Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- CiteFactor
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2015) Volume 6, Issue 1
Periodic Existence of Mycorrhizal Fungi in Roots and Non-root Dissident Portions of Some Bulbous Plants
Abstract
Decaying and senescing scale-like leaves and roots were collected regularly from Botanical Garden, University of the Punjab, Lahore for a period of four months, with an interval of fifteen days. Roots and other portions of both the plants when processed and examined revealed the occurrence of AM fungal structures. However, AM structures were totally missing in Allium cepa, and roots of Amaryllis vittata. Thick hyphal mats with clusters and clumps of spores were often seen in the decaying scale-leaves. The vesicles in these portions were large sized and thick walled. As regard the seasonal variations, the side by side of hyphal, arbuscular and vesicular infections varied among the samples collected throughout the season. Seasonal variations in Glomalean spore dynamics were observed with respect to number while glomeromycetous species richness varied in the rhizosphere soil of the three plants. The recent research was conducted to evaluate the configuration of occurrence of arbuscular mycorrhizal fungal structures in decaying scale like leaves and root system of three bulbous plants i.e., Allium cepa, Amaryllis vittata and Zaphyranthes citrina.
Keywords: Arbuscular mycorrhizae; Scale-leaves; Allium cepa; Amaryllis vittata; Zaphyranthes citrine; Species richness
Introduction
Arbuscular Mycorrhizae (AM) is mutually beneficial relationship amongst fungi and roots of the higher plants. Colonization of roots by mycorrhiza has been revealed to recover development and yield of numerous field crops including leguminous crops, cereals, vegetables and oil crops [1-5]. Mycorrhizal associations increase plant growth and productivity by increasing nutrient element uptake [6] and improving resistance to abiotic [7,8] and biotic [9] stress factors. Plants often benefit from the presence of mycorrhizal associates via a variety of mechanisms including improvement of soil structure, mobilization of essential minerals, enhancement of desiccation resistance, and protection from pathogens and herbivores [10]. Recently some workers have reported that AM also increase the crop tolerance against allelopathy and enhance crop growth under this stress [11,12].
Vesicular arbuscular mycorrhizae are of universal occurrence in roots of 95% of land plants [13,14]. However, during past few decades reports are available about the presence of AM in plant portions other than roots [15,16]. Since than a number of examples have been added to the literature with an emphasis on a much wider occurrence of these associations than reported ever before.
We had planned this study to further add to the information about AM proliferating in roots and non-root portions of some bulbous plants.
Materials and Methods
Sampling of root and non-root portions like scale leaves, dried decaying sheathing leaves on the bulbs of two test bulbous plants viz. Allium cepa, Amaryllis vittata and Zaphyranthes citrina. The sampling was done regularly from February to May. Specific sites (Sites name) were selected for sampling of plants. The root/bulb samples were dug up along with rhizospheric soil. Extreme care was taken to avoid the disturbance of root systems, adhering decayed and semi-decayed scale leaves and epidermis. The samples were brought back to the Lab. in polythene bags. Decaying scale like leaves and fine roots were gently peeled off with the help of forceps, while rest of the bulbous portion along with root system were dipped in a bucket of water for half an hour. The adhering soil was removed with the help of camel hairbrush while washing gently under the tap water. The root system of all samples, scales and other portions were cut up into 1 cm2 pieces and then fixed in F.A.A. (Formaline: Acetic Acid : Alcohol in 5:5:90 ratio by volume) in properly labeled MacCartney bottles separately. The samples were cleared and stained for analysis of colonization of AM fungi using Phillips and Hayman procedure [15]. The roots were cleared in 10% KOH solution in an autoclave, placed in 0.1N HCl for 2-3 minutes for neutralization and then stained with trypan blue solution (0.05% in lactophenole glycerine). The sample pieces were mounted on the glass slides in a drop of lactic acid and were observed under low power (10x) of the light microscope. Extent of AM infection was recorded with the help of an already calibrated ocular micrometer. This was done by randomly focusing the plant material under the microscope and by measuring the hyphae. The number of vesicles and other structures were also recorded in the same way. Microphotography was done with the help of Minolta X 700 camera with the adapter tube.
Soil adhering to the roots and other portions like bulbs were utilized for screening the associated AM spores. Spore extraction was done by following wet sieving decanting method of Nicolson and Gerdemann [11,12]. Density and diversity of spores was recorded. Spores were identified using synoptic key by Morton [16] and Schenck and Perez [17]. All the data was statistically analyzed by computing Standard Error, Least Significant Difference; (LSD) and Duncan’s New Multiple Range Test (Steel and Torrie [18].
