pAUC0-3 hr; **Outside the acceptable limit of 15% and may have been influenced by the variability of pAUC0-3 hr values.
Table 5: Bias results for childrenâ??s simulated mean (N=1000 studies) bioequivalence values versus observed mean values.
Discussion
The major difference between children 6-12 and adults was the much larger relative standard error of the PK based upon intrasubject variability in children’s parameters [8]. This would result in a much different PD result if the adult test drug variability is not controlled. A similar comparison of adults to the previously combined data for children ages 4-5 and 6-12 years also resulted in a larger relative standard error for the children. The other major model structural difference was that the adults did not have an early lag in absorption whereas for the 6-12 year-olds, 14 of 15 (93%) subjects had an early lag and for the 4-5 year-olds 3 of 9 (33%) had an early lag [8]. However, the early lag seemed to have had no impact on efficacy, since a generic Aptensio XR® was approved for Actavis Elizabeth on December 13, 2018 [17]. It appears that the new replicated study design worked well for this approval for which the draft was being implemented for study design though not formally approved until 2021 [18]. This is supported by the lack of reports of generic formulation failure as was previously reported for the Kudco (now UHC/Kremers Urban) ER generic drug product version of Concerta® [18]. The early profiles for children 6-12 years (i.e., presence of an early lag) are more variable than those for adults (i.e., no early lag time) [8]. However, the approval of the generic Aptensio XR® would indicate that the apparent early profile differences and any variability it introduced were well handled by the replicated study design.
The PD parameters for MPH presented in Kimko’s paper are not identical to those obtained for the current 6-12 year-olds since Kimko did not use Aptensio XR® for PD and they had no children’s individual PK data [7]. As a consequence of using mean observed MPH concentrations from adults, inter-individual variability was not included in their analysis. Instead, they assumed that all of the variability was driven by the variability in the ADHD scores. However, since the current study includes individual subject values, the inter-subject variability was better defined in our study. Most of the current study values (variance of α1 at baseline, variance of α1, variance of α2) were greater than those presented by Kimko. Only the variance of the residual error was less in our study (at 0.475 vs. 1.62 in the Kimko study).
Fixed parameters estimated in the current study were similar to those of Kimko but the random parameters differed, reflecting the impact of having individual subject data. Nonetheless, their parameters were used as initial estimates and other than Emax (27.8 Kimko vs. 21.9 current study) and EC50 (7.55 ng/ml Kimko vs. 1.50 ng/ml current study) final estimates were similar to those of Kimko [7]. This means that their model and results were very good despite having only adult data. In fact, these differences were predicted by Kimko in the discussion as possibly being different due to the magnitude of the concentration differences between children and adults.
Parameter bias was good for all model parameters other than Cmax and pAUC0-3 hours which may be related to the delayed absorption for 93% of the subjects and the highly variable times for Tmax, with many of the observed values very near 2 hours post-absorption and some at 1 hour. Since the drug has a short half-life of approximately 5 hours, AUC0-inf was not estimated since we believe that AUC0-T (i.e., 24 hrs) is very representative of AUC0-inf.
Conclusion
In the determination of BE for generic versions of Aptensio XR®, the problem of excessive PK variability seems to have been generally overcome by the use of a replicated design BE study. Prior studies done by Kimko, et al., although lacking individual children’s data, seem to describe SKAMP score PD values very well. The results from this study can possibly be used to determine how well adult data predicts the outcome in children. The current results seem to support the use of adult PK data to predict the outcome in SKAMP scores in children 6-12 years old for Aptensio XR®.
Ethics
This research was carried out in accordance with the clinical research guidelines established by the Basic Principles defined in the U.S. 21 CFR Parts 50, 56, and 312; the principles enunciated in the Declaration of Helsinki (and its amendments); and the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use. All protocols, protocol amendments, informed consent forms and other study documents were reviewed and approved by the Research Ethics Board at the Royal University Hospital, Saskatoon, SK, Canada. Written informed consent was obtained from each subject or parents/legal guardians at the screening visit prior to performing any procedures or collecting any information. The study RP-BP- EF-001 was conducted as a phase 1 study. The data for this study is the property of Rhodes Pharmaceuticals and Purdue Pharma, Canada and can’t be shared to protect study participant privacy.
