Indexed In
- Open J Gate
- Academic Keys
- JournalTOCs
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2024) Volume 2, Issue 2
Perchlorate Reducing Bacterias Having Ecological and Mars Terraforming Significances
Utsav Sunilkumar* and Sanjay LalReceived: 15-Sep-2020, Manuscript No. JAO-24-6494; Editor assigned: 18-Sep-2020, Pre QC No. JAO-24-6494 (PQ); Reviewed: 02-Oct-2020, QC No. JAO-24-6494; Revised: 01-Aug-2024, Manuscript No. JAO-24-6494 (R); Published: 29-Aug-2024
Abstract
Perchlorate is a tireless contaminant of concern that has introduced various issues to the environment, private segment, government organisation and health of individuals. Moreover, the presence of different sodium and magnesium perchlorates in martian regolith is the most common life inhibiting agent on the red planet. In order to remove the life inhibiting chemical, using microbial methods over chemical and physical is much preferred due to economic and environmental reasons. As an outcome, isolated perchlorate reducing strain of Azospirillum sp. was showing perchlorate reduction in separate mediums with 10 mm of ammonium perchlorate, sodium perchlorate and magnesium perchlorate respectively. In addition to that, isolated Pseudomonas sp. was capable of reducing ammonium perchlorate up to 5 mM and sodium perchlorate up to 1 mM. Among these, Azospirillum sp. may reduce different metallic perchlorate into simpler compounds like oxygen and chloride ion on Mars, irrespective of other physical factors. Moreover, both species can definitely be used for the bioremediation purpose at the perchlorate contaminated sites on earth.
Keywords
Perchlorate; Terraforming; Bioremediation; Mars; Enzyme assay; Antimicrobial agent; Metallic salts of perchlorate; Methyl viologen; Chlorate reductase; Perchlorate reductase
Introduction
Perchlorate is both naturally occurring and man-made anion that consists of one chlorine atom bonded to four oxygen atoms (ClO4¯). Perchlorate may occur naturally, particularly in arid regions while it is manufactured as a perchloric acid and metallic salts such as ammonium perchlorate, sodium perchlorate and potassium perchlorate. It is found as a natural impurity in the Atacama desert of Chile and regions of northern Ladakh in India. Perchlorate is commonly used as an oxidizer in solid propellants, ammunitions, fireworks, airbag initiators for vehicles, matches and signal flares. It is also used in some electroplating operations and found in some disinfectants and herbicides. Of the total domestically produced (high grade) perchlorate, 90 percent is manufactured for use in the defence and aerospace industries, primarily in the form of ammonium perchlorate.
Due to high solubility in water, perchlorate is relatively stable on surface and mobile in subsurface aqueous system. Perchlorate released directly to the atmosphere is expected to readily settle through wet or dry deposition studies have shown perchlorate accumulates in some food crop leaves, tobacco plants and in broad-leaf plants the past transfer of weapons in either internment pits or by open consuming and open explosion may have brought about discharges to nature.
Perchlorate is readily absorbed after oral exposure and can migrate from the stomach and intestines to the bloodstream. The thyroid organ is the essential focus of perchlorate harmfulness in people. Thyroid hormones assume an imperative job in directing digestion which is basic for ordinary development and improvement in babies, new-born children and youthful youngsters. Introduction to high portions of perchlorate can result in the decline of body weight, cause hypertrophy of the thyroid organ and decline quality articulation of Thyroglobulin (Tg) also, Thyroperoxidase (TPO), which are associated with the biosynthesis of thyroid hormones. Perchlorate is destructive to the eyes, skin and respiratory tract and also may cause a sore throat, hacking and worked breathing and profound consume, loss of vision, stomach agony, retching or looseness of the bowels.
