Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- Directory of Abstract Indexing for Journals
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research - (2024) Volume 14, Issue 1
Paracrine Secretions and Immunological Activities of Human Mesenchymal Stem Cells: The Key Regenerative Factors of Microenvironment
Seyed Mehdi Hoseini1, Elham Sadat Hosseini1, Pantea Abessi2 and Fateme Montazeri2*2Abortion Research Center, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences and Health Services, Yazd, Iran
Received: 22-Apr-2024, Manuscript No. JSCRT-24-25545; Editor assigned: 24-Apr-2024, Pre QC No. JSCRT-24-25545 (PQ); Reviewed: 08-May-2024, QC No. JSCRT-24-25545; Revised: 15-May-2024, Manuscript No. JSCRT-24-25545 (R); Published: 22-May-2024, DOI: 10.35248/2157-7633.24.14.636.
Abstract
Human Mesenchymal Stem Cells (hMSCs) have immunomodulatory properties, mainly through their paracrine secretions, which contain anti-inflammatory molecules and exosomes. The favourable characteristics of hMSCs in the regeneration of damaged tissues have caused these cells to be considered a therapeutic approach in immune-related diseases. However, the cell therapy approach requires a detailed understanding of the behaviour of hMSCs in the inflammatory microenvironment of target tissues because MSCs may respond differently to this pathological microenvironment compared to normal physiological conditions. In addition, the level of influence that effective mechanisms of hMSCs, including paracrine approaches and cell contact, have on maintaining homeostasis and repairing damaged tissues is a subject of on-going controversy. hMSCs show different characteristics in vitro and in vivo depending on their tissue of origin. This variability is more apparent when the cells are derived from different donors, with fetal and adult sources showing different regenerative capabilities. Furthermore, the fact that hMSCs behave differently depending on the local microenvironment adds to the complexity of understanding the immunological pathways mediated by hMSCs. Previous research has demonstrated that the origin of MSCs (fetal or adult) has a significant impact on their immunological characteristics. This is due to differences in the cells’ mechanisms of cell contact and paracrine secretion, which ultimately affect the microenvironment of the MSCs. This study aims to review the immunomodulatory and immunogenic features of fetal and adult/somatic MSCs to highlight the effect of the cell source on paracrine secretions and the resulting microenvironment, both during in vitro expansion and in vivo after cell administration.
Keywords
Mesenchymal stem cells; Microenvironment; Paracrine secretions; Immune modulation; Immunogenicity
Introduction
Human Mesenchymal Stem Cells (hMSCs), obtained from Bone Marrow (BM-MSCs) at first [1], have received much attention during the last decade due to the ease of use in human clinical trials, as well as available cryopreservation approaches used for preservation and storage [2]. In addition, some of their intrinsic properties are also important in tissue repair and regeneration, including anti-inflammatory and immunosuppressive-immunomodulatory activity, low immunogenicity, differentiation ability, and homing [3-5], as well as their ease of manipulation in the laboratory, like isolation from multiple tissues and the capacity to be adapted to large scale ex vivo culture expansion [6]. As MSCs can be expanded from different tissues of fetal and adult sources, a set of minimal criteria has been defined by the International Society for Cellular Therapy and Gene Therapy (ISCT) [7]. These criteria include: (a) binding to the plastic surface of the culture vessel; (b) expressing the mesenchymal surface antigens of CD105, CD73, and CD90, as well as lacking expression of hematopoietic markers such as CD34 and CD45; and (c) multipotent capability to differentiate into osteoblast, adipocyte, and chondrocyte, under standard conditions. Over the last decade, new approaches have been developed to use ex vivo-expanded MSCs in regenerative medicine. However, the belief that MSCs meeting the minimum criteria would respond the same way to the microenvironment of a damaged tissue is highly misleading.
MSCs’ plasticity is of great significance regarding their immunomodulatory properties, meaning that depending on the environment they are exposed to, they can have either a pro- inflammatory or anti-inflammatory effect on the immune system [8]. MSCs regulate tissue homeostasis and integrity by adopting these contradictory phenotypes through interaction with both innate and adaptive immune cells [8]. For this purpose, MSCs become polarized and express different markers and mediators that prepare them for such contrary functions. In other words, these features enable MSCs to maintain a balance between physiological and pathological states of tissue [9]. A damaged tissue creates special conditions that resident or transplanted. MSCs have to deal with, including inflammatory responses by immune cells, death signals released by necrotic cells, infection circumstances, and oxidative stress [10]. The entire signals make MSCs quit the quiescent state and appropriately respond by initiating the mechanisms of proliferation, migration, and regeneration. The homeostatic microenvironment is regenerated by MSCs in a damaged tissue, through their contact with immune effectors and paracrine secretions, such as microvesicles, exosomes, and other related growth factors and chemokines/cytokines [11,12]. These factors affect the main characteristics of MSCs, including their in vitro multipotency, secretome and senescence,ex vivo survival, as well as their in vivo homing and integration into inflamed tissues [11].
When MSCs are expanded from a particular donor, the resulting cells can have varying phenotypes in each batch. This variability is more apparent when the cells are derived from different donors, with fetal and adult sources showing different regenerative capabilities. Previous research has demonstrated that the origin of MSCs (fetal or adult) has a significant impact on their immunological characteristics. This is due to differences in the cells’ mechanisms of cell contact and paracrine secretion, which ultimately affect the microenvironment of the MSCs. This study aims to review the immunomodulatory and immunogenic features of fetal and adult/ somatic MSCs to highlight the effect of the cell source on paracrine secretions and the resulting microenvironment, both during in vitro expansion and in vivo after cell administration.
Literature Review
Cell source options for regenerative medicine
Multipotent fetal/adult vs. pluripotent embryonic: Stem cells can be categorized into three main divisions based on the developmental hierarchy, including embryonic, fetal and adult/somatic stem cells [13]. There is a clear distinction between developmental stages and characteristics of stem cells obtained from. As Embryonic Stem Cells (ESCs) are derived from the inner cell mass of blastocysts leading to the destruction of a human embryo, using them raises ethical concerns. They are also tumorigenic which causes safety concerns to using ESCs in clinical trials [11]. Unlike ESCs, MSCs become senescent after long-term expansion, which reduces their tumorigenic potential. Therefore, there has been a growing interest in MSCs due to the absence of concerns associated with ESCs, e.g., teratoma formation and ethical issues [14-16].
However, the source of MSCs plays a vital role in determining their regenerative properties. It’s challenging to compare MSCs from different sources and measure their ability to regenerate damaged tissues in the body. Therefore, it’s essential to investigate the unique features of fetal and adult stem cells from different perspectives as they differ in hierarchical levels and may possess distinct regenerative capabilities. Research has shown that Fetal Stem Cells (FSCs) possess intermediate characteristics regarding the expression of stem cell markers related to pluripotency and multipotency identity [16]. Therefore, they are called highly multipotent stem cells in some papers [17]. FSCs, in addition, display a comparable capability to differentiate into all three embryonic germ layers like ESCs [13,16].
Fetal vs. adult: Using Adult Stem Cells (ASCs) in cell therapy has long been common, particularly those isolated from bone marrow for Hematopoietic Stem Cell Transplantation (HSCT). While Hematopoietic Stem Cells (HSCs) would be accessible by an “isolation” process, obtaining MSCs for cell therapy requires a time-consuming process of ex vivo expansion, which is called “derivation” [18]. Accordingly, selecting the appropriate source to derive MSCs can affect the success of the procedure. The derivation of MSCs from adult tissues has some limitations, such as ethical issues and variable outcomes. Adipose Tissue (AT) and bone marrow are the most used sources, but both require an invasive surgical procedure to harvest the cells. Additionally, studies have shown that functional MSCs can vary significantly depending on the donor’s age and health [19]. For example, MSCs derived from elderly, diabetic, obese, or atherosclerotic patients have less immunosuppressive activity than those derived from younger or healthy individuals [11,20].
The use of ASCs in cell therapy has been limited due to various reasons, and recent studies have focused on the more primitive fetal source of MSCs [11,21]. Fetal tissues are more rich in MSCs than adults and they can be easily accessible from normally discarded fetal and extra-fetal tissues at birth, such as umbilical cord and cord blood, placenta, amnion, and amniotic fluid [2,22]. MSCs make up only a small portion of the stem cell population. They are much more common during fetal life compared to adulthood, comprising roughly 1 in 3000 blood cells and 1 in 400 bone marrow cells during the second trimester of pregnancy. However, in a new-born and an 80-year-old healthy individual, the ratio drops to 1 in 10,000 and 1 in 2 million bone marrow cells, respectively [23,24]. Finally, regardless of the benefits mentioned, experimental studies have also shown that fetal/neonatal MSCs have more effective therapeutic properties than adult/somatic ones, including higher anti-inflammatory and immunosuppressive capacity, advanced homing ability and remarkably more efficient plasticity and potency [14-16]. Thus, all these features make fetal MSCs a prominent candidate for developing new strategies in the future of regenerative medicine. In Figure 1, the tissue-origin, and general properties of ESCs, FSCs, and ASCs are schematically illustrated.
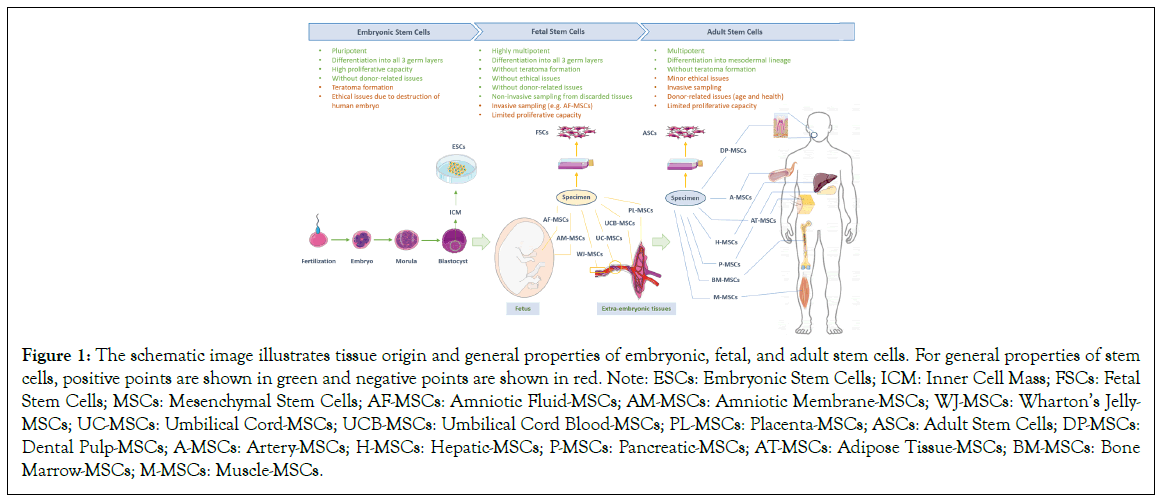
Figure 1: The schematic image illustrates tissue origin and general properties of embryonic, fetal, and adult stem cells. For general properties of stem cells, positive points are shown in green and negative points are shown in red. Note: ESCs: Embryonic Stem Cells; ICM: Inner Cell Mass; FSCs: Fetal Stem Cells; MSCs: Mesenchymal Stem Cells; AF-MSCs: Amniotic Fluid-MSCs; AM-MSCs: Amniotic Membrane-MSCs; WJ-MSCs: Wharton’s Jelly- MSCs; UC-MSCs: Umbilical Cord-MSCs; UCB-MSCs: Umbilical Cord Blood-MSCs; PL-MSCs: Placenta-MSCs; ASCs: Adult Stem Cells; DP-MSCs: Dental Pulp-MSCs; A-MSCs: Artery-MSCs; H-MSCs: Hepatic-MSCs; P-MSCs: Pancreatic-MSCs; AT-MSCs: Adipose Tissue-MSCs; BM-MSCs: Bone Marrow-MSCs; M-MSCs: Muscle-MSCs.
