Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- Directory of Abstract Indexing for Journals
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2024) Volume 14, Issue 3
Percutaneous Application of Adipose Derived Stem Cells (ADSCs) in Non-Union of Long Bone Fractures
Himanshu Bansal1*, Jerry Leon2, Anupama Bansal1 and Iustin Preoteasa32Department of Neurology, University of Puerto Rico, Puerto Rico, USA
3Department of Neurology, Alpha Medica Stem Clinic, Targoviste, Romania
Received: 07-Dec-2020, Manuscript No. JSCRT-24-7430; Editor assigned: 10-Dec-2020, Pre QC No. JSCRT-24-7430 (PQ); Reviewed: 24-Dec-2020, QC No. JSCRT-24-7430; Revised: 16-Aug-2024, Manuscript No. JSCRT-24-7430 (R); Published: 13-Sep-2024, DOI: 10.35248/2157-7633.24.14.647
Abstract
Aim: Non-union is a common clinical complication that causes serious limb debilities and eventually leads to permanent limb impairment if not treated timely. Each bone fracture is unique and no common treatment modality will prove efficacious. To date, stem cells derived from bone marrow tissue are used for the treatment of non-union fractures. Present study reported personalized stem-cell therapy with Adipose tissue Derived Stem Cells (ADSCs)/ Stromal Vascular Fraction (SVF) in seven patients with history of non-union of long bones.
Methods: A total of 11 patients were enrolled for the study and 7 patients could be followed up for the treatment. ADSCs/SVF cells were extracted from adipose tissues and re-suspended in peripheral blood Plasma Rich in Platelets (PRP). The preparation was injected into the non-union site aided by fluoroscopic localization. The outcome was assessed using lower limb core scale and radiological screening. The patients were evaluated critically for any evidence of healing. If no evidence of healing was seen for any 6 month, the procedure was considered as failure.
Results and conclusion: We observed the long bone reunion in 6 of 7 (86%) patients in 4 months of SVF implantation. First evidence of healing was visible in five of the seven (71%) patients in 8 weeks post implantation of ADSCs suggesting the efficacy of the modality in treating atrophic non-union fractures. This is the first study that demonstrated the efficacy of ADSCs to treat non-union fractures. Present study is one of a trailblazer with more possibilities to resolve the practical difficulties of non-union fractures.
Keywords
Stem cell therapy; Non-union fracture; Clinical study; Adipose derived stem cells; Stromal vascular fraction cells; Bone grafts; Growth stimulators
Introduction
Non-union is one of the consequences of a delayed union and is established when at least nine months has passed since an injury without any sign of union. It has been estimated that the total number of non-union fractures account for 5%-10% of total fractures. Bone grafts with or without disconnecting the fibrous union has been gold standard treatment however it has several disadvantages including increased morbidity, pain, paresthesia, blood loss, risk of re-fracture, infection at the donor site, limited amount of cancellous bone and devascularization of the fracture fragments. Allografts, bone morphogenic protein all have their own adverse effects, have major limitations: Rejection, transmission of diseases, lower engraftment capacity and cost and there exists a dearth in promising methods that effectively treat non-union fractures [1]. The importance of osteoprogenitor cells in bone formation has led to increased interest in exploring osteo-inductive properties of mesenenchymal stem cells. MSCs from Bone Marrow Aspirate (BMAC) have been considerably used in the recent past for treating non-union. Adipose tissue as an alternative source of MSCs is being used for autologous cellbased therapies. Although the earliest clinical application of autologous adipose SVF in nonunion was reported in 2004, only a few clinical studies of ADSC in non-union have been reported till date. However, the effect of ADSCs has been extensively studied in animal models with critical size segmental defects and the results show that ADSCs significantly enhance the rate of new bone formation both at the center and the periphery of the defect.
Present study is an approach to evaluate the practicality of minimally invasive percutaneous injection with autologous ADSCs/SVF and explored the osteo-inductive efficacy of ADSCs in the management of non-union. In addition, the possibility of early application with ADSCs before it reaches dormancy (as in delayed union) has also been determined [2].