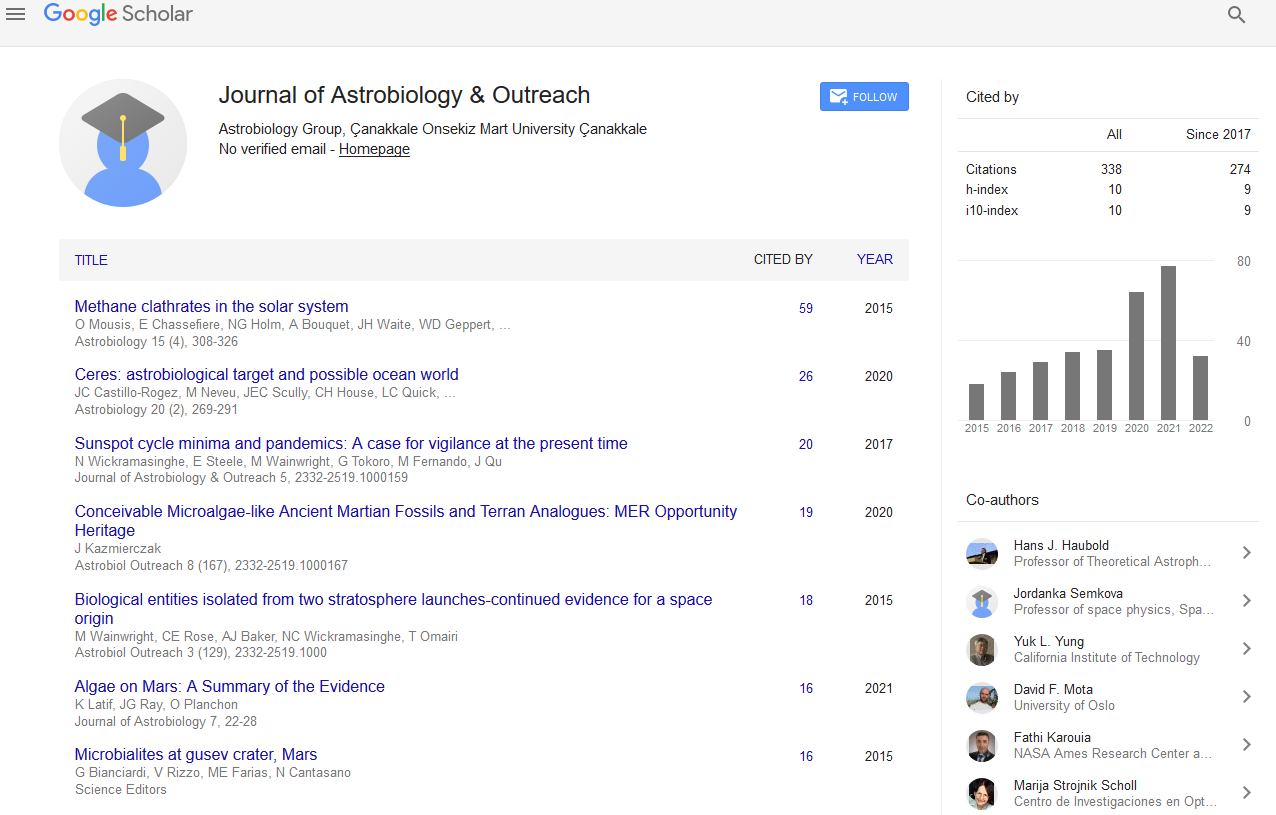
There are many ways to remove different perchlorates which are broadly classified into chemical, physical and biological methods. Among which chemical and physical methods which include Ion exchange chromatography, HPLC, use of dialysis membranes, use of electrodes, fluidized bed biological reactor, liquid phase carbon adsorption using granular activated carbon. But all these methods are highly costly and if removed perchlorate is not disposed of properly then it may enter the environment again. To overcome these disadvantages, biological methods would be the most preferable one with the use of perchlorate degrading microbes which can degrade the contaminant into the simpler form which would not harm the environment (Figure 1).
Figure 1: Pathway for perchlorate reduction.
In the course of recent years, the presence of compound on the surface of Mars has been progressively examined, both remotely and by landers on the planet. The nearness of oxidizers was first suspected by the NASA viking lander missions of the 1970s. This was later affirmed by the Phoenix lander mission where perchlorate groupings of 0.4-0.6 wt% (3.3 mM Mg2+, 2.4 mMClO4¯) were identified by the installed wet chemistry lab and affirmed by the sample analysis at Mars instrument on the Mars curiosity rover [1].
Remote spectral investigation of Palikir Crater and Hale Crater demonstrated the potential for the presence of hydrated salts instead of unadulterated water in repeating slant lineae, predictable with magnesium perchlorate. The Horowitz Crater had bigger repeating slant lineae characteristic of the potential for fluid water and spectra predictable with Martian soil blended with sodium perchlorate along with small traces of ammonium perchlorate.