Result and Discussion
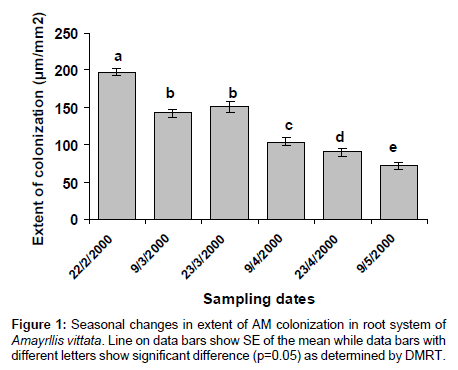
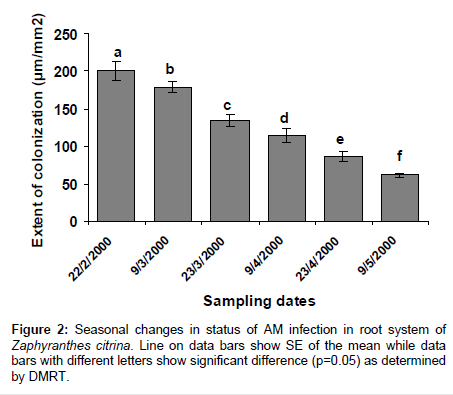
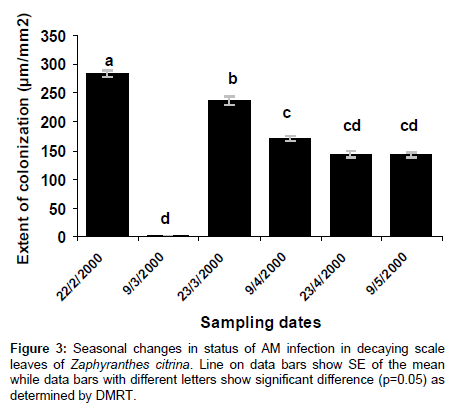
AM colonization was completely absent in scales and roots of Allium cepa, and roots of Amaryllis vittata (members of Amaryllidaceae) may be attributed to existence of immunity to attack by pathogenic fungi. In bulbs of Allium cepa resistance to attack by fungus is due to the presence of catecol and protocatechuic acid in the dry, pigmented, outer scale leaves. Members of Amaryllidaceae also produce fungitoxin (Methylene-buty rolactose) in their bulbs, which also diffuses to plant roots (Figures 1-3) and (Tables 1-3).
| Sample No | Sampling date | No. ofvesicles per mm2 | No. of Intramatrical spores per mm2 | No. of cells filled with DSEF per mm2 |
|---|---|---|---|---|
| 1. | 22/2/2000 | 000.77d ± 0.29 | 002.44e ± 0.94 | 008.66e ± 0.66 |
| 2. | 9/3/2000 | 002.66b ± 0.577 | 003.66d ± 1.33 | 007.33f ± 2.33 |
| 3. | 23/3/2000 | 002.66b ± 1.00 | 005.33c ± 0.19 | 013.66d ± 1.76 |
| 4. | 9/4/2000 | 002.44c ± 0.1 | 007.99b ± 0.51 | 015.66c ± 0.66 |
| 5. | 23/4/2000 | 002.88a ± 0.39 | 012.66a ± 3.38 | 016.00b ± 0.57 |
| 6. | 9/5/2000 | 002.44c ± 1.25 | 012.10a ± 2.27 | 018.00a ± 4.04 |
| L.S.D | 1.36 | 3.40 | 3.93 | |
Table 1: Status of various AM structures in decaying scale like leaves of Amaryllis vittata at different sampling time.
| Sample No | Sampling month | No. ofvesicles per mm2 | No. of Intramatrical spores per mm2 | No. of cells filled withDSEF per mm2 |
|---|---|---|---|---|
| 1. | 22/2/2000 | 011.00a ± 0.57 | 003.66c ± 1.76 | -- |
| 2. | 9/3/2000 | 010.00b ± 1.52 | 003.66c ± 0.33 | -- |
| 3. | 23/3/2000 | 010.33b ± 1.45 | 003.66c ± 0.33 | 001.33c ± 0.33 |
| 4. | 9/4/2000 | 008.88c ± 3.39 | 003.89d ± 0.39 | 006.00b ± 3.00 |
| 5. | 23/4/2000 | 006.33d ± 0.03 | 005.33b ± 0.33 | -- |
| 6. | 9/5/2000 | 005.33e ± 0.57 | 006.33a ± 0.36 | 007.33a ± 0.88 |
| L.S.D | 1.77 | 1.48 | 2.42 | |
Table 2: Status of various AM structures in root systems of Zaphyranthes citrina at different sampling times.