Materials and Methods
Study design and patient characteristics
A total of 11 patients with long bone fractures who failed to unite after standard treatment were enrolled after obtaining approval from institutional ethics committee to evaluate the safety and efficacy with fluoroscopic guided percutaneous implantation of ADSC at the site of fracture as an outpatient procedure between 2012 to 2018. All the patients were well informed and consent was taken prior to treatment (Figure 1) [3].
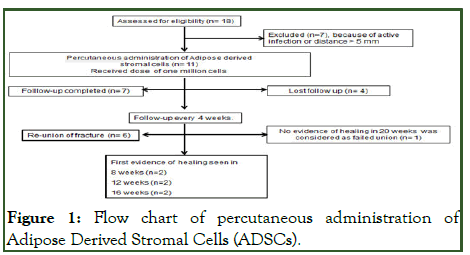
Figure 1: Flow chart of percutaneous administration of Adipose Derived Stromal Cells (ADSCs).
Criteria for non-union and assessment for infection
The indication for this procedure was established as non-union with maximum gap between the fragments to be <5 mm and duration not later than one year between the fracture and the procedure. The other primary criterion was absence of infection at the fracture site. Non-union was considered as established when: i) at least 9 months has passed since the injury and ii) the fracture did not show any visible signs of healing for 3 months. All non-unions were considered atrophic since they showed very little callus formation. Screening tests were conducted to observe the presence of any active systemic infection (Figure 1). The fracture site was subjected to hot fomentation for 7 days to flare up latent infection if any. The intensity of infection was assessed through x-rays and local examination. Patients were enrolled in the study only if they meet the eligibility criteria (Table 1) [4].
| Inclusion criteria | Exclusion criteria |
|---|---|
| Male and female subjects between 25 to 70 years of age | Multiple major fracture or untreated major fracture |
| Non-union or delayed union diagnosed after x-ray examination | Infected fracture |
| More than 4 cm distance from the joint | HIV, hepatitis B or hepatitis C infection at the time of screening |
| Provided written informed consent | Pregnant or lactating women |
| - | Diagnosis of cancer |
| - | Active treatment with immunosuppressive drugs or anticoagulant agents |
| - | Known allergic reaction to components of study treatment and/or study injection procedure |
Table 1: Eligibility criteria for enrolment into the study.
Isolation of stromal vascular fraction cells from adipose tissue
Stromal Vascular Fraction (SVF) cells were isolated from adipose tissue as described earlier. Briefly, around 100 ml abdominal fat was collected from each patient using an aspiration cannula (3 mm) with prior administration of tumescent solution. SVF cells were harvested from the fluid portions of the liposuction aspirates.
The suctioned fluid was centrifuged (400 gm for 10 minutes) and the pellet was mixed with saline and passed through a 100 μm mesh filter (Thermo Fisher Scientific, USA) followed by filtering through a 40 μm filters (Thermo Fisher Scientific, USA) and then through flush back filter (7 μm; Alpha corpuscle, India) to isolate cells ranging from 7 μm to 40 μm in one ml of saline.
Cells were taken to determine the cell quantity, viability and stem cells characterization as described earlier. The suctioned fluid was centrifuged (400 gm for 10 minutes) and the pellet was mixed with saline and passed through a 100 μm mesh filter (Thermo Fisher Scientific, USA) followed by filtering through a 40 μm filters (Thermo Fisher Scientific, USA) and then through flush back filter (7 μm; Alpha corpuscle, India) to isolate cells ranging from 7 μm to 40 μm in one ml of saline. Cells were taken to determine the cell quantity, viability and stem cells characterization as described earlier.
Flow cytometric analysis
Cells isolated from patients were cultured in six well plates in complete DMEM media as described earlier in our report. The cells were trypsinised and washed thrice with PBS followed by incubation with FITC labeled primary antibodies CD90-APC (BD biosciences, USA), CD34 APC‐Alexa 700 (Beckman Coulter, USA), CD73-PE (BD biosciences, USA), CD45 APCCy7 (BD biosciences, USA), CD105‐FITC (BD biosciences, USA) and HLA-DR9-per CP-Cy (BD biosciences, USA). The cells were processed for flow cytometry analysis using BD FACS Calibur.