The average surface temperature on Martian equator is -60°C to -20°C and sometimes it reaches up to +20°C in the noon near the equator which is optimum for the growth of most of the microbes present on the earth. As the presence of perchlorate is a major challenge for the life on Mars, so there is the probability that perchlorate reducers or resistant may have colonized the subsurface (as surface is continuously exposed to UV flux) of Mars. Terraforming of a planet is phenomena in which a planet is transformed from inhabitable to a habitable planet, so removal of perchlorate is one of the major criteria in terraforming a planet. For this, the perchlorate reducers from the earth can be used for the terraforming purpose. Moreover, perchlorate reducers found on earth can also be considered as the strong candidate which may have lived on Mars in past on the surface when the planet was wet or might be living even today itself but on the subsurface of the planet.
Materials and Methods
Culturing and identification
Samples to isolate the perchlorate reducers are collected from land near cracker factory outlet, sewage treatment plant, biogas chamber and corn farm near GSFC (fertilizer factory with its waste pipeline leakage in nearby farms since long) in sterile carbon dioxide scrubbed PBS (Phosphate Buffered Saline) buffer.
From collected samples 0.1 ml of sample was spreaded on the Czapak Dox Agar; mineral salt media and RC Media where half number of the plates were premixed with 1 mM ammonium perchlorate before solidification.
Further, plates were sealed with paraffin tape and kept in anaerobic chamber to maintain anaerobic conditions, incubated for the 72 hrs, at 30°C.
Expected colonies were transferred on the new plates in order to prepare stock cultures. Biochemical identification, gram’s nature and motility of organisms were determined.
Optimization of the level of degradation or tolerance of different metallic perchlorate
Stock cultures were prepared using isolates grown in their respective liquid media (in which they were showing growth during culturing) up to the optical density of 0.5 at the maximum absorbance.
The 0.1 ml of stock culture is spread on the respective media, containing 1 mM, 5 mM and 10 mM ammonium perchlorate, sodium perchlorate and magnesium perchlorate separately on different plates. More the number of colonies, more the isolates show the tolerance against the mentioned metallic perchlorates.
Then the colonies are transferred to liquid media of same compositions that of solid media for presence of perchlorate in media by titrimetric method.
For titration chlorite was analysed by reacting chlorite with iodide at pH 2, forming iodine. The iodine formed was titrated with a sodium thiosulphate solution (10 mM). The equivalence point was determined visually using starch as an indicator [2].
Effect of environmental factors on perchlorate degradation
Isolates on their respective media with 1 mM ammonium perchlorate are incubated at different temperatures. Media with different pH are used to check the tolerance against different pH.
Media with different salinity of MgSO4 i.e., 0.1 M, 0.5 M and 1.0 M were used to check the tolerance against the salinity. UV (A) tolerance for the time period of 5 minutes is checked.
Enzyme assay of perchlorate reductase and chlorate reductase
For this experiment sodium perchlorate was used in the media. Isolate 2 (as it was giving better results in SPC experiment) were grown in their respective liquid media up to the O.D of 0.5 at the maximum absorbance. 0.1 ml of culture is mixed in the respective media, containing 5 mM sodium perchlorate as well as sodium chlorate.
Since this experiment is having more importance for the terraforming aspect, so sodium perchlorate and chlorate were used for the experiment. The enzyme activity were determined in whole cell lysate; cell fraction (periplasm); spheroplast (membrane); enzyme after ammonium sulphate precipitation and after ammonium sulfate precipitation followed by purified with a dialysis membrane. Purification of both the enzymes was done by native gel electrophoresis irrespective of the condition that it will reduce the enzyme activity [3].
Cell fractionation and spheroplast formation
The culture was centrifuged at 11,000 g for 45 min in PBS buffer and pellets were re-suspended in 25 ml scrubbed PBS buffer (100 mM, pH 7.5). Cells were disrupted in the ultrasonicator in an icebox followed by fractionation via centrifugation at 110,000 g for 60 mins. The supernatant (periplasm) and the re-suspended membranes (pallet) were kept at 4°C. Then enzyme assay was done for both the enzymes.