Microenvironment: The foundation of tissue regeneration
Numerous cellular and non-cellular components constitute the local microenvironment, including immune cells and vascular elements such as pericytes and endothelial cells, tissue-resident fibroblasts and MSCs, structural proteins, glycoproteins, and proteoglycans of Extracellular Matrix (ECM) and its concomitant soluble factors like cytokines and chemokines, growth factors, angiogenic factors, etc. [25,26]. MSCs play an important role in restoring tissue homeostasis after disruption caused by chronic injury or acute trauma. To this end, MSCs regenerate the local microenvironment by secreting various biologically active substances such as cytokines, chemokines, growth factors and miRNAs. These molecules, collectively known as the MSC’s secretome, play a key role in the regulation of immune responses and the activation of local progenitor cells. The regenerated local microenvironment by MSC’s secretome determines the cell fate of other tissue progenitors by regulating their self-renewal and differentiation, which enhances the regenerative response [12].
It is generally believed that MSCs utilize two approaches for tissue regeneration. The first mechanism involves supplying cells that can help to “reconstruct” the tissue, while the second mechanism involves “empowering” other cells to promote the healing process by regulating inflammation [27]. Despite many reports proving the efficacy of MSC engraftment in preclinical and clinical studies [28-30], the low rate of engrafted MSCs and their short-lived effect has demonstrated that the “empowerment” process may be even more efficient than the “reconstitution” to regenerate an injured tissue [31-33].
Moreover, studies in animal models of myocardial infarction, multiple sclerosis, renal failure and liver fibrosis, have revealed the success of MSC-based treatment despite the lack or low rate of transplanted MSCs in the target tissue [34-37]. Likewise, in autologous engrafted MSCs, these positive therapeutic effects have been observed in the immunocompetent models treated with allogeneic and xenogeneic sources of MSCs [38,39]. These findings lead to a similar conclusion that long-term transplantation is unnecessary in the regeneration, suggesting more efficiency of the enriched microenvironment than the cell replacement. It is attributed to the “MSC secretome” or “empowerment” process, as cases of inflammatory diseases have been cured with only the supernatant of MSC culture [37,40].
Based on many in vitro and in vivo studies, both derived MSCs and tissue-resident MSCs, respectively, need to be “licensed” by inflammatory mediators to exhibit their immunomodulatory function. When MSCs encounter an inflammatory microenvironment in the damaged site, they respond by modifying paracrine activity to promote cell mobilization, proliferation, and immune cell recruitment to the site of injury, thereby facilitating tissue repair [40]. However, this interaction can also affect some essential characteristics of MSCs that are critical for their regenerative capabilities, including immunomodulation and immunogenicity. These and their possible differences between fetal and adult MSCs will be discussed later in below sections.
Immunomodulation
As one of the decisive characteristics for tissue regeneration, the immunomodulatory and immunosuppressive features are renowned activity in MSCs that can be exploited to deal with inflammatory conditions as cellular therapies for immune-related diseases and transplantations. MSCs can alleviate the immune system by affecting innate responses by impeding the maturation of Dendritic Cells (DCs) and cytotoxicity of Natural Killer Cells (NKCs), as well as adaptive responses through disrupting the function of B cells and CD4+, CD8+ T cells [6]. It has been disclosed that MSCs, regardless of donor origin, suppress the proliferation of stimulated allogeneic leukocytes in a dose-dependent manner [41]. These properties are more correlated with the autocrine and paracrine effects of MSCs by releasing a wide range of cytokines, chemokines, and anti-inflammatory mediators [42,43]. However, consistent with prior research indicating distinct functions of MSCs from different origins, the immunomodulatory abilities may also differ based on the source of derivation [44-46].
Pathological conditions related to MSC’s immunomodulation: The regeneration of damaged tissue is highly dependent on immunoregulatory mechanisms that result from the interaction of MSCs with innate and adaptive immune cells in the microenvironment [6]. The immunoregulatory properties of human MSCs can alter inflammatory conditions and impact different effector cells, moving from lymphoid cells (T-and B-cells, NKCs), to myeloid components (monocytes, DCs) [47]. Results from many investigations illustrate the ability of MSCs to control the functioning of the immune system. It has also been suggested that dysregulation of MSCs might be involved in a range of pathological conditions, such as autoimmunity and chronic inflammatory diseases [48]. Adult MSCs play an important role in regulating the immune response in inflamed tissues. They can either act locally as tissue-resident MSCs or be recruited from the bloodstream via circulating cells released by the bone marrow [49]. These cells also assist in subsequent regenerative processes by acting as biosensors that can detect disease-specific inflammatory markers and migrate to the site of injury. As a result, they have been suggested to be involved in a range of autoimmune and inflammatory-related diseases [48].
Recent research in the field of cancer has highlighted the importance of the immunomodulatory function of adult MSCs in the development and progression of the disease. Tissue-resident stromal cells are a major class of cellular components in the Tumour Microenvironment (TME), and their paracrine activity involves complex interactions with cancer cells, immune cells, and the Extracellular Matrix (ECM). These stromal cells play a critical role in tumour metabolism, growth, metastasis, immune evasion, and treatment resistance [50]. These cross-talks can be involved in the development of new lineages of malignant cells and heterogeneity of cancer stem cells, leading to the generation of drug-resistant lines. Recently, there has been an increased focus on identifying and targeting interactions between malignant and non-malignant cells in the TME for the development of therapeutic strategies [51].
Fetal MSCs play a critical role during pregnancy by releasing paracrine factors that potentially affect the prenatal period [52]. Compared to adult MSCs, FSCs possess an increased capacity for immunomodulatory activities. However, various conditions can affect FSCs’ characteristics, especially in pathogenic situations. Our recent study on AF-MSCs (as representative of fetal progenitors released by different tissues in the amniotic fluid) revealed that MSCs derived from fetuses of couples with a history of unexplained recurrent miscarriage (a possible immunologic disorder) exhibit a defect in their immunomodulatory capacity compared to AF-MSCs from couples without miscarriage problems. This difference is mainly due to the inherent characteristics of these cells that impact their paracrine factors [53]. The idea that the paracrine secretions of fetal cells may play a role in the modulation of the maternal immune system has been suggested in some other clinical findings. For example, pregnant women with autoimmune disorders like Multiple Sclerosis (MS) [54], Rheumatoid Arthritis (RA) [55], and autoimmune hepatitis [56], have experienced an inexplicable decrease in disease severity or improvement in symptoms.
Immunomodulation and cell origin: Apart from the intermediate state of FSCs between ESCs and adult MSCs regarding the stemness and related pluripotency markers [16,57], it has been established that FSCs possess higher immunomodulatory properties than adult MSCs [58-62]. Numerous reports have recorded higher, longer-lasting immunomodulatory properties of FSCs in comparison with BM-MSCs as the most commonly investigated adult source [6,60,62-66]. It seems that the origin of FSCs is responsible for the large array of paracrine factors they produce, which are significant to their immunomodulatory capabilities. These soluble immunosuppressive and tolerogenic factors make FSCs more efficient at modulating the immune system [59,62,67,68].
Various signals that play a role in pregnancy-related processes are released from the placenta and fetal tissues, such as adrenal glands and liver, those are involved in the immunological function. From implantation to delivery, these signals are secreted into the mother’s blood by the fetus and placenta, thus modifying the production of maternal hormones. These compounds can be generally divided into two groups: Fetoplacental steroids that can alleviate the mother’s immune system, including progesterone, estrogens, androgens, and glucocorticoids; and complex peptide molecules that carry the immunosuppressive signals from fetus to mother, such as α-Fetoprotein (α-FP) released by fetal liver [69,70]. Recently, much attention has been paid to the fetomaternal immunological relationship between maternal immune systems and the fetus. This has led to the development of new therapeutic strategies for immune-related diseases [71,72].
Overall, studies have shown that fetal cells have a different gene expression profile compared to adult cells, which supports stronger immunomodulatory and immunosuppressive properties in fetal cells. For instance, it was demonstrated that fetal Dendritic Cells (DCs) are more efficient than adult DCs in reducing CD8+ T-cell proliferation and inducing Treg cells. This is attributed to more than three thousand genes differentially expressed in fetal and adult DCs [73]. Similar comparative studies on fetal and adult MSCs exhibit different patterns of expression for genes related to adhesion, chemokines, and pro-inflammatory agents, despite their same mesodermal differentiation ability. These characteristics make FSCs more effective in immunomodulation [59]. Recent studies have confirmed that fetal liver-derived MSCs are more effective than adult BM-MSCs in suppressing activated conventional T cells and inducing Treg cells [6,65]. In another study by Najar et al. [74], it was observed that AT-MSCs and BM-MSCs derived from adult sources express varying profiles of immunomodulatory genes compared to that derived from fetal Wharton’s Jelly (WJ-MSCs), including surface markers (e.g., CD73, HLA-G, HO-1) and soluble markers (e.g., HGF, PGE2, and IGFBP-3). The results suggest that WJ-MSCs have more immunosuppressive properties than the other two adult sources.
The expression profile of MSCs can differ depending on the source they are derived from. This can affect the proteins present on their surface and those secreted from the cells. As a result, two key aspects of MSCs in immune regulation, which are cell contact and paracrine activity, can vary based on the cell origin. While cell contact has been shown to play an important role in MSC-based immunosuppression, in this case, we will be focusing on how the paracrine activity of MSCs influences the microenvironment of the injured tissue.
Immunomodulation and microenvironment: MSCs can regulate the immune system by either contacting other cells or by releasing diverse factors. One of their main functions is to prevent T cells from activating inappropriately and to create a healing environment that promotes immune tolerance. This means that MSCs act as key regulators of the microenvironment in orchestrating the inflammatory responses through establishing a coordinated relationship with various immune cells. During this interaction, MSCs acquire a specific secretory profile, which is called licensing or priming. This allows them to act as either pro-inflammatory or anti-inflammatory cells, thereby modifying the microenvironment to maintain the immune homeostasis of an inflamed tissue [75].
The paracrine activity of MSCs includes a range of cytokines/ chemokines, growth factors, anti-inflammatory agents, and Extracellular Vesicles (EVs). By conveying regulatory messages to the cells of a damaged site, MSCs initiate tissue repair [12]. MSCs primarily have an immunosuppressive effect, acting as inhibitors of pro-inflammatory T helper 1 (Th1) pathways. However, they also play an essential role in favour of restoring immune homeostasis through the induction of Treg cells, differentiation of anti-inflammatory Th2 cells from naïve T cells and Th1 cells, and promotion of a shift from pro-inflammatory M1 macrophages to anti-inflammatory M2 cells [76]. These mechanisms involve various signalling molecules that help maintain immune homeostasis in situations where the immune response is either over-activated or under-activated. This network is illustrated in Figure 2, and discussed in this section regardless of their fetal or adult origin.
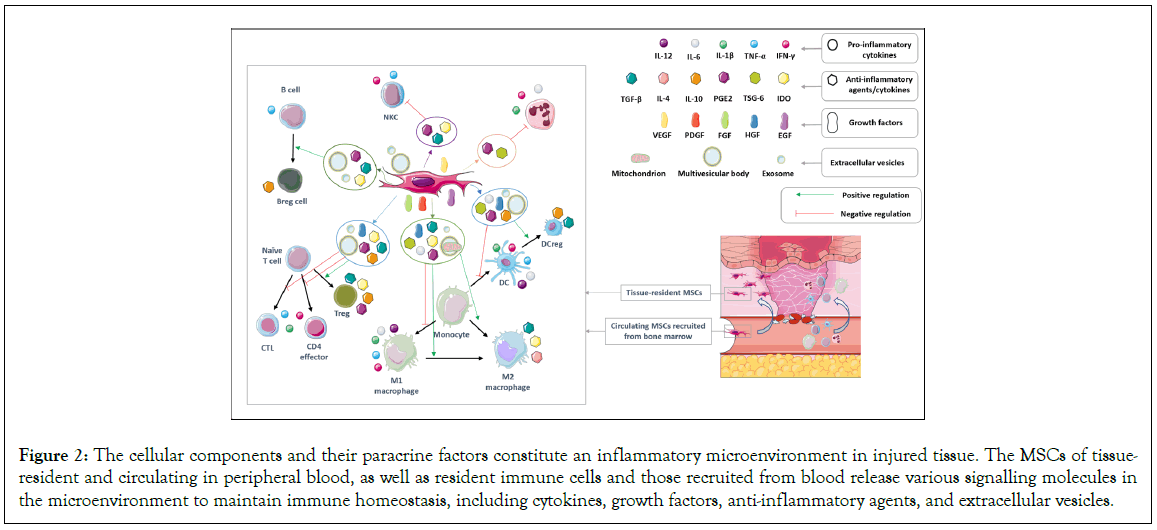
Figure 2: The cellular components and their paracrine factors constitute an inflammatory microenvironment in injured tissue. The MSCs of tissue resident and circulating in peripheral blood, as well as resident immune cells and those recruited from blood release various signalling molecules in the microenvironment to maintain immune homeostasis, including cytokines, growth factors, anti-inflammatory agents, and extracellular vesicles.