Platelet Rich Plasma (PRP) preparation
Platelet Rich Plasma (PRP) was derived from the peripheral blood of the same patient as the adipose tissue. Briefly, 20 ml of peripheral blood was collected into vaccutainers (Bectan Dickinson and company, NJ, USA) and centrifuged at 1800 g for 10 minutes. The plasma fraction was collected and again centrifuged at 10,000 × g for 20 min to obtain a platelet pellet. Platelets were re-suspended in 7 ml of supernatant [5].
Preparation of fracture site and fluoroscopic localization
One ml of SVF cells were re-suspended in 7 ml of PRP and injected into the non-union site and around the bones (18 G needles; Bectan Dickinson and company, NJ, USA) without removing the fibrous tissue. Compression strapping was done to prevent ooze or hematoma.
Assessments of patient recovery
Healing at the injury site was evaluated using clinical and radiological examination at 1, 2, 6 and 12 months posttreatment. Antero-posterior radiographs with fully extended joint and patient standing at 1 m from the x-ray source, was obtained as standard procedure. Two independent, blind experts (orthopedic surgeon and radiologist) reviewed the x-ray images to determine whether there was any evidence of persistent nonunion or signs of union of the fracture. The radiological progression was determined as per scoring pattern and the scores were used to quantify non-union and healing. Patient reported outcomes were also assessed using Lower Extremity Functional Scale (LEFS) and Short Form 12 (SF-12) for physical component summary scale. Tolerability and safety assessments included any adverse events reported by the patient and laboratory based hematological and biochemical assays. Aspirate from fracture site was analyzed using microscopic examination at day 0 and at 3 months post procedure for bacterial growth and atypical cell counts. Adverse events were categorized as isolated, intermittent or continuous and depending on their occurrences as mild, moderate or severe based on the interference with the patient’s daily activities. Possible causal relationship with the supplement in terms of definite/possible/probable/non-assessable/none was also assessed (Table 2).
| Features | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Radiological features | No callus at all | Minimum ensheathing callus | Good ensheathing callus or internal callus with bridging of at least 2 cortex | Good internal callus with bridging of all 4 cortex |
Table 2: Radiological grading for fracture healing.
Statistical analysis
The statistical significance was assessed by student’s t test using 2-tailed Mann-Whitney U test. Data are expressed as medians (range). P values less than 0.05 were considered statistically significant [6].
Results
Stromal vascular fraction/ADSCs
Isolation process yielded approximately 1 million of ADSCs/ml of lipoaspirate (range from 0.87 × 106/ml to 1.24 × 106/ml). The cells mainly consist of mature adipocytes and intercellular tissue or SVF.
The ADSCs isolated in this manner were shown to share immunophenotype markers and analuysis revealed CD29+ (91.21%), CD73+ (92. 1%), CD90+ (90.6%), CD105+ (88.65%) positive and CD14-, CD34- and HLA-DR- negative SVC (Figure 2).
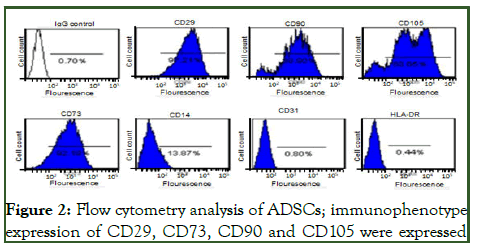
Figure 2: Flow cytometry analysis of ADSCs; immunophenotype expression of CD29, CD73, CD90 and CD105 were expressed
Patients
A total of 11 patients were enrolled for present study. Four patients were lost to follow-up so our result comprised of follow up of 7 patients after ADSCs implantation. The age of the patients who completed the treatment ranged from 25-58 years; median 48 years and 5 of the 7 were male and 2 were females.