For spheroplast formation, the pellet was resuspended in scrubbed 100 mM PBS buffer (pH 7.5) containing 100 mM sucrose and 5 mM magnesium chloride. The supernatant consists of cytoplasm and periplasm were resuspended in 100 mM PBS buffer (pH 7.5) (40 ml) containing 100 mM sucrose, 5 mM Na. EDTA and 100 mM lysozyme and incubated at 37°C for 1 h.
Spheroplasts were harvested by centrifugation at 11,000 g (4°C) for 30 min. The supernatant was saved as the periplasmic fraction. The pellet was resuspended in buffer (30 mM Tris-HCl (pH 7.5), 30% sucrose) and then broken by sonication (30 s). Centrifugation at 11,000 × g for 30 min yielded a supernatant that contained the membrane and cytoplasmic portions of the cell. Cell manipulations were not performed under anoxic conditions. Then enzyme assay was done for both the enzymes.
Purified enzyme
Enzymes were separated by ammonium sulphate precipitation in an anaerobic chamber. Ammonium sulphate (saturated solution of 4.1 M in MOPS buffer; final concentration 20%, 40%, 55%, 67% and 76% saturation) was introduced into the different fraction of samples with slow stirring for 10 min on magnetic stirrer maintaining cold and anaerobic conditions.
The precipitates were seperated by ultracentrifugation (25,000 g, 10 min, 4°C) and further passed through dialysis membrane (dialysis membrane-150 HI media) and the proteins were resolubilized in 5 ml of MOPS buffer. Further seperation of both the enzymes was done by native gel electrophoresis irrespective of the condition that enzyme activity will decline and analyzed for chlorate and perchlorate reductase activities using reduced methyl viologen assays [4].
Sample analysis for chlorate and perchlorate reductase activities using methyl viologen assay
Movement of perchlorate was estimated in cell separates by observing the oxidation of diminished methyl viologen at 600 nm for 1 minute in anerobic conditions. The activity was determined by the difference between the absorbance with substrate and without substrate (blank). A 1 ml of mixture contains 0.5 ml methyl viologen, 0.35 mM sodium thioglycolate along with the addition of 100 μl of both the enzymes separately purified from the different cell fractions in MOPS (50 mM, 7. 5 pH) buffer.
On addition of substrate cuvate with stocker was used and substrate was transferred in anaerobic conditions. To affirm that the cytoplasm stayed flawless during spheroplast arrangement, malate dehydrogenase assayed using Hi-media's malate dehydrogenase activity assay kit.
The reaction initiates by the addition of substrate present in 3 forms. Specific activity was defined as units of activity per mass of protein (U/mg) based on the oxidation of 1 mm of methyl viologen per min (600 nm). A previously determined millimolar extinction coefficient of 13 for methyl viologen at 600 nm was used to relate absorbance to concentration. Protein concentrations were measured using the Bradford assay reagent.
Identification of isolates by sequencing
Test organisms were grown in their respective liquid media and incubated at 30˚C for 36 hrs on the shaker. The bacterial culture was used for isolating genomic DNA by the phenol chloroform method. The primers used for the detection of 1.5 kb of 16s rRNA gene were 1492 forward and 27 reverse primers. Each polymerase chain reaction contained 10 × buffers, each of four dNTP, Primer, Taq DNA polymerase, genomic DNA and sterile ultrapure water. PCR products were detected in 1.5% agarose gel. For detection of 16 srRNA band 1 kb molecular marker was along with the sample. Later the obtained bands adjacent to 1 kb molecular marker were sent for the further sequencing of 16 srRNA product [5].
Results
Culturing and identification
Morphological and cultural characterisation of the two organisms isolated using Czapak Dox and RC media were done to determine the genus of the isolated organisms. Although these characterisations are not itself sufficient to identify the microbial isolates, that’s why biochemical tests were also done and results are mentioned further (Tables 1 and 2) (Figures 2 and 3).
| Morphological and cultural characters of isolated organisms | ||
|---|---|---|
| Isolates | Isolate 1 | Isolate 2 |
| Size | Small | Small |
| Color | Pale yellow | Light pink |
| Shape | Round | Round |
| Margin | Regular | Regular |
| Texture | Smooth | Rough |
| Elevation | Elevated | Flat |
| Opacity | Opaque | Translucent |
| Pigmentation | Nil | Nil |
| Gram’s nature | Gram’s negative | Gram’s negative |
| Motility | Motile | Highly motile |
Table 1: Morphological and cultural characters of isolated organisms.