Cytokine/chemokine network: The cytokine network is a highly complex system of immune molecular messengers. It has multiple layers of activation and control mediated through soluble receptors, receptor antagonists, diverse serum mediators, and gene polymorphisms. Proteomic methods have revealed further layers of complexity and control in cytokine production and expression. These involve long coding RNAs, siRNAs, and miRNAs, which make cytokine production and control in the inflammatory process challenging to interpret [77,78]. Many cytokines can act in multiple ways or paradoxically at different times. Moreover, many act in feedback loops with the ability to control their production. The expression of cytokines is also influenced by local cellular microenvironments, suggesting that multiple pathways exist to achieve homeostatic immunologic control and effectiveness [78]. As mentioned earlier, the local microenvironment can contrarily intensify chronic immune activation due to dysregulated MSCs.
Chemokines and their cognate receptors play an important role in the recruitment and chemotaxis of immune cells to areas of inflammation. As a result, these molecules have become potential biomarkers and therapeutic targets for various autoimmune disorders [79,80]. Pro-inflammatory cytokines such as IFNγ and TNFα are responsible for creating an inflammatory environment in damaged tissues. Additionally, the activation of IFN-Stimulated Genes (ISGs) leads to the production of several chemokines in the damaged tissue (such as CXCL9/CXCL10/CXCR3 and CCL3/ CCL4/CCR5 axis) that recruit immune cells there [81]. IFNγ is also vital in polarizing MSCs from an unlicensed to an activated state [8]. Therefore, the high levels of IFNγ in the microenvironment around the inflamed area can cause the expression of chemokines and induce the activation of MSCs [81,82]. This elaborate cross-talk among these components (resident cells, immune cells and MSCs) in the inflamed microenvironment is believed to be implicated in the pathogenesis of autoimmune diseases. Our recent study found that the interaction of preconditioned and non-preconditioned human AF-MSCs (IFNγ+ and IFNγ-, respectively) with Peripheral Blood Mononuclear Cells (PBMCs) from patients with Type 1 Diabetes (T1D) resulted in different expression of chemokines and their receptors compared to PBMCs of healthy donors. We also detected the same opposite trend in their paracrine activity through the induction of T regulatory (Treg) cells [83]. By controlling the homing of autoreactive CTLs to pancreatic islets and by mediating antigen presentation on β-cells in the islet inflammatory microenvironment, IFNγ appears to be a major contributor to the development of autoimmune pathology in T1D [84]. However, the complexity of the relationship between chemokine production, cytokine function, and the stimulation of MSCs at the site of inflammation, particularly in autoimmunity, is yet to be well understood.
Growth factors and immunosuppressive agents: MSCs have been found to enrich their microenvironment by releasing growth factors and immunosuppressive agents. Some studies have even suggested that the administration of these factors alone can be sufficient to elicit a therapeutic effect [36,37]. Various growth factors and immunosuppressive agents, such as HGF, EGF, FGF, PDGF, VEGF, IGF, SDF1, IDO, PGE2, and NO, work together to establish an anti- inflammatory microenvironment. MSCs’ secretome, which contains these agents, modulates the inflammatory response, promotes the proliferation and autocrine activities in endothelial and fibroblast cells, and facilitates the proliferation and differentiation of tissue progenitor cells in situ [27]. Therefore, the therapeutic benefits of MSCs are more attributed to their “empowerment” approach that alleviates inflammatory responses by restoring tissue homeostasis.
Extracellular vesicles and exosomes: It has become increasingly clear that Extracellular Vesicles (EVs) are released by nearly all cell types and are present in high quantities in biological fluids such as blood, Cerebrospinal Fluid (CSF), saliva, and urine. These vesicles act as effective transporters of bioactive molecules, playing an important role in facilitating cell-to-cell communication. They usually share a common cargo composition of proteins, lipids, mRNAs, miRNAs, and DNA fragments that can be regulated based on the secreting cell type and its microenvironment [85]. This makes EVs a valuable tool to modulate the biology and fate of recipient cells, particularly for MSC-based therapy, as they can enhance safety by eliminating the need for the presence of MSCs themselves [12].
Exosomes are EVs that are formed when Multivesicular (MVs) bodies fuse with the plasma membrane. These exosomes have been extensively studied for their therapeutic effects in models of acute kidney injury, liver damage, and myocardial ischemia [86-88]. It has been acknowledged that the therapeutic effects of exosomes derived from fetal sources (e.g., WJ-MSCs) on animal models of acute kidney injury are mediated by miR-15a, miR-15b, and miR-16. These miRNA molecules act as inhibitors of the CX3CL1 ligand, which is a potent chemical adsorbent for macrophages and is mainly expressed in endothelial cells. As a result, the accumulation of pro-inflammatory (M1 phenotype) macrophages in the kidneys is suppressed [89].
In addition to exosomes, MSCs also release larger MVs that contain lysosomal-like vesicles and complete mitochondria. This suggests that MVs containing these organelles are released from MSCs and may target adjacent cells for mitophagy [90]. Recent studies indicate that mitophagy and healthy mitochondrial function are critical to the survival of stem cells, and may affect the interaction of MSCs with macrophages as fundamental components of the stem cell niche in bone marrow. It has been revealed that MSCs can manage their intracellular oxidative stress by directing depolarized mitochondria to the plasma membrane using arrestin domain-containing protein 1-mediated microvesicles. These microvesicles are then engulfed and reused by macrophages, leading to enhanced bioenergetics. Additionally, it has been found that MSCs release microRNA-containing exosomes that prevent macrophage activation through the suppression of the Toll-like receptor signalling pathway. As a result, macrophages become desensitized to the ingested mitochondria. These findings provide further insight into the regulatory activity of MSCs to regulate the innate immune system, particularly macrophages [90-92].
Immunogenicity
It has long been thought that MSCs are “immune privileged” or “hypo-immunogenic”, making them capable of avoiding detection by the immune system. However, in vivo studies and clinical trials have provided compelling evidence describing the generation of antibodies against allogeneic MSCs that led to their rejection. Hence it has been suggested that MSCs are immune-evasive, not immune-privileged [93]. A ‘hit and run’ mechanism has been suggested to enable MSCs to fulfil their therapeutic purpose by avoiding immune detection and recipient sensitization to donor antigens. This mechanism is mediated by MSC’s secretome, i.e., through their “empowerment” approach, during the early phase of injection [33,93]. The immune-invasive nature of MSCs, specifically those from fetal sources, points to their low immunogenicity, making them “immunologically safe” for use in allogeneic clinical applications [94,95].
Allogeneic MSC transplantation faces the challenge of immune rejection in which both innate and adaptive immune responses are involved. The key molecules implicated in this process are classic Major Histocompatibility Complex (MHC) and costimulatory. While MHC molecules bearing antigens are recognized by T Cell Receptor (TCR) on naïve T cells and leading to their activation, costimulatory molecules amplify the MHC activation signal. Class I and II MHC molecules are recognized by CD8+ Cytotoxic T Lymphocytes (CTLs) (the main factors of cell transplant rejection) and CD4+ effector cells, respectively [96]. Acute graft rejection is mainly attributed to the recognition of MHC class I molecules by CTLs (days or weeks after transplantation), whereas chronic graft rejection is due to the function of CD4+ effector cells as a result of the recognition of Class II MHC molecules on donor cells [97]. The low level of class I MHC on MSCs, particularly those derived from human fetal sources such as AF-MSCs, makes these cells protected from CTLs and NKCs-mediating phagocytosis and reduces the risk of transplant rejection [98]. Also, MSCs could be barely recognized by CD4+ effector cells due to the lack of class II MHC on their surface [99].
Pathological conditions related to MSC’s immunogenicity: Despite possessing these features and the anti-inflammatory qualities of MSCs, the results of several in vivo studies and clinical trials have indicated that the expression of mismatched MHC molecules by donor MSCs can trigger recipient lymphocytes to differentiate into MHC-specific effector and memory cells [100]. Additionally, antibodies produced after B cell activation by allo-antigens and cytokine-dependent upregulation of HLA molecules in transplanted cells lead to the rejection of allogeneic cells [101]. According to a study conducted on horses, injecting MHC-mismatched MSCs intradermally caused the production of cytotoxic anti-MHC I isoantibodies, while a similar effect was not observed for MHC-matched MSCs [102]. Despite most clinical trials demonstrating that those injected with allogeneic MSCs did not generate substantial alloantibodies, 10% of patients did possess alloantibodies [103,104]. Thus, checking the immunogenic status of MSCs before injection can positively impact the success of cell therapy.
It is important to consider hemocompatibility when intravascular infusion of MSCs is used in clinical trials. The use of incompatible MSCs with circulating innate immune cells can lead to fatal adverse events such as thrombosis and embolism [105,106]. In addition, in vitro expanded MSCs have the capacity to activate both coagulation and complement pathways, which can result in innate immune attacks known as Instant Blood-Mediated Inflammatory Reaction (IBMIR). The expression of tissue factor (TF or CD142) on MSCs is responsible for these attacks and can cause thrombosis when these cells come in contact with peripheral blood [105]. A recent clinical trial on critically ill patients with COVID-19 was the first study to evaluate the Pro-Coagulant Activity (PCA) of human TF+ immature dental pulp stromal cells (hIDPSC, NestaCell product) before administration. Although the product cells varied from 0.2% to 63.9% in terms of the TF+ percentage of the population, cells with a TF+ proportion of less than 25% were selected for treatment. It was found that thromboelastography (in vitro) was insufficient to predict the risk of TF+ MSC treatments. Also, it was determined that, apart from TF, other unnamed factors are likely involved in the PCA of hIDPSC [107].
TF/CD142 plays an important role in the coagulation process and is the primary cause of IBMIR. It has been suggested that the impact of TF/CD142 on coagulation and compatibility should be assessed before MSC injection. As such, TF/CD142 expression should be included in early evaluations as a phenotypic immune marker [106,108]. Recent in vivo studies have revealed varying levels of highly pro-coagulant TF/CD142 in different sources of MSCs [106,109]. Clinical trials have also reported side effects, particularly thrombogenic events, after infusion of MSCs from both fetal [19,110], and adult [111,112], sources. While comparing these studies can be misleading due to differences in experimental conditions, comparative studies conducted by Moll and colleagues over the last decade have shown that PCA is observed in both adult BM-MSCs and placenta-derived Decidual Stromal Cells (DSCs) [113-115]. However, TF/CD142 expression levels are higher in DSCs, which leads to stronger PCA than BM-MSCs. These findings may be due to the high levels of TF/CD142 expression in blood cells and vascularized organs such as the placenta [116]. Another comparison between two adult sources of MSCs, AT- and BM-MSCs, has shown that TF/CD142 expression levels were lower in BM-MSCs, resulting in lower PCA [117]. Although the intensity of PCA is directly correlated with the amount of TF/CD142 expressed by donor cells, it is also a dose-dependent property that increases with ex vivo expansion and cryopreservation [96].