The clinical characteristics of these patients have been described in Table 3. All patients underwent initial treatment with closed/open reduction and internal/external fixation at the time of primary management (Table 4). A fair conservative attempt including dynamization in functional cast brace was tried before embarking upon percutaneous ADSC injection. All patients were categorized as grade 1 of non-union radiologically [7].
| S. no | Characteristics | Values |
|---|---|---|
| 1 | Age (years) | 25-58 years |
| 2 | Sex | Male-5; female-2 |
| 3 | Mean (SD) | 45 (12) years |
| 4 | Median | 48 years |
Table 3: Summary of patient characteristics.
| Patient no | Type of fracture | Initial treatment | Time to injection |
|---|---|---|---|
| 1 | Tibia open II | Debridement and external fixator | 14 months |
| 2 | Tibia open IIIa | Debridement and external fixator | 10 months |
| 3* | Tibia closed | Locked IM nail | 9 months 10 days |
| 4 | Tibia closed | Locked IM nail | 9 months |
| 5* | Femur closed | Locked IM nail | 9 months |
| 6 | Femur closed | Locked plating | 12 months |
| 7 | Operated femur; infected | External fixator after removal of locked IM nail | 16 months |
Table 4: Clinical characteristic of patients completing the study.
Open tibial fractures (patient number 1 and 2) were treated earlier with debridement and external fixator followed by locked nailing and bone grafting post wound healing. Two closed shaft tibia fractures (patient number 3 and 4) were treated earlier by closed interlock nailing before presenting as non-union. Two patients (number 5 and 6) were treated earlier by interlocked nailing and the other patient number 6 had fracture supracondular femur treated with plates and screws but by 12 months presented with non-union and implant failure post implantation showed first evidence of healing by 8 weeks and consolidation by another 6 weeks. The most difficult situation (patient number 7) with infected operated fracture shaft femur presented with non-union on external fixator after nail removal needed more time to heal as first evidence of healing noted by 20 weeks and good consolidation needed another 40 weeks and a repeat procedure. The infection was healed at the time of ADSCs implantation. All conservative attempts like functional bracing, partial weight bearing and dynamization were already being attempted in all these patients to facilitate union (Figures 3 and 4) [8].
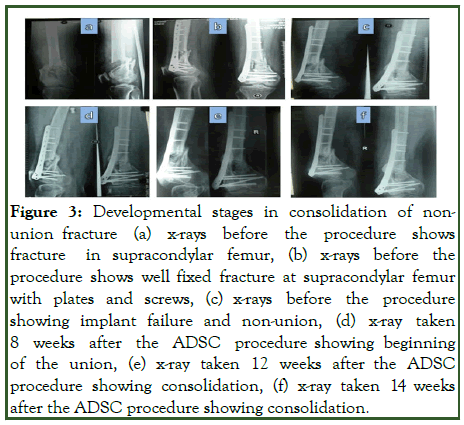
Figure 3: Developmental stages in consolidation of nonunion fracture (a) x-rays before the procedure shows fracture in supracondylar femur, (b) x-rays before the procedure shows well fixed fracture at supracondylar femur with plates and screws, (c) x-rays before the procedure showing implant failure and non-union, (d) x-ray taken 8 weeks after the ADSC procedure showing beginning of the union, (e) x-ray taken 12 weeks after the ADSC procedure showing consolidation, (f) x-ray taken 14 weeks after the ADSC procedure showing consolidation.
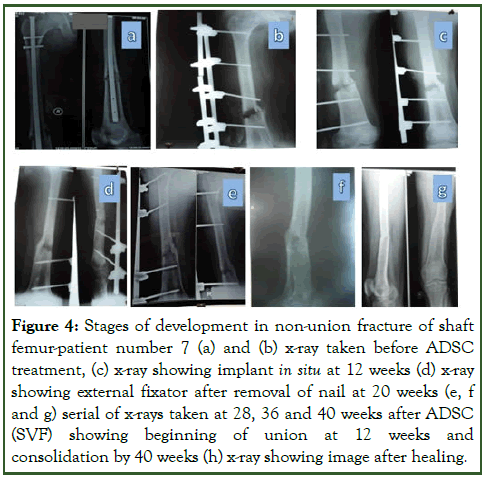
Figure 4: Stages of development in non-union fracture of shaft femur-patient number 7 (a) and (b) x-ray taken before ADSC treatment, (c) x-ray showing implant in situ at 12 weeks (d) x-ray showing external fixator after removal of nail at 20 weeks (e, f and g) serial of x-rays taken at 28, 36 and 40 weeks after ADSC (SVF) showing beginning of union at 12 weeks and consolidation by 40 weeks (h) x-ray showing image after healing.