Figure 2: Isolate 1 on Czapak Dox agar.
Figure 3: Isolate 2 on RC media.
| Biochemical characters of isolated organisms | |||
|---|---|---|---|
| S. no | Name of test | Isolate 1 | Isolate 2 |
| 1 | Carbohydrate utilizing test | ||
| Glucose | - | + | |
| Fructose | - | + | |
| Sucrose | - | - | |
| Xylose | - | - | |
| Maltose | - | + | |
| Dextrose | - | + | |
| Mannitol | - | + | |
| 2 | Methyl red test | - | - |
| 3 | Indole production test | - | - |
| 4 | Voges proskauer test | - | - |
| 5 | Citrate utilization test | + | + |
| 6 | Nitrate reduction test | + | - |
| 7 | Triple sugar iron test | + | - |
| 8 | H2S production test | - | - |
| 9 | Urea hydrolysis test | + | + |
| 10 | Gelatine test | - | - |
Table 2: Biochemical characters of isolated organisms.
The biochemical characterization infer almost precise result in the identification of genus of isolated microbes. From the above test it can be concluded that isolate 1 is of Pseudomonas genus and isolate 2 is of Azospirillum genus, still this test is having a drawback for identification of species.
Optimization of the level of degradation or tolerance of different metallic perchlorate
Formula for CFU/ml=(no. of colonies * dilution factor)/volume of culture plated. The dilution factor of culture is 10-3 (Table 3).
| SPC of isolates in different metallic perchlorate and concentrations | ||||||
|---|---|---|---|---|---|---|
| 1 mM | 5 mM | 10 mM | ||||
| Isolate 1 | Isolate 2 | Isolate 1 | Isolate 2 | Isolate 1 | Isolate 2 | |
| Ammonium perchlorate | 4.8 Ã? 102 | 6.4 Ã? 102 | 3.2 Ã? 102 | 4.7 Ã? 102 | - | 1.3 Ã? 102 |
| Sodium perchlorate | 2.6 Ã? 102 | 7.8 Ã? 102 | - | 5.3 Ã? 102 | - | 3.9 Ã? 102 |
| Magnesium perchlorate | - | 5.3 Ã? 102 | - | 3.2 Ã? 102 | - | 1.6 Ã? 102 |
Note: All the values are in Colony-Forming Unit per Millilitre (CFU/ml)
Table 3: SPC of isolates in different metallic perchlorate and concentrations.
Isolate 1 is capable of degrading up to 5 mM of ammonium perchlorate and is also capable of degrading up to 1 mM of sodium perchlorate as no spot of perchlorate is found during non-aqueous titration. Kim and Logan has isolated another perchlorate reducing bacteria named Wolinella succinogenes which can degrade ammonium perchlorate up 25 mM which is far better than isolate 1. Wolinella succinogenes can be used for the purpose of bioremediation of perchlorate at contaminated sites [6].
Isolate 2 is capable of degrading up to 10 mM of ammonium perchlorate, sodium perchlorate and magnesium perchlorate has isolated another perchlorate degrading microorganism named Halorubrum lacusprofundi from the salty lakes of Antarctica which was showing the degradation of ammonium perchlorate up to 10 mM; sodium perchlorate up to 7.5 mM and magnesium perchlorate up to 5.5 mM and this microorganism was considered as a perfect candidate which can grow on the martian surface.
Effect of environmental factors on perchlorate degrading isolates
Isolate 1 can grow between the temperature range of 15°C to 40°C; with a pH range of 6-8 and tolerate salinity of MgSO4 up to 0.1 M with no U.V tolerance, so it can be considered for the degradation of perchlorate at perchlorate contaminated sites. Due to this capability, microorganism may present on Mars in past or present either in live or fossilized form. Since this microorganism cannot tolerate or degrade sodium perchlorate or magnesium perchlorate which is the major perchlorates present on the planet’s soil so this microbe cannot be used for the martian terraforming purpose. Picardal et al., has isolated Dechlorospirillum sp. which is having temperature range for growth of 20°C-55°C and pH range of 4-9 and can tolerate up to 0.5 M salinity of magnesium sulphate can be used as perchlorate reducing strain for biodegradation of perchlorate from water bodies or marshes. Dechlorospirillum is far better than isolate 1 as per environmental factor tolerance but still it can also be considered for the terraforming purpose.