Immunogenicity and cell origin: Several factors may affect the immunogenicity of MSCs, such as the source of the cells, the donor status, the culture conditions, and the host status [96]. Like other characteristics of MSCs, the immunogenicity features are influenced by two main reasons-the origin of the cells (cell source and donor status) and the microenvironment (culture conditions and host status). Different studies have reported varying immunogenicity levels among MSCs according to their tissue or fetal/adult sources. In most studies, the expression of Human Leukocyte Antigens (HLA), which represent the MHC class I and II receptors, is used to determine the immunogenicity of MSCs. The HLA-ABC surface antigens are the major contributors to the production of alloreactive memory-CD8+ T cells [118]. Studies have demonstrated that adult tissues display variable levels of immunogenicity. For example, a study on AT-MSCs and BM-MSCs at the single-cell and cultivation levels showed that AT-MSCs had lower expression levels of HLA class I [119]. Additionally, it has been found that the percentage of MHC II expression in adult MSCs from young donors was lower than that from old donors [120].
It has been suggested for a while now that fetal MSCs are less likely to cause an immune response than adult cells. A study conducted on human UC-MSCs and adult BM-MSCs found that fetal cells had significantly lower HLA class I expression, meaning they were less likely to be recognized by the host immune system. When tested in an immunocompetent animal model, the fetal cells were also shown to have lower allogeneic and xenogeneic immune activation and were rejected more slowly than the adult cells [121]. Another study, based on the analysis of immunogenicity by Mixed Lymphocyte Reaction (MLR) in vitro between fetal UC-MSCs and adult AT-MSCs, demonstrated that both fetal and adult sources lacked MHC II. UC-MSCs also expressed lower levels of MHC class I receptors and IFNγ receptors than AT-MSCs [122]. Similarly, many other studies also report lower surface levels of HLA class I in FSCs than in ASCs [123-125].
Furthermore, the lack of HLA class II has been reported in FSCs, neither on their surface nor in intracellular form. In contrast, ASCs express the intracellular form of these antigens when they are in the basal immune state without the stimulatory effect of pro- inflammatory cytokines (e.g., IFN-γ). In this regard, the response of fetal and adult MSCs to IFN-γ stimulation is different, resulting in surface induction of HLA class II at different rates (only after 1 day in ASCs and after 7 days in FSCs) [125,126]. Other reports indicate that HLA class I and II expression is reduced in fetal cells compared to adult MSCs after being exposed to IFN-γ [127]. Overall, FSCs are widely held to be less immunogenic, at least initially, than ASCs [24,125,128,129], thus indicating the potential advantages of using FSCs in certain clinical procedures.
Immunogenicity and microenvironment: Aside from the source of cells, the microenvironment in which MSCs are located can also affect their immunogenicity. This includes the culture media used during in vitro expansion and the host environment following in vivo administration. In this section, we discuss some well-recognized factors of the microenvironment involved in the immunogenicity of MSCs, including culture medium, cytokines, and oxygen level.
Culture medium: Studies have shown that the culture media used for expansion have varying effects on the immunogenicity of FSCs and ASCs [130-132]. Indeed, in vitro microenvironment has a substantial impact on the immunogenicity of cultured cells. Recently, a new line of MSCs called IACs have been derived from human adipose tissue that are immune-privileged and pro- angiogenic. These cells are produced by using a specific chemical cocktail in the culture system that contains cytokines, small molecules, structural proteins, and other essential components. IACs have demonstrated an increased pro-regenerative potential and elicited a milder immune response compared to conventional AT-MSCs [133].
Cytokines: It has been known that different cytokines and their levels in the environment can affect the expression of MHC and the phenotype of MSCs [96]. For instance, increased expression of HLA-DR was observed in BM-MSCs cultured with media containing human serum [134], with a correlation between HLA-DR and levels of interleukin (IL)-17F and IL-33 [135]. In addition, HLA-DR expression was induced by incubating MSCs with IFNγ and other pro- inflammatory cytokines in vitro [134,136]. Nonetheless, numerous in vitro studies have shown that preconditioning MSCs with pro- inflammatory cytokines, particularly IFN-γ and TNF-α, increases the expression of anti-inflammatory molecules and promotes their immunosuppressive properties [12,137]. These findings have also been confirmed in animal models in which preconditioned MSCs enhance islet allograft survival [138]. Furthermore, TGFβ2 has been shown to downregulate the expression of MHC I and II molecules induced by IFNγ on MSCs, without changing their morphology and surface markers. Also, the treatment of MSCs by TGFβ2, in a medium lacking IL-1β and TNFα, has been found to decrease their immunogenicity [139]. While more research is necessary, these studies suggest that preconditioning plays an important role in balancing the immunosuppressive properties of MSCs with their immunogenicity.
Oxygen level: The oxygen level during in vitro expansion is an important factor in MSCs’ microenvironment affecting their immunogenicity. Several studies have confirmed the positive effects of hypoxic in vitro conditions on the survival, immunomodulatory properties, and angiogenic capacity of MSCs from both fetal and adult sources [137,140]. In addition, hypoxic conditions can increase the proliferation potential of MSCs, improve their antioxidant capacity, and enhance their therapeutic effect on radiation-induced lung damage in mouse models [141]. Despite the advantages of hypoxic conditions on the beneficial characteristics of MSCs for cell therapy, numerous studies have indicated that it can also cause MSCs to lose their immune privilege and become immunogenic [142]. This is attributed to proteasome activity, whereby intracellular degradation of MHC II mediated by 26S proteasome under normoxic conditions is abolished during hypoxia. Accordingly, the down regulation of Heat Shock Protein-(HSP-) 90α under hypoxic conditions leads to the inactivation of proteasome and consequently the loss of immune privilege in allogeneic MSCs due to upregulated MHC II [140]. Hypoxia also increases the biosynthesis of MHC II in MSCs through upregulation of Sug1 (ATPase subunit of 19S proteasome) and enhanced binding to class II transactivator (CIITA), a transcriptional coactivator of MHC II, which ultimately results in increased biosynthesis of MHC II in MSCs [141]. Additionally, exposure of MSCs to hypoxia leads to the downregulation of PGE2 and the loss of MSC immune privilege due to proteasome-mediated degradation of COX2, the rate- limiting enzyme in PGE2 biosynthesis [142]. While there have been several studies on the immunogenicity of MSCs, understanding the impact of various components of the microenvironment on MSCs’ immunogenicity requires further investigation.
Discussion
Differentially Expressed Genes (DEGs) between fetal and adult stem cells
Stem cells can be isolated at all stages of ontogeny, from early embryos to post-reproductive adults. While fetal and adult MSCs may appear similar in terms of morphology and surface markers, studies indicate that MSCs derived from fetal tissues are more adaptable and exhibit greater self-renewal capacity, both in vivo and in vitro [143,144]. FSCs can safeguard against ageing and possess a greater capacity to multiply than adult stem cells. These cells can expand more easily in laboratory conditions and display no obvious change in phenotype after a long culture. Long-term culture is necessary to obtain suitable numbers of MSCs for clinical use, even if senescence and impaired function may occur during ex vivo expansion [145,146]. Although adult stem cells are less potent compared to FSCs, they still have a vital role in maintaining overall health. Additionally, the use of fetal tissue is still being debated and not widely accepted. Therefore, understanding the differences between FSCs and ASCs, as well as their regulatory mechanisms, could provide valuable insights into the clinical application of adult stem cells. However, the precise molecular mechanisms responsible for these disparities are still not entirely comprehended [147,148].
Several studies have shown that MSCs derived from different sources, in different culture conditions, from donors with different ages and diseases have distinct biological properties [149-161]. For example, one previous study compared the gene expression profile of MSCs derived from the fetal liver and adult bone marrow. These cells can be easily expanded in vitro and senesced later in culture. Both MSCs have immunomodulatory properties and are non- immunogenic, even though some differences have been observed [162]. However, to our knowledge, there are not many studies to analyze the differentially expressed genes between FSCs and ASCs. A recent study discovers robust DEGs between BM-MSCs of fetal and adult sources using the two existing GEO datasets. This study reported 388 up-regulated and 289 down-regulated DEGs in ASCs compared with FSCs. After data mining and network analysis, the most considerably over-expressed genes in ASCs were MYC, KIF20A, HLA-DRA, and HLA-DPA1 [148]. These four hub genes, the MYC (a senescence-associated gene), KIF20A (involved in the cell cycle) and two HLA-DRA and HLA-DPA1 genes, induced during age-related inflammatory conditions. More experiments and network analysis are necessary to determine DEGs between FSCs and ASCs and understand differences in function and differentiation potential that exist between them. Some of the studies conducted in this field are listed in Table 1, which are worth studying and bioinformatics analysis. Overall, it seems that analysis of the mentioned GEO datasets in this Table 1 could help to unravel the molecular basis of the senescence phenomenon. Finally, it can open the way to improve ASCs performance compared to FSCs, which may lead to upgrading of clinical protocols.
| Ref. | Cell type and N. of samples | Aim | Result | Method and GSE-number |
|---|---|---|---|---|
| [149] | Primary human lung MVPC and Fetal lung MSC/N=9 | Finding hallmark signaling pathway of ageing and chronic lung disease | A significant role for mTOR in the maintenance of MVPC function, microvascular niche homeostasis and lung ageing | Transcriptome analysis by array with Platform: GPL6244/GSM7055827,GSE225760 |
| [150] | Adult human Sertoli cells and precursor of testicular germ cell tumours type II/N=16 | Understanding the exact pathogenesis of germ cells in young men | Interactions between Sertoli cells and tumour cells/impaired in Sertoli cells associated with GCNIS represent adult cells undergoing progressive dedifferentiation | Whole Human Genome Microarray/ Platforms: GPL6480, GPL17077/GSE169557 |
| [151] | Tonsil-derived MSCs (T-MSCs)/N=4 | To identify specific biomarkers for senescent cells | Activation of ECM-receptor signalling, can regulate stem cell senescence in T-MSCs/increase the therapeutic efficacy of T-MSCs in clinical applications. | Transcriptome analysis by array with Platform: GPL23126/GSE149588 |
| [152] | Human iPSCs-derived MSCs (iMSCs)/N=18 | Determining the primary MSCs is fraught with aging-related shortfalls | iMSCs irrespective of donor age and cell source acquire a rejuvenation gene signature/iMSC could allow circumventing the drawbacks associated with the use of ASCs | Transcriptome analysis by array with Platform: GPL10558/GSE97311 |
| [153] | Wharton's jelly-derived MSCs/adult BM-MSCs and multipotent adult progenitor cells (MAPC)/N=15 | MSCs from different sources show differences in their surface marker and gene expression profiles | Gene ontology analysis revealed that genes associated with cell adhesion, proliferation, and immune system functioning are enriched in WJ-MSC | Transcriptome analysis by array with Platform: GPL6244/GSE77685 |
| [154] | Fetal Neural Stem Cell (fNSC)/adult Neuroprogenitor–Cell (ANPC)/adult Brain Perivascular Mesodermal Cell (aBPMC)/N=17 | Understanding the "intrinsic" MSC population of the human brain | The lack of an innate neuronal but high mesodermal differentiation potential in aBPMC is of great interest for possible autologous therapeutic use | Transcriptome analysis by array with Platform: GPL96/GSE62505 |
| [155] | fetal and adult BM-MSCs (fBM-MSCs, aBM-MSCs)/N=6 | Studying the differences between aBM-MSCs and fBM-MSCs | While fBM-MSCs produced factors, including Wnt signals, that enhanced MSC proliferation, aBM-MSCs produced Wnt factors in a setting that enhanced hematopoietic support | Transcriptome analysis by array with Platform: GPL5188/GSE68374 |
| [156] | Adult BM-MSC and amniotic membrane-derived MSCs (AM-MSCs) of fetal origin/N=24 | Determining the paracrine properties of AM-MSCs | Several proangiogenic factors were detected in AM-MSCs-CM/Our results strongly support the administration of AM-MSCs-CM favours the repair process | Transcriptome array and cytokine array with Platform: GPL6947/GSE61153 |
| [157] | BM-MSC of elderly patients (79-94 years old) with osteoporosis and BM-MSC of elderly and middle-aged patients without symptoms of osteoporosis/N=9 | To unravel whether MSC biology is directly involved in the pathophysiology of the disease, Effects of ageing | Finding new candidates, e.g., MAB21L2, a novel repressor of BMP-induced transcription. Such transcriptional changes may reflect epigenetic changes, which are part of a specific osteoporosis-associated ageing process | Expression profiling by an array with Platform: GPL570/GSE35955 |
| [158] | the cord blood-derived neonatal unrestricted somatic stem cell (USSC), adult BM-MSCs, and adult AT-MSCs (aAT-MSCs)/ N=9 | Understanding differences in function and differentiation potential exist between distinct stem cell populations | BM-MSC and aAT-MSCs are more closely related to each other than to USSC/USSC expressed genes involved in the cell cycle at much higher levels/The BM-MSC signature indicates that they are primed towards developmental processes of tissues / aAT-MSCs appear to be highly enriched in immune-related gene | Expression profiling by an array with Platform: GPL5175/GSE18698 |
Table 1: A list of studies conducted on the MSCs of fetal (FSCs) and adult (ASCs) sources, cell types and number (N) of samples, the purpose and the results obtained and GSE number.