In all the cases, adipose tissue was harvested under local anesthesia with mild sedation of midazolam (1 mg/kg slow intravenous) and 8 ml of ADSC concentrate was injected percutaneously with 18 G needle into the site of non-union at 3-4 points around the non-union zone, under c-arm guidance. The patients showed evidence of healing as early as two months with a full consolidation in an average of 6-8 weeks after the first sign (Table 4). The time from initial injury to non-union or delayed union and ADSC treatment ranged from 9 months to 16 months. After ADSC implantation, six of the seven fractures united within 4 months of injection. One fracture could not get healed and needed an open procedure. A single injection was found to be sufficient in the majority of cases, only in case no 7, where the procedure was repeated [9].
Follow-up screening was done every 8 weeks for a minimum of 18 months to a maximum of 6 years after the injection. Improvements in LEFS were significant (54.6-86.7; P<0.02) and though the assessment for short form 12 for physical component summary score (27.2-48.4) also improved from the baseline, the differences was not statistically significant (P>0.05).
Discussion
Skeletal reconstruction has recently seen a major paradigm shift towards tissue engineering with application of growth factors and Mesenchymal Stem Cells (MSCs). Cells from raw bone marrow or BMAC are the main stay for cell based treatment of nonunion. Besides pain in aspiration, limitation of volume, low yield of MSCs and inconsistency in progenitor cell counts is the main concern with bone marrow based cell therapy. The success of cell based procedures greatly depends on the number of Colony Forming Unit-Fibroblasts (CFU-F).
To overcome limitation of low MSC counts in bone marrow, in vitro culture expansion of MSCs from bone marrow is used these days with good clinical outcome. However expanded MSCs are considered a biological drug and needs regulatory agencies approval for its use. Stromal Vascular Fraction (SVF) or Adipose Derived Stem Cells (ADSCs) is alternative option. Adipose tissue can be harvested with minimal patient discomfort and has a 500- fold greater mesenchymal stem cell count compared to an equal volume of bone marrow.
The number of nucleated cells in adipose tissue can range from 5 × 105 to 2 × 106 cells per gram (g) with approximately 1%-10%of ADSCs in them. Roughly, 1 × 107 ADSCs can routinely be isolated from 300 ml of lipoaspirate, with >95% purity. ADSCs yield roughly about 5000 fibroblast CFU per gram of adipose tissue. This is comparatively greater than BMMSCs which produce approximately 100-1000CFU-F/ml. Most importantly, adipose tissue can be processed within a short period of time (<2 h) and can be applied immediately in a one-step surgical procedure [10].
In our study six of the seven patients achieved union and five patients showed the first evidence of healing by eight weeks after the procedure. The clinical improvement corresponded well with x-ray imaging. Open tibial fractures (patient number 1 and 2) were treated earlier with debridement and external fixator followed by locked nailing and bone grafting post wound healing. Two closed shaft tibia fractures (patient number 3 and 4) were treated earlier by closed interlock nailing before presenting as non-union. Two patients (number 5 and 6) were treated earlier by interlocked nailing and the other patient number 6 had fracture supracondular femur treated with plates and screws but by 12 months presented with non-union and implant failure post implantation showed first evidence of healing by 8 weeks and consolidation by another 6 weeks. The most difficult situation (patient number 7) with infected operated fracture shaft femur presented with non-union on external fixator after nail removal needed more time to heal as first evidence of healing noted by 20 weeks and good consolidation needed another 40 weeks and a repeat procedure. The infection was healed at the time of ADSCs implantation. All conservative attempts like functional bracing, partial weight bearing and dynamization were already being attempted in all these patients to facilitate union.