Isolate 2 is having an optimum temperature for growth of 30°C with a temperature range of 5°C-45°C. It is also having a pH range of 5-9 and can grow in salinity of up to 0.5 M magnesium sulphate with no U.V tolerance. So this isolated microorganism can be used for the biodegradation purpose in perchlorate contaminated sites. Due to this capability, perchlorate reducers could be found as an extant or extinct form. This microorganism can also be used for the martian terraforming by seeding the microbes on the sub-surface of Mars near the equator. This isolated microorganism gives a better result than isolate 1 as per environmental aspect as well as martian aspect. Picardal et al., has isolated Magnetospirillum sp. which is having temperature range for growth of 20°C-55°C and pH range of 4-9 and can tolerate up to 0.5 M salinity of magnesium sulphate, which is not up to the mark to tolerate martian conditions. As compared to isolate 2, Magnetospirillum sp. is not quite good and it also cannot be considered for the life detection or terraforming purpose of Mars.
Enzyme assay of perchlorate reductase and chlorite dismutase
Perchlorate and chlorate reductase activity for isolate 2 was found to be greater in the periplasm fraction as compared to whole cell lysate based on analysis of enzyme activities in whole-cell lysate and membrane fraction are obtained through the following equation (Table 4).
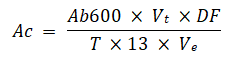
Where, 13 is the millimolar extraction coefficient of methyl viologen at 600 nm.
Ab600=Absorbance at 600 nm Vt=Total volume DF=Dilution factor T=Reaction time and Ve=Volume of enzyme
| Activity (U/mg protein) of enzymes from Isolate 2 with chlorate or perchlorate at each step of the enzyme purification | ||
|---|---|---|
| Substrate for enzyme activity-cell fractions used | Sodium perchlorate for perchlorate reductase assay | Sodium chlorate for chlorate reductase assay |
| Whole cell lysate | 0.73 ± 0.01 | 0.9 ± 0.1 |
| Periplasam | 0.95 ± 0.06 | 1.6 ± 0.3 |
| Spheroplast (membrane) | 0.61 ± 0.02 | 1.0 ± 0.2 |
| After ammonium sulphate precipitation | 1.17 ± 0.11 | 2.8 ± 0.8 |
| Purified with dialysis membrane after ammonium sulfate precipitation | 1.56 ± 0.13 | 3.1 ± 0.2 |
Note: One unit equals the oxidation of 1 mmol of methyl viologen per minute
Table 4: Activity (U/mg protein) of enzymes from isolate 2 with chlorate or perchlorate at each step of the enzyme purification.
Malate dehydrogenase assays showed that spheroplasts from on chlorate or perchlorate were 79% and 89% similar. Enzyme activity was larger in the periplasm fraction than in the cytoplasm fraction for chlorate and perchlorate, independent of whether cells were originally grown on chlorate or perchlorate. Based on these results, the periplasm fraction was selected for further analysis in which ammonium sulphate salt precipitation (40%-55%) was done for the purification of enzyme, then, further purification was done by filtration using dialysis membrane. Purification done by precipitation+filtration gave higher enzyme activity for both the enzyme as compared to purification done by precipitation only.
Chlorate and perchlorate reductase activity was localized within the periplasmic fractions for isolate 2 indicating these enzymes are soluble and not membrane-bound. The location of these enzymes appears consistent with several other perchlorate respiring strains [7].
There can be a major effect of oxygen on enzyme activity of both the enzymes, as chlorate reductase activity of the periplasmic fraction from isolate 2 was reduced by 45% after 72 hrs, while perchlorate reductase activity was reduced by 73% after 72 hrs. While on the other hand no change was observed in activity seen in any of the anoxic controls kept in icebox in the anaerobic conditions. Hence this proves that oxygen have a significant effect on enzyme activity.
Identification of isolates by sequencing
From the sequencing of both the isolated bands of 16 srRNA the obtained sequence was sequence matched using BLAST tool, it concluded that isolate 1 is Pseudomonas stutzeri and isolate 2 is Azospirillum brasilense (Figure 4).
