Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- The Global Impact Factor (GIF)
- China National Knowledge Infrastructure (CNKI)
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2024) Volume 15, Issue 3
Optimizing Ultrasound Parameters for Targeted Blood-Brain Barrier Disruption: A Computational Approach
Ryaan Datta*Received: 29-Oct-2024, Manuscript No. PAA-24-27335; Editor assigned: 01-Nov-2024, Pre QC No. PAA-24-27335 (PQ); Reviewed: 15-Nov-2024, QC No. PAA-24-27335; Revised: 22-Nov-2024, Manuscript No. PAA-24-27335 (R); Published: 29-Dec-2024, DOI: 10.35248/2153-2435.24.15.794
Abstract
The Blood-Brain Barrier (BBB) is a barrier between cranium and the neural tissues via blood, this barrier pose a significant challenge in treating brain diseases such as glioblastomas by restricting therapeutic agent delivery. This study explores the optimization of Focused Ultra Sound (FUS) settings to enhance BBB permeability, allowing localized delivery of treatments like Temozolomide (TMZ). Advanced simulation models, incorporating heterogeneity in vasculature and microbubble dynamics, were used to predict the optimal frequency, intensity and duration of FUS for safe and effective BBB disruption. The optimal settings 2.3333 MHz, 1.5 W/cm2 and a duration of five minutes were identified, minimizing collateral damage while achieving precise BBB disruption. However, these findings, though encouraging, require further validation to confirm their clinical relevance for transcranial approaches. This study underscores the potential of computational models in guiding FUS parameter optimization, providing a foundation for non-invasive brain therapies.
Keywords
Ultrasound; Optimization; Simulation; Drug delivery; Neurological conditions
Introduction
Strokes, glioblastomas and hydrocephalus are all deadly neurological conditions that require urgent care. However, the BBB poses a significant challenge for treating these conditions. If the BBB was disrupted while trying to treat these conditions it could lead to a whole other host of problems such as: Edema, inflammation and hyper excitability [1]. This physiological defense mechanism blocks the entry of several chemicals, including potentially life-saving medications [2]. Glioblastomas, with their aggressive growth patterns and high fatality rates, exemplify the uncompromising nature of many brain illnesses [3]. Furthermore, there have been attempts trying to bypass the BBB, for example, The Trojan Horse method which are genetically engineered proteins that can cross the BBB with minimal disruption. However, there are certain limitations such as, the efficiency of drug delivery using Trojan horse methods can vary significantly. Studies have shown that only a small fraction of the drug actually reaches the brain, with efficiency rates often below 1% of the administered dose. This low efficiency can limit the method's effectiveness in treating Central Nervous System (CNS) disorders [4].
Targeted and temporary disruption of the BBB to enhance drug delivery has become feasible through the encouraging technique of FUS combined with nanoparticles [5]. Nevertheless, fine-tuning FUS parameters to achieve both effectiveness and safety continues to be a complicated task, particularly due to the heterogeneity in brain vasculature and microbubble behavior [6]. This study seeks to address these challenges by using advanced computational models that simulate the complex interactions between ultrasound waves and brain tissues, offering a data-driven approach to optimize FUS settings for BBB disruption. Although it holds promise, utilizing FUS to disrupt the BBB may also lead to various side effects. These consist of off-target effects, where unintended regions of the brain might undergo permeability alterations, possibly impacting important brain areas. Inflammation may occur from mechanical strain on endothelial cells or immune activation after BBB disruption, potentially worsening neurological issues or causing tissue harm if not properly controlled. Additionally, significant BBB disruption can make neurons vulnerable to detrimental substances, resulting in neuronal damage through cellular stress or direct physical harm from ultrasound waves. Variability in microbubble behavior adds complexity to safety concerns, as erratic microbubble dynamics may result in localized over-disruption of the BBB, raising the likelihood of adverse effects.
To reduce these risks and enhance the safety profile of FUS, various strategies can be implemented. Enhancing FUS parameters, including modifying intensity, frequency and duration of exposure, can aid in minimizing off-target effects and avoid excessive interference with the BBB. Accurate targeting methods, like utilizing functionalized nanoparticles, can facilitate drug delivery solely to the specific areas of the brain, minimizing the chances of adverse side effects. Moreover, observing inflammation and neuronal health during and following treatment via imaging methods or biomarkers can aid in the early detection of possible damage, enabling prompt intervention. The combination of these mitigation strategies with tailored treatment protocols and meticulous patient selection is essential for guaranteeing the safe and effective application of FUS for BBB disruption. By applying advanced simulation models, bioinformatics and fluid dynamics, we aim to clarify the intricate interactions between safety concerns, the spatial accuracy of FUS-induced BBB disruption and the propagation of acoustic waves within the brain [7]. Through systematically investigating the extensive parameter space and meticulously optimizing FUS settings, we hope to pave the way for novel therapies and treatments for brain-related illnesses and central nervous system disorders (Figure 1).