References
- Drug Price Competition And Patent Term Restoration Act. 1984.
- Pawar G, Wu F, Zhao L, Fang L, Burckart GJ, Feng K, et al. Development of a pediatric bioavailability /bioequivalence database and identification of putative risk factors associated with evaluation of pediatric oral products. AAPS J. 2021;23(3):57.
[Crossref] [Google Scholar] [PubMed]
- Best Pharmaceuticals for Children Act (BPCA). 2018.
- Pediatric Research Equity Act (PREA). 2003.
- GDUFA Regulatory Science Initiatives Public Meeting: Sherwin CMT. 2016.
- Swanson J, Gupta S, Guinta D, Flynn D, Agler D, Lerner M, et al. Acute tolerance to methylphenidate in the treatment of attention deficit hyperactivity disorder in children. Clin Pharmacol Ther. 1999;66(3):295-305.
[Crossref] [Google Scholar] [PubMed]
- Kimko H, Gibiansky E, Gibiansky L, Starr HL, Berwaerts J, Massarella J, et al. Population pharmacodynamics modeling of various extended-release formulations of methylphenidate in children with attention deficit hyperactivity disorder via meta-analysis. J Pharmacokinet Pharmacodyn. 2012;39(2):161-176.
[Crossref] [Google Scholar] [PubMed]
- Jackson AJ, Brundage R, Ette E, Chaudhary I. Semi-physiological population pharmacokinetic model for methylphenidate hydrochloride multi-layer extended release (Aptensio XR) capsules in children ages 4-12 and adults. J Bioequiv and Bioavail. 2022;14(6):1-10.
- Jackson AJ, Foehl HC. A simulation study to compare performance of partial area under the curve (pAUC) and partial area under the effect curve (pAUEC) metrics in crossover versus replicated bioequivalence studies for Concerta and Ritalin LA. AAPS J. 2022;24(4):80.
[Crossref] [Google Scholar] [PubMed]
- Teuscher NS, Adjei AL, Findling RL, Greenhill LL, Kupper RJ, Wigal S. Population pharmacokinetics of methylphenidate hydrochloride extended-release multiple-layer beads in pediatric subjects with attention deficit hyperactivity disorder. Drug Des Devel Ther. 2015; 9:2767-2775.
[Crossref] [Google Scholar] [PubMed]
- Yang X, Morris SM, Gearhart JM, Ruark CD, Paule MG, Slikker W, et al. Development of a physiologically based model to describe the pharmacokinetics of methylphenidate in juvenile and adult humans and nonhuman primates. Plos One. 2014; 9(9):1-30.
[Crossref] [Google Scholar] [PubMed]
- Yang X, Duan J, Fisher J. Application of physiologically based absorption modeling to characterize the pharmacokinetic profiles of oral extended release methylphenidate products in adults. PLoS One. 2016;11(10): e0164641.
[Crossref] [Google Scholar] [PubMed]
- Jackson, AJ. A semi-physiologically-based model for methylphenidate pharmacokinetics in adult humans. J Bioequi Bioava. 2019;11(2):29-37.
[Crossref]
- Proost JH. Combined proportional and additive residual error models in population pharmacokinetic modelling. Eur J Pharm Sci. 2017;109:S78-S82.
[Crossref] [Google Scholar] [PubMed]
- Center For Drug Evaluation And Research. 2019.
- Ette EI, Williams PJ, Kim YH, Lane JR, Liu MJ, Capparelli EV. Model appropriateness and population pharmacokinetic modeling. J Clin Pharmacol. 2003;43(6):610-623.
[Crossref] [Google Scholar] [PubMed]
- Generic Aptensio XR Availability. 2015.
- Questions and Answers Regarding Methylphenidate Hydrochloride Extended Release Tablets (generic Concerta) made by Mallinckrodt and UCB/Kremers Urban (formerly Kudco). 2016.
Citation: Jackson AJ, Chaudhary I (2023) Pharmacokinetic/Pharmacodynamic Model for Aptensio XR® (an Extended-Release Methylphenidate Formulation) in 6-12 year-old Children. J Bioequiv Availab. 15:539.
Copyright: © 2023 Jackson AJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