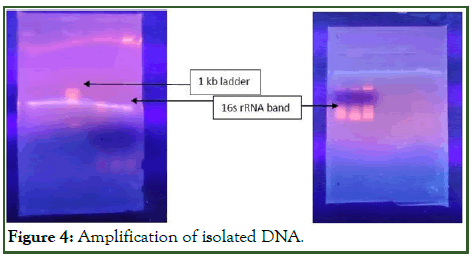
Figure 4: Amplification of isolated DNA.
Identification of both the isolates by 16 srRNA sequencing was significant because the morphological and biochemical test can only predict about the genus of the sample microbe, while on the other hand the sequencing gives the exact name of microbe present in the sample along with the genus and species name.
Taxonomic correlation between Pseudomonas stutzeri and Azospirillum brasilense and other perchlorate reducing strains mentioned
Phylogenetic analysis gives the evolutionary and taxonomic relationship among various biological species. Here, 8 different perchlorate reducers that are capable of reducing perchlorate were considered for the analysis, among which 2 were isolated by me and other 6 were isolated by others undergoing similar type of research. The main reason to include 6 extra reducers was to draw the ancestral relationship between the organisms as drawing phylogenetic relationship between 2 organisms doesn’t gives a significant evolutionary comparison (Figure 5) [8].
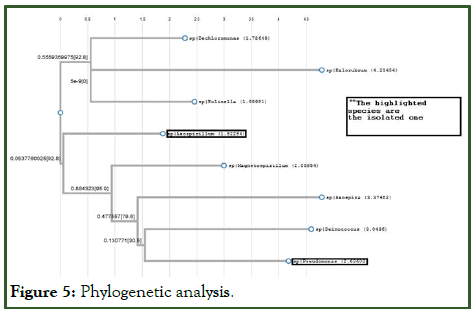
Figure 5: Phylogenetic analysis.
Discussion
Pseudomonas stutzeri
As per the environmental aspects are concerned with this organism which was isolated from the sewage treatment plant of GSFC Vadodara can be used for the biodegradation purpose at the contaminated sites by perchlorate. Since most of the contamination of perchlorate on earth is by ammonium perchlorate so this organism is capable of that. Kim and Logan has isolated another perchlorate reducing bacteria named Wolinella succinogenes from the tank of sewage treatment plant which can degrade ammonium perchlorate up 25 mM which is far better than the isolated Pseudomonas stutzeri as it can degrade only up to 5 mM. Wolinella succinogenes can be used for the purpose of biodegradation of perchlorate at contaminated sites. To increase the biodegradation ability of the isolated Pseudomonas stutzeri some of the mutation techniques can be applied to the organism, either by physical or chemical means which may increase the biodegradation capability of organism used in place of Wolinella succinogenes.
Pseudomonas stutzeri is having one benefit over Wolinella succinogenes that it is microaerophile and while Wolinella succinogenes is a strict anaerobe, tough this organism is having high ability than the isolated one it will need extra supplementations before inoculating them to the contaminated site, while the isolated one doesn’t require that. Vibrio dechloraticans, Dechloromonas sp., Dechlorosoma sp. and Pseudomonas chloritidismutans are the other perchlorate degrading microbes mentioned by Xu et al. The incubation temperature range for the Pseudomonas stutzeri was found to be 15°C-40°C and it can tolerate the salinity of MgSO4 up to the concentration of 0.1 M, due to this feature this organism can also be used for the biodegradation of perchlorate in case sea, marshes or any other water body gets contaminated [9]. As per the Martian terraforming aspect, this organism is not a good candidate as mars are having concentration of sodium and magnesium perchlorate and this organism is not having an ability to degrade these both metallic perchlorate. Laye and DasSarma has isolated another perchlorate degrading microorganism named Halorubrum lacusprofundi from the salty lakes of Antarctica which was showing the degradation of ammonium perchlorate up to 10 mM; sodium perchlorate up to 7.5 mM and magnesium perchlorate up to 5.5 mM, so this is better than the isolated one.
Azospirillum brasilense
As per the environmental aspects, this organism which was isolated from the corm farm near GSFC outlet can also be used for the biodegradation of ammonium perchlorate which can degrade it up to 10 mM, if we consider the no of organism by concentration it may degrade the ammonium perchlorate up to the concentration of 15 mM. As compared to Wolinella succinogenes which can degrade 25 mM, Azospirillum brasilense is not as much capable as Wolinella succinogenes. As Wolinella succinogenes is a strict anaerobe it will again face a problem regarding its inoculation at the contaminated sites. When compared with the 1st isolate Pseudomonas stutzeri, Azospirillum brasilense is better to use for the degradation purpose as it can degrade up to the 10 mM (by experiment) and 15 mM (by prediction).