Conclusion
MSCs possess immunomodulatory properties, primarily through their paracrine secretions that contain anti-inflammatory molecules and exosomes. This makes them a potential candidate for treating various immune disorders. Many clinical trials have been conducted to investigate the use of human MSCs in treating immune-related diseases such as Graft-versus-Host Disease (GvHD), inflammatory bowel disease, multiple sclerosis, rheumatoid arthritis, type 1 and type 2 diabetes, and systemic lupus erythematosus. They also have been used to regenerate bone, cartilage, musculoskeletal system, liver, and lung. Although MSC therapy shows potential as a treatment option for many pathological conditions, the cellular and molecular mechanisms underlying MSC-mediated immunomodulation are not yet fully understood. Studies have demonstrated various immunomodulatory changes following the administration of MSCs, but a clear understanding is still lacking, and study results are often inconsistent. This may be due in part to the fact that MSCs from different sources and under different culture conditions express different surface markers, show varying cytokine secretion profiles, and differ in telomere length and methylation patterns that control senescence and epigenetic status, respectively.
Meeting the minimal criteria set out by the International Society for Cellular Therapy (ISCT) is not enough to ensure consistency in the phenotype and function of hMSCs. There is often significant batch-to-batch variation in these cells, which can be attributed to differences in donor and tissue source, as well as culture conditions. These variations have real implications for tissue engineering and cell therapy, as they affect cell heterogeneity, senescence, secretome, multipotent potential, in vivo homing, survival, and integration in damaged tissues. It must be considered that MSCs grown in different environments will result in diverse MSC products, despite meeting the minimum requirements established by the ISCT.
The polarization of MSCs toward anti-inflammatory cells due to exposure to pro-inflammatory stimuli diminishes inflammation and promotes tissue homeostasis in inflamed, damaged tissue. However, HLA molecules are induced by incubating MSCs with pro-inflammatory cytokines in vitro. While more research is necessary, current knowledge suggests that preconditioning plays an important role in balancing the immunosuppressive properties of MSCs with their immunogenicity. Indeed, the plasticity of MSCs in controlling immunoregulatory pathways involved in the maintenance of immune homeostasis depends on the intensity and complexity of inflammatory stimuli. Thus, enhanced comprehension of the controlling mechanisms will prepare for novel therapeutic approaches.
MSCs can be obtained from different adult and fetal tissues. In clinical research, MSCs derived from adipose tissue in adults, and placenta/umbilical cord delivered by fetus is commonly used due to their easy accessibility. However, the diverse range of potential sources makes it difficult to compare study results, as MSCs exhibit varying characteristics in vitro and in vivo depending on their tissue of origin. Moreover, the fact that MSCs behave differently depending on the local microenvironment adds to the complexity of understanding the immunological pathways mediated by MSCs. In summary, the paracrine factors released by fetal MSCs made them a fascinating field of research and a potential candidate for therapeutic applications.
References
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143-147.
[Crossref] [Google Scholar] [PubMed]
- Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells-current trends and future prospective. Biosci Rep. 2015;35(2):e00191.
[Crossref] [Google Scholar] [PubMed]
- Lee MW, Ryu S, Kim DS, Sung KW, Koo HH, Yoo KH. Strategies to improve the immunosuppressive properties of human mesenchymal stem cells. Stem Cell Res Ther. 2015;6:1-10.
[Crossref] [Google Scholar] [PubMed]
- Li H, Shen S, Fu H, Wang Z, Li X, Sui X, et al. Immunomodulatory functions of mesenchymal stem cells in tissue engineering. Stem Cells Int. 2019: 9671206.
[Crossref] [Google Scholar] [PubMed]
- Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2(2):141-150.
[Crossref] [Google Scholar] [PubMed]
- Yu Y, Valderrama AV, Han Z, Uzan G, Naserian S, Oberlin E. Human fetal liver MSCs are more effective than adult bone marrow MSCs for their immunosuppressive, immunomodulatory, and Foxp3+ T reg induction capacity. Stem Cell Res Ther. 2021;12:131-138.
[Crossref] [Google Scholar] [PubMed]
- Dominici ML, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for cellular therapy position statement. Cytotherapy. 2006;8(4):315-317.
[Crossref] [Google Scholar] [PubMed]
- Bernardo ME, Fibbe WE. Mesenchymal stromal cells: Sensors and switchers of inflammation. Cell Stem Cell. 2013;13(4):392-402.
[Crossref] [Google Scholar] [PubMed]
- Le Blanc K, Davies LC. Mesenchymal stromal cells and the innate immune response. Immunol Lett. 2015;168(2):140-146.
[Crossref] [Google Scholar] [PubMed]
- Garcia-Sanchez D, Fernandez D, Rodríguez-Rey JC, Pérez-Campo FM. Enhancing survival, engraftment, and osteogenic potential of mesenchymal stem cells. World J Stem Cells. 2019;11(10):748.
[Crossref] [Google Scholar] [PubMed]
- Yin JQ, Zhu J, Ankrum JA. Manufacturing of primed mesenchymal stromal cells for therapy. Nature Biomed Eng. 2019;3(2):90-104.
[Crossref] [Google Scholar] [PubMed]
- Najar M, Martel-Pelletier J, Pelletier JP, Fahmi H. Novel insights for improving the therapeutic safety and efficiency of mesenchymal stromal cells. World J Stem Cells. 2020;12(12):1474-1491.
[Crossref] [Google Scholar] [PubMed]
- Pappa KI, Anagnou NP. Novel sources of fetal stem cells: Where do they fit on the developmental continuum?. Regen Med. 2009;4(3):423-433.
[Crossref] [Google Scholar] [PubMed]
- Antonucci I, Pantalone A, Tete S, Salini V, V Borlongan C, Hess D, et al. Amniotic fluid stem cells: A promising therapeutic resource for cell-based regenerative therapy. Curr Pharm Des. 2012;18(13):1846-1863.
[Crossref] [Google Scholar] [PubMed]
- Di Trapani M, Bassi G, Fontana E, Giacomello L, Pozzobon M, Guillot PV, et al. Immune regulatory properties of CD117pos amniotic fluid stem cells vary according to gestational age. Stem Cells Dev. 2015;24(1):132-143.
[Crossref] [Google Scholar] [PubMed]
- Loukogeorgakis SP, De Coppi P. Concise review: Amniotic fluid stem cells: The known, the unknown, and potential regenerative medicine applications. Stem Cells. 2017;35(7):1663-1673.
[Crossref] [Google Scholar] [PubMed]
- Murphy SV, Atala A. Amniotic fluid and placental membranes: Unexpected sources of highly multipotent cells. Semin Reprod Med. 2013;31(1):62-68.
[Crossref] [Google Scholar] [PubMed]
- Hoseini SMK, Montazeri F, Zarein F, Sadeghian F, Ghasemi-Esmailabad S, Hajimaghsoudi E. The effect of isolation method on derivation of mesenchymal stem cells from human amniotic fluid: A methodological comparison. Biologicals. 2023. Under Review.
- François M, Romieu-Mourez R, Li M, Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2, 3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther. 2012;20(1):187-195.
[Crossref] [Google Scholar] [PubMed]
- Serena C, Keiran N, Ceperuelo-Mallafre V, Ejarque M, Fradera R, Roche K, et al. Obesity and type 2 diabetes alters the immune properties of human adipose derived stem cells. Stem Cells. 2016;34(10):2559-2573.
[Crossref] [Google Scholar] [PubMed]
- Hoseini SM, Moghaddam-Matin M, Bahrami AR, Montazeri F, Kalantar SM. Human amniotic fluid stem cells: General characteristics and potential therapeutic applications. J Shahid Sadoughi Uni Med Sci. 2021;28(12):3252-3375.
- Sousa BR, Parreira RC, Fonseca EA, Amaya MJ, Tonelli FM, Lacerda SM, et al. Human adult stem cells from diverse origins: An overview from multiparametric immunophenotyping to clinical applications. Cytometry A. 2014;85(1):43-77.
[Crossref] [Google Scholar] [PubMed]
- Caplan AI. The mesengenic process. Clin Plast Surg. 1994;21(3):429-435.
[Crossref] [Google Scholar] [PubMed]
- Sagar R, Walther-Jallow L, David AL, Götherström C, Westgren M. Fetal mesenchymal stromal cells: An opportunity for prenatal cellular therapy. Curr Stem Cell Rep. 2018;4:61-68.
[Crossref] [Google Scholar] [PubMed]
- Runa F, Hamalian S, Meade K, Shisgal P, Gray PC, Kelber JA. Tumor microenvironment heterogeneity: Challenges and opportunities. Curr Mol Biol Rep. 2017;3:218-229.
[Crossref] [Google Scholar] [PubMed]
- Kuznetsov HS, Marsh T, Markens BA, Castaño Z, Greene-Colozzi A, Hay SA, et al. Identification of luminal breast cancers that establish a tumor-supportive macroenvironment defined by proangiogenic platelets and bone marrow–derived cells. Cancer Discov. 2012;2(12):1150-1165.
[Crossref] [Google Scholar] [PubMed]
- Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nat Immunol. 2014;15(11):1009-1016.
[Crossref] [Google Scholar] [PubMed]
- Han F, Wang CY, Yang L, Zhan SD, Zhang M, Tian K. Contribution of murine bone marrow mesenchymal stem cells to pancreas regeneration after partial pancreatectomy in mice. Cell Biol Int. 2012;36(9):823-831.
[Crossref] [Google Scholar] [PubMed]
- Pan HC, Cheng FC, Chen CJ, Lai SZ, Lee CW, Yang DY, et al. Post-injury regeneration in rat sciatic nerve facilitated by neurotrophic factors secreted by amniotic fluid mesenchymal stem cells. J Clin Neurosci. 2007;14(11):1089-1098.
[Crossref] [Google Scholar] [PubMed]
- Zomorodian E, Baghaban Eslaminejad M. Mesenchymal stem cells as a potent cell source for bone regeneration. Stem Cells Int. 2012;2012.
[Crossref] [Google Scholar] [PubMed]
- Ge W, Jiang J, Arp J, Liu W, Garcia B, Wang H. Regulatory T-cell generation and kidney allograft tolerance induced by mesenchymal stem cells associated with indoleamine 2,3-dioxygenase expression. Transplantation. 2010;90(12):1312-1320.
[Crossref] [Google Scholar] [PubMed]
- Leuning DG, Reinders ME, Li J, Peired AJ, Lievers E, de Boer HC, et al. Clinical-grade isolated human kidney perivascular stromal cells as an organotypic cell source for kidney regenerative medicine. Stem Cells Transl Med. 2017;6(2):405-418.
[Crossref] [Google Scholar] [PubMed]
- Von Bahr L, Batsis I, Moll G, Hägg M, Szakos A, Sundberg B, et al. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells. 2012;30(7):1575-1578.
[Crossref] [Google Scholar] [PubMed]
- Togel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289(1):F31-42.
[Crossref] [Google Scholar] [PubMed]
- Sakaida I, Terai S, Yamamoto N, Aoyama K, Ishikawa T, Nishina H, et al. Transplantation of bone marrow cells reduces CCl4‐induced liver fibrosis in mice. Hepatology. 2004;40(6):1304-1311.