| Sample No | Sampling date | No. ofvesicles per mm2 | No. of Intramatrical spores per mm2 | No. of cells filled withDSEF per mm2 |
|---|---|---|---|---|
| 1. | 22/2/2000 | 032.55a ± 1.25 | 004.00e ± 0.57 | 008.66a ± 2.40 |
| 2. | 9/3/2000 | 030.77b ± 0.83 | 005.33d ± 0.66 | 004.66c ± 0.33 |
| 3. | 23/3/2000 | 026.21c ± 0.29 | 011.33e ± 1.45 | 005.33b ± 1.53 |
| 4. | 9/4/2000 | 023.88d ± 1.68 | 022.00d ± 1.52 | 004.00c ± 1.73 |
| 5. | 23/4/2000 | 018.32e ± 1.33 | 030.33ab ± 1.85 | 003.66d ± 0.88 |
| 6. | 9/5/2000 | 015.55f ± 1.56 | 032.00a ± 0.57 | 003.33d ± 1.20 |
| L.S.D | 2.35 | 2.30 | 2.76 | |
Table 3: Status of various AM structures in scale leaves of Zaphyranthes citrina at different sampling times.
References
- Javaid A, Iqbal SH, Hafeez FY (1994) Effect of different strains of Bradyrhizobium and two types of vesicular arbuscularmycorrhizae (VAM) on biomass and nitrogen fixation in Vigna radiate (L.) Wilzek var. NM 20-21.Sci Inter (Lahore) 6: 265-267
- Yao MK, Tweddell RJ, Désilets H (2002) Effect of two vesicular-arbuscularmycorrhizal fungi on the growth of micropropagated potato plantlets and on the extent of disease caused by Rhizoctoniasolani. Mycorrhiza 12: 235-242.
- Kapoor R, Giri B, Mukerji KG (2004) Improved growth and essential oil yield and quality in Foeniculumvulgare mill on mycorrhizal inoculation supplemented with P-fertilizer. BioresourTechnol 93: 307-311.
- Subramanian KS, Santhanakrishnan P, Balasubramanian P (2006) Responses of field grown tomato plants toarbuscularmycorrhizal fungal colonization under varying intensities of drought stress. SciHortic 107: 245-253
- Wang FY, Lin XG, Yin R, Wu LH (2006) Effect of arbuscularmycorrhizal inoculation on the growth of Elsholtziasplendens and Zea mays and the activities of phosphatase and urease in a multi-metal-contaminated soil under unsterilized conditions. Appl Soil Ecol 31: 110-119.
- Al-Karaki GN (2002) Benefit, cost and phosphorus use efficiency of mycorrhizal field grown garlic at different soil phosphorus levels. J Plant Nutrition 25: 1175-1184.
- Feng G, Zhang FS, Li XL, Tian CY, Tang C, et al. (2002) Improved tolerance of maize plants to salt stress by arbuscularmycorrhiza is related to higher accumulation of soluble sugars in roots. Mycorrhiza 12: 185-190.
- Chen BD, Zhu YG, Smith FA (2006) Effects of arbuscularmycorrhizal inoculation on uranium and arsenic accumulation by Chinese brake fern (Pterisvittata L.) from a uranium mining-impacted soil. Chemosphere 62: 1464-1473.
- St. Arnaud M, Hamel C, Caron M, Fortin JA (1994) Endomycorrhize VA et sensibilite des plantes aux maladie. In: FortinJA, Charest Cand Piche Y(eds.)La SymbioseMycorhizienne, Etat des Connaisances, Orbis, Quebec.
- Bajwa R, Javaid A, Haneef A (1999) EM and VAM technology in Pakistan N: Response of Cowpea to co-inoculation with EM and VAM under allelopathicstress. Pak. J. Botany. 31: 387-369
- Nicolson TH (1967) Vesicular arbuscularmycorrhiza, a universal plant symbionts
- Gerdemann JW (1968) Vesicular arbuscularmycorrhizae and plant growth. Annual Review of Phytopathology 6:39-418
- Bagyaraj DJ, Manjunath A, Patal RB (1979) Occurrence of vesicular arbuscularmycorrhiza in some tropical aquatic plants. Trans Brit MycolSoc 72: 164-167
- Nasim G (1990) Vesicular arbuscularmycorrhiza in portions other than roots. In: Bushan L.Jalali, ChandL(eds.) Current trends in mycorrhizal research. Proceedings of the national conference on mycorrhiza. A Haryana AgrUniHisar14-16: 4-6.
- Philips JM, Gerdeman DS (1970) Improved procedure for clearing and staining parasitic and VAM fungi for the rapid assessment of infection. Irans. Brit. Mycol. Soc., 55:158-161
- Morton JB (1988) Taxonomy of mycorrhizal fungi. Classification, nomenclature and identification, Mycotoxicon 100: 267-3240
- Schenck NC, Perez Y (1987) Manual for the identification of VAM fungi. In: VAM, 1453
- Steel RDG,TorrieJH(1980) principles and procedure of statistics. McGraw Hill Book Co., Inc. New York USA.
Copyright: © 2015 Ali M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.