The incubation temperature range for the Azospirillum brasilense was found to be 5°C- 45°C and it can tolerate the salinity of MgSO4 up to the concentration of 0.5 M, due to this feature this organism can also be used for the biodegradation of perchlorate in case sea, marshes or any other water body gets contaminated. This microbe is also having quite wide range of pH for growth that is pH 5-9, which also increases its application over the different biospheres [10].
Other probable applications of perchlorate/or its degradation
• Oxygen/water/carbon dioxide production form it. (Refer its
degradation pathway)
• In oil reservoirs for bio souring control
• Xenobiotic bioremediation
• Biochemical oxidation of certain compounds
Conclusion
As per the martian terraforming aspect, this organism is one of the good candidate as martian soil is having concentration of sodium and magnesium perchlorate and this organism is able to degrade the above metallic perchlorates up to the concentration of 10 mM in both the cases. This microbe is also showing growth in the temperature range of 5°C-45°C and has a quite wide range of pH for growth. The equator and its nearby areas of the mars are having a temperature range of 15°C-25°C during the day time and its quite warmer in the subsurface with the MgSO4 salinity of up to 0.1 M, the U.V.(A) can penetrate up to an inch in the soil.
Due to these climatic factors, the isolated microbe Azospirillum brasilense can be seeded in the subsurface nearby the areas of equator for the metallic perchlorates degradation which may remove the toxicity from the soil, as this toxicity restricts any life form to grow there. By using this organism form the earth one of the terraforming criteria of mars may get accomplished.
Acknowledgment
I have no conflict of interest regarding this research. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki declaration of 1964 and its later amendments. Informed consent was obtained from all patients for being included in the study. This article does not contain any studies with animal subjects performed by the any of the authors. All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- CockellCharles S. Trajectories of martian habitability. Astrobiology. 2014.
[Crossref] [Google Scholar] [PubMed]
- Goncharuk VV, Zui OV, Kushchevskaya NF. Methods of determining perchlorates. J Water Chem Technol. 2009;31(3):186-194.
- Gurwell MA, Bergin EA, Melnick GJ, Tolls V. Mars surface and atmospheric temperature during the 2001 global dust storm. Icarus. 2005;175(1):23-31.
- Hutchison JM, Zilles JL. Biocatalytic perchlorate reduction: Kinetics and effects of groundwater characteristics. Environ Sci: Water Res Technol. 2015;1(6):913-921.
- Kim K, Logan BE. Microbial reduction of perchlorate in pure and mixed culture packed-bed bioreactors. Water Res. 2001;35(13):3071-3076.
[Crossref] [Google Scholar] [PubMed]
- Laye VJ, DasSarma S. An Antarctic extreme halophile and its polyextremophilic enzyme: Effects of perchlorate salts. Astrobiology. 2018;18(4):412-418.
[Crossref] [Google Scholar] [PubMed]
- Picardal FW, Zaybak Z, Chakraborty A, Schieber J, Szewzyk U. Microaerophilic, Fe (II)-dependent growth and Fe (II) oxidation by a Dechlorospirillum species. FEMS Microbiol Lett. 2011;319(1):51-57.
[Crossref] [Google Scholar] [PubMed]
- Caceres EA. Improved medium for isolation of Azospirillum spp. Appl Environ Microbiol. 1982;44(4):990-991.
[Crossref] [Google Scholar] [PubMed]
- Steinberg LM, Trimble JJ, Logan BE. Enzymes responsible for chlorate reduction by Pseudomonas sp. are different from those used for perchlorate reduction by Azospira sp. FEMS Microbiol Lett. 2005;247(2):153-159.
[Crossref] [Google Scholar] [PubMed]
- Xu J, Song Y, Min B, Steinberg L, Logan BE. Microbial degradation of perchlorate: Principles and applications. Environ Eng Sci. 2003;20(5):405-422.
Citation: Sunilkumar U, Lal S (2024) Perchlorate Reducing Bacterias Having Ecological and Mars Terraforming Significances. J Astrobiol Outreach. 12:341.
Copyright: �© 2024 Sunilkumar U, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.