[Crossref] [Google Scholar] [PubMed]
- Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5(1):54-63.
[Crossref] [Google Scholar] [PubMed]
- Bai L, Lennon DP, Caplan AI, DeChant A, Hecker J, Kranso J, et al. Hepatocyte growth factor mediates mesenchymal stem cell–induced recovery in multiple sclerosis models. Nat Neurosci. 2012;15(6):862-870.
[Crossref] [Google Scholar] [PubMed]
- Prockop DJ, Kota DJ, Bazhanov N, Reger RL. Evolving paradigms for repair of tissues by adult stem/progenitor cells (MSCs). J Cell Mol Med. 2010;14(9):2190-2199.
[Crossref] [Google Scholar] [PubMed]
- Wolf D, Reinhard A, Wolf D, Reinhard A, Seckinger A, Gross L, et al. Regenerative capacity of intravenous autologous, allogeneic and human mesenchymal stem cells in the infarcted pig myocardium–complicated by myocardial tumor formation. Scand Cardiovasc J. 2009;43(1):39-45.
[Crossref] [Google Scholar] [PubMed]
- Prockop DJ, Oh JY. Mesenchymal Stem/Stromal Cells (MSCs): Role as guardians of inflammation. Mol Ther. 2012;20(1):14-20.
[Crossref] [Google Scholar] [PubMed]
- Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30(1):42-48.
[Crossref] [Google Scholar] [PubMed]
- Moorefield EC, McKee EE, Solchaga L, Orlando G, Yoo JJ, Walker S, et al. Cloned, CD117 selected human amniotic fluid stem cells are capable of modulating the immune response. PloS One. 2011;6(10):e26535.
[Crossref] [Google Scholar] [PubMed]
- Le Blanc K, Ringden O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007;262(5):509-525.
[Crossref] [Google Scholar] [PubMed]
- Kuçi Z, Seiberth J, Latifi-Pupovci H, Wehner S, Stein S, Grez M, et al. Clonal analysis of multipotent stromal cells derived from CD271+ bone marrow mononuclear cells: functional heterogeneity and different mechanisms of allosuppression. Haematologica. 2013;98(10):1609.
[Crossref] [Google Scholar] [PubMed]
- Ren G, Su J, Zhang L, Zhao X, Ling W, L'huillie A, et sl. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. 2009;27(8):1954-1962.
[Crossref] [Google Scholar] [PubMed]
- Elahi KC, Klein G, Avci-Adali M, Sievert KD, MacNeil S, Aicher WK. Human mesenchymal stromal cells from different sources diverge in their expression of cell surface proteins and display distinct differentiation patterns. Stem Cells Int. 2016;2016.
[Crossref] [Google Scholar] [PubMed]
- Castro-Manrreza ME, Montesinos JJ. Immunoregulation by mesenchymal stem cells: Biological aspects and clinical applications. J Immunol Res. 2015;2015.
[Crossref] [Google Scholar] [PubMed]
- Fayyad-Kazan M, Najar M, Fayyad-Kazan H, Raicevic G, Lagneaux L. Identification and evaluation of new immunoregulatory genes in mesenchymal stromal cells of different origins: Comparison of normal and inflammatory conditions. Med Sci Monit Basic Res. 2017;23:87-96.
[Crossref] [Google Scholar] [PubMed]
- Hoseini SM, Kalantar SM, Bahrami AR, Zareien F. Mesenchymal stem cells: Interactions with immune cells and immunosuppressive-immunomodulatory properties. Sci J Iran Blood Transfus Organ. 2020;17(2):147-169.
- Zhao Y, Shen M, Wu L, Yang H, Yao Y, Yang Q, et al. Stromal cells in the tumor microenvironment: Accomplices of tumor progression?. Cell Death Dis. 2023;14(9):587.
[Crossref] [Google Scholar] [PubMed]
- Nilendu P, Sarode SC, Jahagirdar D, Tandon I, Patil S, Sarode GS, et al. Mutual concessions and compromises between stromal cells and cancer cells: Driving tumor development and drug resistance. Cell Oncol (Dordr). 2018;41:353-367.
[Crossref] [Google Scholar] [PubMed]
- Montazeri F, Tajamolian M, Hosseini ES, Hoseini SM. Immunologic factors and genomic considerations in recurrent pregnancy loss: A Review. Int J Med Lab. 2023;10(4):279-305.
- Hoseini SM, Kalantar SM, Sheikhha MH, Aflatoonian B, Ghasemi N, Hosseini ES, et al. The immunomodulatory capacity of human amniotic fluid-derived mesenchymal stem cells from women with a history of idiopathic recurrent miscarriage suggests the possible role of fetal cells in the disease. 2023.
- Finkelsztejn A, Brooks JB, Paschoal Jr FM, Fragoso YD. What can we really tell women with multiple sclerosis regarding pregnancy? A systematic review and meta‐analysis of the literature. BJOG. 2011;118(7):790-797.
[Crossref] [Google Scholar] [PubMed]
- Østensen M, Villiger PM. The remission of rheumatoid arthritis during pregnancy. Semin Immunopathol. 2007;29(2):185-191.
[Crossref] [Google Scholar] [PubMed]
- Buchel E, Van Steenbergen W, Nevens F, Fevery J. Improvement of autoimmune hepatitis during pregnancy followed by flare-up after delivery. Am J Gastroenterol. 2002;97(12):3160-3165.
[Crossref] [Google Scholar] [PubMed]
- Guillot PV, Gotherstrom C, Chan J, Kurata H, Fisk NM. Human first-trimester fetal MSC express pluripotency markers and grow faster and have longer telomeres than adult MSC. Stem Cells. 2007;25(3):646-654.
[Crossref] [Google Scholar] [PubMed]
- Le Blanc K. Immunomodulatory effects of fetal and adult mesenchymal stem cells. Cytotherapy. 2003;5(6):485-489.
[Crossref] [Google Scholar] [PubMed]
- Wang Q, Yang Q, Wang Z, Tong H, Ma L, Zhang Y, et al. Comparative analysis of human mesenchymal stem cells from fetal-bone marrow, adipose tissue, and Warton's jelly as sources of cell immunomodulatory therapy. Hum Vaccin Immunother. 2016;12(1):85-96.
[Crossref] [Google Scholar] [PubMed]
- Chen PM, Yen ML, Liu KJ, Sytwu HK, Yen BL. Immunomodulatory properties of human adult and fetal multipotent mesenchymal stem cells. J Biomed Sci. 2011;18(1):49.
[Crossref] [Google Scholar] [PubMed]
- Castro-Manrreza ME, Mayani H, Monroy-García A, Flores-Figueroa E, Chávez-Rueda K, Legorreta-Haquet V, et al. Human mesenchymal stromal cells from adult and neonatal sources: A comparative in vitro analysis of their immunosuppressive properties against T cells. Stem Cells Dev. 2014;23(11):1217-1232.
[Crossref] [Google Scholar] [PubMed]
- Götherström C. Human foetal mesenchymal stem cells. Best Pract Res Clin Obstet Gynaecol. 2016;31:82-87.
[Crossref] [Google Scholar] [PubMed]
- Bollini S, Gentili C, Tasso R, Cancedda R. The regenerative role of the fetal and adult stem cell secretome. J Clin Med. 2013;2(4):302-327.
[Crossref] [Google Scholar] [PubMed]
- Giuliani M, Fleury M, Vernochet A, Ketroussi F, Clay D, Azzarone B, et al. Long-lasting inhibitory effects of fetal liver mesenchymal stem cells on T-lymphocyte proliferation. PloS One. 2011;6(5):e19988.
[Crossref] [Google Scholar] [PubMed]
- Fan Y, Herr F, Vernochet A, Mennesson B, Oberlin E, Durrbach A. Human fetal liver mesenchymal stem cell-derived exosomes impair natural killer cell function. Stem Cells Dev. 2019;28(1):44-55.
[Crossref] [Google Scholar] [PubMed]
- Mattar P, Bieback K. Comparing the immunomodulatory properties of bone marrow, adipose tissue, and birth-associated tissue mesenchymal stromal cells. Front Immunol. 2015;6:164869.
[Crossref] [Google Scholar] [PubMed]
- Christodoulou I, Kolisis FN, Papaevangeliou D, Zoumpourlis V. Comparative evaluation of human mesenchymal stem cells of fetal (Wharton's jelly) and adult (adipose tissue) origin during prolonged invitro expansion: Considerations for cytotherapy. Stem Cells Int. 2013;2013.
[Crossref] [Google Scholar] [PubMed]
- Chan CK, Wu KH, Lee YS, Hwang SM, Lee MS, Liao SK, et al. The comparison of interleukin 6–associated immunosuppressive effects of human ESCs, fetal-Type MSCs, and adult-type MSCs. Transplantation. 2012;94(2):132-138.
[Crossref] [Google Scholar] [PubMed]
- Stern CM. Immunology of pregnancy and its disorders. Springer Science and Business Media. 2012.
- Morel Y, Roucher F, Plotton I, Goursaud C, Tardy V, Mallet D. Evolution of steroids during pregnancy: Maternal, placental and fetal synthesis. Ann Endocrinol (Paris). 2016;77(2): 82-89.
[Crossref] [Google Scholar] [PubMed]
- Deshmukh H, Way SS. Immunological basis for recurrent fetal loss and pregnancy complications. Annu Rev Pathol. 2019;14:185-210.
[Crossref] [Google Scholar] [PubMed]
- Luo X, Miller SD, Shea LD. Immune tolerance for autoimmune disease and cell transplantation. Annu Rev Biomed Eng. 2016;18:181-205.
[Crossref] [Google Scholar] [PubMed]
- McGovern N, Shin A, Low G, Low D, Duan K, Yao LJ, et al. Human fetal dendritic cells promote prenatal T-cell immune suppression through arginase-2. Nature. 2017;546(7660):662-666.
[Crossref] [Google Scholar] [PubMed]
- Najar M, Raicevic G, Kazan HF, De Bruyn C, Bron D, Toungouz M, et al. Immune-related antigens, surface molecules and regulatory factors in human-derived mesenchymal stromal cells: The expression and impact of inflammatory priming. Stem Cell Rev Rep. 2012;8:1188-1198.
[Crossref] [Google Scholar] [PubMed]
- Lu D, Xu Y, Liu Q, Zhang Q. Mesenchymal stem cell-macrophage crosstalk and maintenance of inflammatory microenvironment homeostasis. Front Cell Dev Biol. 2021;9:681171.
[Crossref] [Google Scholar] [PubMed]
- Huang Y, Wu Q, Tam PK. Immunomodulatory mechanisms of mesenchymal stem cells and their potential clinical applications. Int J Mol Sci. 2022;23(17):10023.
[Crossref] [Google Scholar] [PubMed]
- Battle A, Khan Z, Wang SH, Mitrano A, Ford MJ, Pritchard JK, et al. Impact of regulatory variation from RNA to protein. Science. 2015;347(6222):664-667.
[Crossref] [Google Scholar] [PubMed]
- Rea IM, Gibson DS, McGilligan V, McNerlan SE, Alexander HD, Ross OA. Age and age-related diseases: Role of inflammation triggers and cytokines. Front Immunol. 2018;9:334076.
[Crossref] [Google Scholar] [PubMed]
- Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: Positioning cells for host defense and immunity. Annu Rev Immunol. 2014;32:659-702.
[Crossref] [Google Scholar] [PubMed]
- Sarkar SA, Lee CE, Victorino F, Nguyen TT, Walters JA, Burrack A, et al. Expression and regulation of chemokines in murine and human type 1 diabetes. Diabetes. 2012;61(2):436-446.
[Crossref] [Google Scholar] [PubMed]
- Fallahi P, Corrado A, Di Domenicantonio A, Frenzilli G, Antonelli A, Martina Ferrari S. CXCR3, CXCR5, CXCR6, and CXCR7 in Diabetes. Curr Drug Targets. 2016;17(5):515-519.
[Crossref] [Google Scholar] [PubMed]
- Burke SJ, Collier JJ. Transcriptional regulation of chemokine genes: A link to pancreatic islet inflammation?. Biomolecules. 2015;5(2):1020-1034.