Our results are correspond well with previous studies using bone marrow concentrate which is still the most frequently used cellbased bone regeneration procedures. Studies have reported union in 78% to 100% patients in closed tibial non-unions and 53.5% in open tibial fractures. The median time to healing in our study was 10.6 weeks corresponded with these studies. ADSC promotes healing almost at the same pace as BMAC. These studies reported healing with fracture gap of less than 4 mm whereas in our study the mean gap was <5 mm.
It is essential to emphasize that our selected patients had injury within 16 months. All of them had occluded marrow cavity and did not possess dense sclerosed bone ends. It can be said thatunion in these cases would have not occurred without the procedure, as the mean duration between the procedure and the injury was 11.7 months.
No major local or systemic complications were observed during the 2 year follow-up period. One patient experienced short lived local pain and swelling at the lipo-aspiration site. One patient developed effusion in the joint which resolved with conservative treatment.
Present proof of concept study is the first report that efficacy presents of ADSCs as better alternative to BMAC in treating non-union of long bones. The literature is void with data on ADSC in nonunion. Ours is first study using ADSC in long bone non-union so direct comparison is not possible. The first successful case with ADSCs implantation was reported for extensive calvarial injury in a seven year old girl followed by few reports of success in treating skull and jaw defects. Consolidation in 80% and 50% of patients of post sarcoma long bone defects was reported using ADSC and bone matrix. The first ever nonunion treated with ADSC was reported for sternal restoration showing healing by 3 months on 3D CT imaging. Positive outcome of SVF implantation in 8 cases of fresh proximal humeral fractures with severe osteoporotic bone has also been observed.
The non-enzymatic method to isolate SVF is considered to exhibit minimal manipulation hence compliant with regulatory laws. Our method of ADSC isolation was non enzymatic and based on filtration [11].
We performed implantation of ADSC in patients without removing the fibrous tissue, the fibrous tissue interposed between the bone ends was ossified. The exact mechanism of transformation of fibrous tissue into callus is not clear. Although the intrinsic potential of a local tissue is generally impaired at the atrophic nonunion site, their generative cells can be re-stimulated to form bone structures under appropriate conditions. This phenomenon suggests that progenitor cells in atrophic non-union tissues regenerate even though the microenvironment in the non-union site might retard their function. Some physical or biological agents (like growth factors or cells) are required to reactivate the endogenous progenitor cells to revive them towards bone regeneration in atrophic non-union. Biopsy of fracture site at 12 months after the procedure indicated the presence of new bone ossicles that were very distinct from osteoconducted bone in its shape and structure. These “cocktail” preadipocytes, pericytes, MSCs and various endothelial progenitor cells present in SVF provide angiogenic, anti-inflammatory and immune modulation effects. MSCs residing in the SVF possess strong osteogenic potential not only by their ability to differentiate into osteogenic cells following induction, but also by their paracrine effects through the secretion of numerous growth factors and cytokines. These factors influence the encompassing progenitor cells. Some evidence based studies recommend that these infused stem cells get engrafted into the tissue and differentiate into tissue [12].
Conclusion
Our results strongly suggest ADSCs/SVF administration is a safe, effective and propitious therapeutic tool in treating nonunion fractures of long bone. Its application could be extended to delayed union and also in cases with primary fixation where high chances of non-union exist. The study demonstrated that ADSC treatment is a high grade option to bone marrow stem cell therapy. Although our study showed profound results in treating non-union or delayed union fractures, phase II clinical studies with a greater number of subjects are warranted to study the efficacy and other practical challenges of the treatment.
Limitations
Present study is a proof of concept study. Further studies with RCT is needed. One patient was lost to follow up. However, the number of patients in this study is too small to reach any definitive conclusions.