[Crossref] [Google Scholar] [PubMed]
- Hoseini SMF, Hosseini ES, Miresmaeili SM, Vahidi Mehrjardi MY, Dehghani MR, Sheikhha MH, et al. The impact of IFNγ-preconditioning on mesenchymal stem cells' interaction with peripheral blood mononuclear cells differs between patients with type 1 diabetes and healthy individuals. 2023.
- De George DJ, Ge T, Krishnamurthy B, Kay TW, Thomas HE. Inflammation versus regulation: How interferon-gamma contributes to type 1 diabetes pathogenesis. Front Cell Dev Biol. 2023;11:1205590.
[Crossref] [Google Scholar] [PubMed]
- Azhdari MH, Goodarzi N, Doroudian M, MacLoughlin R. Molecular insight into the therapeutic effects of stem cell-derived exosomes in respiratory diseases and the potential for pulmonary delivery. Int J Mol Sci. 2022;23(11):6273.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Chopp M, Liu XS, Katakowski M, Wang X, Tian X, et al. Exosomes derived from mesenchymal stromal cells promote axonal growth of cortical neurons. Mol Neurobiol. 2017;54:2659-2673.
[Crossref] [Google Scholar] [PubMed]
- Yu B, Kim HW, Gong M, Wang J, Millard RW, Wang Y, et al. Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. Int J Cardiol. 2015;182:349-360.
[Crossref] [Google Scholar] [PubMed]
- Lou G, Song X, Yang F, Wu S, Wang J, Chen Z, Liu Y. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J Hematol Oncol. 2015;8:1-11.
[Crossref] [Google Scholar] [PubMed]
- Zou X, Zhang G, Cheng Z, Yin D, Du T, Ju G, et al. Microvesicles derived from human Wharton’s Jelly mesenchymal stromal cells ameliorate renal ischemia-reperfusion injury in rats by suppressing CX3CL1. Stem Cell Res Ther. 2014;5:1-13.
[Crossref] [Google Scholar] [PubMed]
- Phinney DG, Di Giuseppe M, Njah J, Sala E, Shiva S, St Croix CM, et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun. 2015;6(1):8472.
[Crossref] [Google Scholar] [PubMed]
- Morrison TJ, Jackson MV, Cunningham EK, Kissenpfennig A, McAuley DF, O’Kane CM, et al. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am J Respir Crit Care Med. 2017;196(10):1275-1286.
[Crossref] [Google Scholar] [PubMed]
- Jackson MV, Morrison TJ, Doherty DF, McAuley DF, Matthay MA, Kissenpfennig A, et al. Mitochondrial transfer via tunnelling nanotubes is an important mechanism by which mesenchymal stem cells enhance macrophage phagocytosis in the in vitro and in vivo models of ARDS. Stem Cells. 2016;34(8):2210-2223.
[Crossref] [Google Scholar] [PubMed]
- Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat Biotechnol. 2014;32(3):252-260.
[Crossref] [Google Scholar] [PubMed]
- Prockop DJ. Concise review: Two negative feedback loops place mesenchymal stem/stromal cells at the center of early regulators of inflammation. Stem Cells. 2013;31(10):2042-2046.
[Crossref] [Google Scholar] [PubMed]
- Lee M, Jeong SY, Ha J, Kim M, Jin HJ, Kwon SJ, et al. Low immunogenicity of allogeneic human umbilical cord blood-derived mesenchymal stem cells in vitro and in vivo. Biochem Biophys Res Commun. 2014;446(4):983-989.
[Crossref] [Google Scholar] [PubMed]
- Chen W, Lv L, Chen N, Cui E. Immunogenicity of mesenchymal stromal/stem cells. Scand J Immunol. 2023;97(6):e13267.
- Abrahimi P, Qin L, Chang WG, Bothwell AL, Tellides G, Saltzman WM, et al. Blocking MHC class II on human endothelium mitigates acute rejection. JCI Insight. 2016;1(1).
[Crossref] [Google Scholar] [PubMed]
- Abdulrazzak H, Moschidou D, Jones G, Guillot PV. Biological characteristics of stem cells from foetal, cord blood and extraembryonic tissues. J R Soc Interface. 2010;7(suppl_6):S689-S706.
[Crossref] [Google Scholar] [PubMed]
- Haddad R, Saldanha-Araujo F. Mechanisms of T-cell immunosuppression by mesenchymal stromal cells: What do we know so far?. Biomed Res Int. 2014;2014.
[Crossref] [Google Scholar] [PubMed]
- Berglund AK, Fortier LA, Antczak DF, Schnabel LV. Immunoprivileged no more: Measuring the immunogenicity of allogeneic adult mesenchymal stem cells. Stem Cell Res Ther. 2017;8:1-17.
[Crossref] [Google Scholar] [PubMed]
- Souidi N, Stolk M, Rudeck J, Strunk D, Schallmoser K, Volk HD, et al. Stromal cells act as guardians for endothelial progenitors by reducing their immunogenicity after co-transplantation. Stem Cells. 2017;35(5):1233-1245.
[Crossref] [Google Scholar] [PubMed]
- Berglund AK, Schnabel LV. Allogeneic major histocompatibility complex‐mismatched equine bone marrow‐derived mesenchymal stem cells are targeted for death by cytotoxic anti‐major histocompatibility complex antibodies. Equine Vet J. 2017;49(4):539-544.
[Crossref] [Google Scholar] [PubMed]
- Sanabria-de la Torre R, Quiñones-Vico MI, Fernández-González A, Sanchez-Diaz M, Montero-Vílchez T, Sierra-Sanchez A, et al. Alloreactive immune response associated to human mesenchymal stromal cells treatment: A systematic review. J Clin Med. 2021;10(13):2991.
[Crossref] [Google Scholar] [PubMed]
- Golpanian S, DiFede DL, Khan A, Schulman IH, Landin AM, Tompkins BA, et al. Allogeneic human mesenchymal stem cell infusions for aging frailty. J Gerontol A Biol Sci Med Sci. 2017;72(11):1505-1512.
[Crossref] [Google Scholar] [PubMed]
- Coppin L, Sokal E, Stéphenne X. Thrombogenic risk induced by intravascular mesenchymal stem cell therapy: Current status and future perspectives. Cells. 2019;8(10):1160.
[Crossref] [Google Scholar] [PubMed]
- Moll G, Ankrum JA, Kamhieh-Milz J, Bieback K, Ringdén O, Volk HD, et al. Intravascular mesenchymal stromal/stem cell therapy product diversification: Time for new clinical guidelines. Trends Mol Med. 2019;25(2):149-163.
[Crossref] [Google Scholar] [PubMed]
- Araldi RP, Prezoto BC, Gonzaga V, Policiquio B, Mendes TB, D’Amélio F, et al. Advanced cell therapy with low tissue factor loaded product NestaCell® does not confer thrombogenic risk for critically ill COVID-19 heparin-treated patients. Biomed Pharmacother. 2022;149:112920.
[Crossref] [Google Scholar] [PubMed]
- Moll G, Ankrum JA, Olson SD, Nolta JA. Improved MSC minimal criteria to maximize patient safety: A call to embrace tissue factor and hemocompatibility assessment of MSC products. Stem Cells Transl Med. 2022;11(1):2-13.
[Crossref] [Google Scholar] [PubMed]
- Wu Z, Zhang S, Zhou L, Cai J, Tan J, Gao X, et al. Thromboembolism induced by umbilical cord mesenchymal stem cell infusion: A report of two cases and literature review. Transplant Proc. 2017;49(7):1656-1658.
[Crossref] [Google Scholar] [PubMed]
- Melmed GY, Pandak WM, Casey K, Abraham B, Valentine J, Schwartz D, et al. Human placenta-derived cells (PDA-001) for the treatment of moderate-to-severe Crohn's disease: A phase 1b/2a study. Inflammatory bowel diseases. 2015;21(8):1809-1816.
[Crossref] [Google Scholar] [PubMed]
- Jung JW, Kwon M, Choi JC, Shin JW, Park IW, Choi BW, et al. Familial occurrence of pulmonary embolism after intravenous, adipose tissue-derived stem cell therapy. Yonsei Med J. 2013;54(5):1293-1296.
[Crossref] [Google Scholar] [PubMed]
- Sokal EM, Stéphenne X, Ottolenghi C, Jazouli N, Clapuyt P, Lacaille F, et al. Liver engraftment and repopulation by in vitro expanded adult derived human liver stem cells in a child with ornithine carbamoyltransferase deficiency. JIMD Rep. 2014;13:65-72.
[Crossref] [Google Scholar] [PubMed]
- Moll G, Ignatowicz L, Catar R, Luecht C, Sadeghi B, Hamad O, et al. Different procoagulant activity of therapeutic mesenchymal stromal cells derived from bone marrow and placental decidua. Stem Cells Dev. 2015;24(19):2269-2279.
[Crossref] [Google Scholar] [PubMed]
- Moll G, Alm JJ, Davies LC, von Bahr L, Heldring N, Stenbeck-Funke L, et al. Do cryopreserved mesenchymal stromal cells display impaired immunomodulatory and therapeutic properties?. Stem Cells. 2014;32(9):2430-2442.
[Crossref] [Google Scholar] [PubMed]
- Moll G, Rasmusson-Duprez I, von Bahr L, Connolly-Andersen AM, Elgue G, Funke L, et al. Are therapeutic human mesenchymal stromal cells compatible with human blood?. Stem cells. 2012;30(7):1565-1574.
[Crossref] [Google Scholar] [PubMed]
- Chu AJ. Tissue factor, blood coagulation, and beyond: An overview. Int J Inflam. 2011;2011.
[Crossref] [Google Scholar] [PubMed]
- Christy BA, Herzig MC, Montgomery RK, Delavan C, Bynum JA, Reddoch KM, et al. Procoagulant activity of human mesenchymal stem cells. J Trauma Acute Care Surg. 2017;83(1):S164-S169.
[Crossref] [Google Scholar] [PubMed]
- Chang SH, Kim HJ, Park CG. Allogeneic ADSCs induce the production of alloreactive memory-CD8 T cells through HLA-ABC antigens. Cells. 2020;9(5):1246.
[Crossref] [Google Scholar] [PubMed]
- Zhou W, Lin J, Zhao K, Jin K, He Q, Hu Y, et al. Single-cell profiles and clinically useful properties of human mesenchymal stem cells of adipose and bone marrow origin. Am J Sports Med. 2019;47(7):1722-1733.
[Crossref] [Google Scholar] [PubMed]
- Stolzing A, Jones E, Mcgonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for cell therapies. Mech Ageing Dev. 2008;129(3):163-173.
[Crossref] [Google Scholar] [PubMed]
- Deuse T, Stubbendorff M, Tang-Quan K, Phillips N, Kay MA, Eiermann T, et al. Immunogenicity and immunomodulatory properties of umbilical cord lining mesenchymal stem cells. Cell Transplant. 2011;20(5):655-667.
[Crossref] [Google Scholar] [PubMed]
- Tan K, Zheng K, Li D, Lu H, Wang S, Sun X. Impact of adipose tissue or umbilical cord derived mesenchymal stem cells on the immunogenicity of human cord blood derived endothelial progenitor cells. PloS One. 2017;12(5):e0178624.
[Crossref] [Google Scholar] [PubMed]
- Zhang ZY, Teoh SH, Chong MS, Schantz JT, Fisk NM, Choolani MA, et al. Superior osteogenic capacity for bone tissue engineering of fetal compared with perinatal and adult mesenchymal stem cells. Stem Cells. 2009;27(1):126-137.
[Crossref] [Google Scholar] [PubMed]
- `Götherström C, Ringden O, Westgren M, Tammik C, Le Blanc K. Immunomodulatory effects of human foetal liver-derived mesenchymal stem cells. Bone Marrow Transplant. 2003;32(3):265-272.
[Crossref] [Google Scholar] [PubMed]
- Götherström C, Ringdén O, Tammik C, Zetterberg E, Westgren M, Le Blanc K. Immunologic properties of human fetal mesenchymal stem cells. Am J Obstet Gynecol. 2004;190(1):239-245.