Ethical Approval and Consent to Participate
Ethical approval was obtained by the institutional committee for stem cell research and therapy, Anupam hospital, Rudrapur, Uttarkhand, India. All patients were recruited following written informed consent.
Consent for Publication
Not applicable.
Funding
Funding is acknowledged from Anupam hospital, Uttarkhand, India. (Grant no. 2012/02/UK/ADM/023).
Competing Interests
The authors declare that they have no competing interests.
Availability of Data and Materials
Not applicable.
Acknowledgements
We are thankful to the patients for their kind collaboration. The authors are thankful to the laboratory staff for collecting and biobanking of patients’ samples.
References
- Oryan A, Monazzah S, Bigham-Sadegh A. Bone injury and fracture healing biology. Biomed Environ Sci. 2015;28(1):57-71.
[Crossref] [Google Scholar] [PubMed]
- Giannoudis PV, Einhorn TA, Marsh D. Fracture healing: The diamond concept. Injury. 2007;38:S3-S6.
[Crossref] [Google Scholar] [PubMed]
- Ekegren CL, Edwards ER, de Steiger R, Gabbe BJ. Incidence, costs and predictors of non-union, delayed union and mal-union following long bone fracture. Int J Environ Res Public Health. 2018;15(12):2845.
[Crossref] [Google Scholar] [PubMed]
- Oryan A, Alidadi S, Moshiri A, Maffulli N. Bone regenerative medicine: Classic options, novel strategies and future directions. J Orthop Surg Res. 2014;9:1-27.
[Crossref] [Google Scholar] [PubMed]
- Sen MK, Miclau T. Autologous iliac crest bone graft: Should it still be the gold standard for treating nonunions? Injury. 2007;38(1):S75-S80.
[Crossref] [Google Scholar] [PubMed]
- Dimitriou R, Mataliotakis GI, Angoules AG, Kanakaris NK, Giannoudis PV. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: A systematic review. Injury. 2011;42:S3-S15.
[Crossref] [Google Scholar] [PubMed]
- Connolly JF, Guse R, Tiedeman J, Dehne R. Autologous marrow injection as a substitute for operative grafting of tibial nonunions. Clin Orthop Relat Res. 1991;266:259-270.
[Google Scholar] [PubMed]
- Bhargava R, Sankhla SS, Gupta A, Changani RL, Gagal KC. Percutaneous autologus bone marrow injection in the treatment of delayed or nonunion. Indian J Orthop. 2007;41(1):67.
[Crossref] [Google Scholar] [PubMed]
- Le Nail LR, Stanovici J, Fournier J, Splingard M, Domenech J, Rosset P. Percutaneous grafting with bone marrow autologous concentrate for open tibia fractures: Analysis of forty three cases and literature review. Int Orthozp. 2014;38:1845-1853.
[Crossref] [Google Scholar] [PubMed]
- Hernigou PH, Poignard A, Beaujean F, Rouard H. Percutaneous autologous bone-marrow grafting for nonunions: Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am. 2005;87(7):1430-1437.
[Crossref] [Google Scholar] [PubMed]
- Nicoletti GF, de Francesco F, D'Andrea F, Ferraro GA. Methods and procedures in adipose stem cells: State of the art and perspective for translation medicine. J Cell Physiol. 2015;230(3):489-495.
[Crossref] [Google Scholar] [PubMed]
- Müller AM, Mehrkens A, Schafer DJ, Jaquiery C, Guven SI, Lehmicke M, et al. Towards an intraoperative engineering of osteogenic and vasculogenic grafts from the stromal vascular fraction of human adipose tissue. Eur Cell Mater. 2010;19:127-135.
[Crossref] [Google Scholar] [PubMed]
Citation: Bansal H, Leon J, Bansal A, Preoteasa I (2024) Percutaneous Application of Adipose Derived Stem Cells (ADSCs) in Non-Union of Long Bone Fractures. J Stem Cell Res Ther. 14:647.
Copyright: © 2024 Bansal H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : Funding is acknowledged from Anupam hospital, Uttarkhand, India. (Grant no. 2012/02/UK/ADM/023).