[Crossref] [Google Scholar] [PubMed]
- Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57(1):11-20.
[Crossref] [Google Scholar] [PubMed]
- Hocking AM, Gibran NS. Mesenchymal stem cells: Paracrine signalling and differentiation during cutaneous wound repair. Exp Cell Res. 2010;316(14):2213-2219.
[Crossref] [Google Scholar] [PubMed]
- Götherström C. Immunomodulation by multipotent mesenchymal stromal cells. Transplantation. 2007;84(1):S35-S37.
[Crossref] [Google Scholar] [PubMed]
- Götherström C, Lundqvist A, Duprez IR, Childs R, Berg L, le Blanc K. Fetal and adult multipotent mesenchymal stromal cells are killed by different pathways. Cytotherapy. 2011;13(3):269-278.
[Crossref] [Google Scholar] [PubMed]
- Le HM, Nguyen LT, Hoang DH, Bach TQ, Nguyen HT, Mai HT, et al. Differential development of umbilical cord-derived mesenchymal stem cells during long-term maintenance in fetal bovine serum-supplemented medium and xeno-and serum-free culture medium. Cell Reprogram. 2021;23(6):359-369.
[Crossref] [Google Scholar] [PubMed]
- Nguyen LT, Tran NT, Than UT, Nguyen MQ, Tran AM, Do PT, et al. Optimization of human umbilical cord blood-derived mesenchymal stem cell isolation and culture methods in serum-and xeno-free conditions. Stem Cell Res Ther. 2022;13(1):15.
[Crossref] [Google Scholar] [PubMed]
- Dam PT, Hoang VT, Bui HT, Hang LM, Hoang DM, Nguyen HP, et al. Human adipose-derived mesenchymal stromal cells exhibit high HLA-DR levels and altered cellular characteristics under a xeno-free and serum-free condition. Stem Cell Rev Rep. 2021;17:2291-2303.
[Crossref] [Google Scholar] [PubMed]
- Yi J, Zhang J, Zhang Q, Chen X, Qi R, Liang R, et al. Chemical-empowered human adipose-derived stem cells with lower immunogenicity and enhanced pro-angiogenic ability promote fast tissue regeneration. Stem Cells Transl Med. 2022;11(5):552-565.
[Crossref] [Google Scholar] [PubMed]
- Grau-Vorster M, Laitinen A, Nystedt J, Vives J. HLA-DR expression in clinical-grade bone marrow-derived multipotent mesenchymal stromal cells: A two-site study. Stem Cell Res Ther. 2019;10:164.
[Crossref] [Google Scholar] [PubMed]
- Grau-Vorster M, Rodríguez L, Torrents-Zapata S, Vivas D, Codinach M, Blanco M, et al. Levels of IL-17F and IL-33 correlate with HLA-DR activation in clinical-grade human bone marrow–derived multipotent mesenchymal stromal cell expansion cultures. Cytotherapy. 2019;21(1):32-40.
[Crossref] [Google Scholar] [PubMed]
- Zachar L, Bačenková D, Rosocha J. Activation, homing, and role of the mesenchymal stem cells in the inflammatory environment. J Inflamm Res. 2016:231-240.
[Crossref] [Google Scholar] [PubMed]
- Sarsenova M, Kim Y, Raziyeva K, Kazybay B, Ogay V, Saparov A. Recent advances to enhance the immunomodulatory potential of mesenchymal stem cells. Front Immunol. 2022;13:1010399.
[Crossref] [Google Scholar] [PubMed]
- Oliveira RL, Chagastelles PC, Sesterheim P, Pranke P. In vivo immunogenic response to allogeneic mesenchymal stem cells and the role of preactivated mesenchymal stem cells cotransplanted with allogeneic islets. Stem Cells Int. 2017;2017.
[Crossref] [Google Scholar] [PubMed]
- Berglund AK, Fisher MB, Cameron KA, Poole EJ, Schnabel LV. Transforming growth factor-β2 downregulates Major Histocompatibility Complex (MHC) I and MHC II surface expression on equine bone marrow-derived mesenchymal stem cells without altering other phenotypic cell surface markers. Front Vet Sci. 2017;4:84.
[Crossref] [Google Scholar] [PubMed]
- Bader AM, Klose K, Bieback K, Korinth D, Schneider M, Seifert M, et al. Hypoxic preconditioning increases survival and pro-angiogenic capacity of human cord blood mesenchymal stromal cells in vitro. PLoS One. 2015;10(9):e0138477.
[Crossref] [Google Scholar] [PubMed]
- Li B, Li C, Zhu M, Zhang Y, Du J, Xu Y, et al. Hypoxia-induced mesenchymal stromal cells exhibit an enhanced therapeutic effect on radiation-induced lung injury in mice due to an increased proliferation potential and enhanced antioxidant ability. Cell Physiol Biochem. 2017;44(4):1295-1310.
[Crossref] [Google Scholar] [PubMed]
- Abu-El-Rub E, Sequiera GL, Sareen N, Yan W, Moudgil M, Sabbir MG, et al. Hypoxia-induced 26S proteasome dysfunction increases immunogenicity of mesenchymal stem cells. Cell Death Dis. 2019;10(2):90.
[Crossref] [Google Scholar] [PubMed]
- Abu‐El‐Rub E, Sareen N, Lester Sequiera G, Ammar HI, Yan W, ShamsEldeen AM, et al. Hypoxia‐induced increase in Sug1 leads to poor post‐transplantation survival of allogeneic mesenchymal stem cells. FASEB J. 2020;34(9):12860-12876.
[Crossref] [Google Scholar] [PubMed]
- Sareen N, Abu‐El‐Rub E, Ammar HI, Yan W, Sequiera GL, ShamsEldeen AM, et al. Hypoxia‐induced downregulation of cyclooxygenase 2 leads to the loss of immunoprivilege of allogeneic mesenchymal stem cells. FASEB J. 2020;34(11):15236-15251.
[Crossref] [Google Scholar] [PubMed]
- Hu Y, Ma L, Ma G, Jiang X, Zhao C. Comparative study of human fetal and adult bone marrow derived mesenchymal stem cells. Zhonghua Xue Ye Xue Za Zhi. 2002;23(12):645-648.
[Google Scholar] [PubMed]
- Song I, Rim J, Lee J, Jang I, Jung B, Kim K, et al. Therapeutic potential of human fetal mesenchymal stem cells in musculoskeletal disorders: A narrative review. Int J Mol Sci. 2022;23(3):1439.
[Crossref] [Google Scholar] [PubMed]
- Jovic D, Yu Y, Wang D, Wang K, Li H, Xu F, et al. A brief overview of global trends in MSC-based cell therapy. Stem Cell Rev Rep. 2022;18(5):1525-1545.
[Crossref] [Google Scholar] [PubMed]
- Liu X, Yin M, Liu X, Da J, Zhang K, Zhang X, et al. Analysis of hub genes involved in distinction between aged and fetal bone marrow mesenchymal stem cells by robust rank aggregation and multiple functional annotation methods. Front Genet. 2020;11:573877.
[Crossref] [Google Scholar] [PubMed]
- Mason EC, Menon S, Schneider BR, Gaskill CF, Dawson MM, Moore CM, et al. Activation of mTOR signaling in adult lung microvascular progenitor cells accelerates lung aging. J Clin Invest. 2023;133(24): e171430.
[Crossref] [Google Scholar] [PubMed]
- Schulz B, Schumacher V, Ngezahayo A, Maier-Begandt D, Schadzek N, Wilhelm J, et al. Analysis of connexin 43, connexin 45 and N-cadherin in the human sertoli cell line FS1 and the human seminoma-like cell line TCam-2 in comparison with human testicular biopsies. BMC Cancer. 2023;23(1):232.
[Crossref] [Google Scholar] [PubMed]
- Choi DH, Oh SY, Choi JK, Lee KE, Lee JY, Park YJ, et al. A transcriptomic analysis of serial-cultured, tonsil-derived mesenchymal stem cells reveals decreased integrin α3 protein as a potential biomarker of senescent cells. Stem Cell Res Ther. 2020;11:359.
[Crossref] [Google Scholar] [PubMed]
- Spitzhorn LS, Megges M, Wruck W, Rahman MS, Otte J, Degistirici Ö, et al. Human iPSC-derived MSCs (iMSCs) from aged individuals acquire a rejuvenation signature. Stem Cell Res Ther. 2019;10:100.
[Crossref] [Google Scholar] [PubMed]
- Donders R, Bogie JF, Ravanidis S, Gervois P, Vanheusden M, Maree R, et al. Human Wharton's jelly-derived stem cells display a distinct immunomodulatory and proregenerative transcriptional signature compared to bone marrow-derived stem cells. Stem Cells Dev. 2018;27(2):65-84.
[Crossref] [Google Scholar] [PubMed]
- Lojewski X, Srimasorn S, Rauh J, Francke S, Wobus M, Taylor V, et al. Perivascular mesenchymal stem cells from the adult human brain harbor no instrinsic neuroectodermal but high mesodermal differentiation potential. Stem Cells Transl Med. 2015;4(10):1223-1233.
[Crossref] [Google Scholar] [PubMed]
- Paciejewska MM, Maijenburg MW, Gilissen C, Kleijer M, Vermeul K, Weijer K, et al. Different balance of Wnt signaling in adult and fetal bone marrow-derived mesenchymal stromal cells. Stem Cells Dev. 2016;25(12):934-947.
[Crossref] [Google Scholar] [PubMed]
- Danieli P, Malpasso G, Ciuffreda MC, Cervio E, Calvillo L, Copes F, et al. Conditioned medium from human amniotic mesenchymal stromal cells limits infarct size and enhances angiogenesis. Stem Cells Transl Med. 2015;4(5):448-458.
[Crossref] [Google Scholar] [PubMed]
- Benisch P, Schilling T, Klein-Hitpass L, Frey SP, Seefried L, Raaijmakers N, et al. The transcriptional profile of mesenchymal stem cell populations in primary osteoporosis is distinct and shows overexpression of osteogenic inhibitors. PLoS One. 2012;7(9):e45142.
[Crossref] [Google Scholar] [PubMed]
- Jansen BJ, Gilissen C, Roelofs H, Schaap-Oziemlak A, Veltman JA, Raymakers RA, et al. Functional differences between mesenchymal stem cell populations are reflected by their transcriptome. Stem Cells Dev. 2010;19(4):481-490.
[Crossref] [Google Scholar] [PubMed]
- Hoseini SM, Montazeri F, Bahrami AR, Kalantar SM, Rahmani S, Zarein F, et al. Investigating the expression of pluripotency-related genes in human amniotic fluid cells: A semi-quantitative comparison between different subpopulations, from primary to cultured amniocytes. Reprod Biol. 2020;20(3):338-347.
[Crossref] [Google Scholar] [PubMed]
- Hoseini SM, Montazeri F, Moghaddam-Matin M, Bahrami AR, Meimandi HH, Ghasemi-Esmailabad S, et al. Comparison of chromosomal instability of human amniocytes in primary and long-term cultures in AmnioMAX II and DMEM media: A cross-sectional study. Int J Reprod Biomed. 2020;18(10):885-898.
[Crossref] [Google Scholar] [PubMed]
- Czapla J, Matuszczak S, Kulik K, Wiśniewska E, Pilny E, Jarosz-Biej M, et al. The effect of culture media on large-scale expansion and characteristic of adipose tissue-derived mesenchymal stromal cells. Stem Cell Res Ther. 2019;10:235.
[Crossref] [Google Scholar] [PubMed]
- Gotherstrom C, West A, Liden J, Uzunel M, Lahesmaa R, Le Blanc K. Difference in gene expression between human fetal liver and adult bone marrow mesenchymal stem cells. Haematologica. 2005;90(8):1017-1026.
[Google Scholar] [PubMed]
Citation: Hoseini SM, Hosseini ES, Abessi P, Montazeri F (2024) Paracrine Secretions and Immunological Activities of Human Mesenchymal Stem Cells: The Key Regenerative Factors of Microenvironment. J Stem Cell Res Ther. 14:636.
Copyright: © 2024 Hoseini SM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