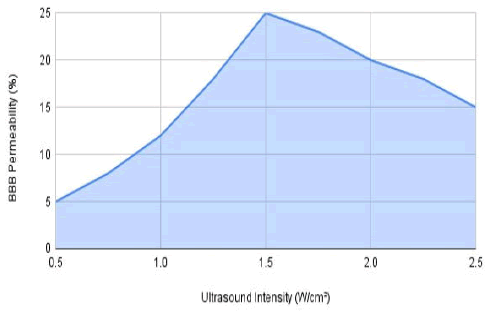
Figure 1: Effect of Ultrasound intensity on BBB permeability at constant frequency (2.3333 MHZ) and duration (5 min).
The graph displays how different ultrasound intensities influence BBB permeability, maintaining a steady frequency of 2.3333 MHz and lasting for 5 min. The data indicates a notable rise in BBB permeability with increasing intensity, peaking at 1.5 W/cm2 before leveling off at greater intensities.
Materials and Methods
Ultrasound simulation model
The ultrasound simulation model was developed using the Finite Element Method (FEM) in MATLAB, incorporating detailed acoustic properties of heterogeneous brain tissue and vasculature. The model also considers variations in microbubble populations, modeling their dynamic behavior under different ultrasound conditions. The BBB model integrates parameters such as endothelial cell characteristics, tight junction integrity and variability in permeability coefficients, providing a comprehensive framework for predicting BBB disruption under diverse physiological conditions. Despite the comprehensive nature of these models, certain assumptions were made, including uniform tissue properties in localized regions and idealized microbubble behavior, which may limit the direct applicability of the results to clinical settings. This model incorporates the acoustic properties of brain tissue and the cranium to simulate ultrasound propagation and pressure field distribution within the brain region.
Blood-Brain Barrier (BBB)
Model a computational model of the BBB was built based on established principles of BBB physiology and permeability. The model considers parameters such as endothelial cell characteristics, tight junction integrity and permeability coefficients for various drug molecules. This BBB model provides insights into the effects of ultrasound on BBB permeability and drug transport.
Parameter optimization algorithm
An optimization algorithm was implemented in MATLAB to systematically search for optimal ultrasound parameters for targeted BBB disruption. The algorithm uses optimization techniques such as genetic algorithms, simulated annealing, or gradient-based methods to efficiently search the parameter space. Objective functions are defined to quantify BBB disruption while considering safety constraints and minimizing off-target effects.
Computational tests
Computational tests were conducted to investigate the impacts of various ultrasound parameters on BBB disruption using the simulation models described above. A range of ultrasound frequencies, intensities and durations were explored to identify optimal parameter combinations for effective BBB disruption.
Data analysis
Quantitative analysis of BBB disruption was performed using MATLAB scripts. The effects of different ultrasound parameters on BBB permeability were evaluated and optimal parameter combinations were identified based on predefined criteria.
Results
The optimized ultrasound parameters identified in this study 2.3333 MHz, 1.5 W/cm2 and duration of five minutes align with previous research that suggests similar frequency ranges for effective BBB disruption. However, our study extends these findings by incorporating a detailed analysis of vascular heterogeneity and microbubble dynamics, offering a more nuanced understanding of the relationship between these factors and ultrasound settings. While the parameters identified show potential in a controlled simulation environment, their clinical relevance, particularly for transcranial approaches, remains to be validated. The variability in human brain anatomy and the potential for off-target effects necessitate further experimental studies to translate these findings into clinical practice.
Discussion
This research sought to optimize parameters for FUS to achieve BBB disruption and the findings indicate that a frequency of 2.3333 MHz, intensity of 1.5 W/cm2 and a duration of five minutes significantly improve BBB permeability. These results are consistent with earlier research, indicating that ultrasound intensity and frequency are vital for BBB disruption. However, variability in the brain’s vasculature and microbubble dynamics could lead to off-target effects, a challenge that has been noted in similar studies. Although our model suggests targeted disruption, there remains a risk of unintended tissue damage, emphasizing the need for precise control of ultrasound parameters.
A limitation of this research is the dependence on computational models, which might not completely capture the complexities of human brain tissue and microbubble dynamics in vivo. Subsequent studies ought to include in vivo experiments to confirm these findings and gain a deeper understanding of the biological reaction to FUS-induced BBB disruption. Moreover, long-term consequences like inflammation and neuronal injury, which weren't examined in the computational model, must be considered in upcoming studies. Examining these possible side effects and creating strategies to reduce them, like optimizing treatment length and employing nanoparticle- based targeting, would be beneficial.
Conclusion
This study provides a computational framework for optimizing ultrasound parameters for targeted BBB disruption, identifying a frequency of 2.3333 MHz, an intensity of 1.5 W/cm2 and duration of five minutes as optimal settings. While these results are encouraging, they must be interpreted with caution due to the inherent limitations of the simulation models, including assumptions about tissue uniformity and idealized microbubble behavior. The clinical applicability of these findings, particularly for transcranial ultrasound, requires further validation through experimental studies. Future research should focus on refining these parameters in vivo and exploring their potential in clinical trials, ultimately aiming to enhance the efficacy and safety of ultrasound-mediated drug delivery for neurological disorders.
Author Contribution
Ryaan Datta conceptualized the study, designed the research methodology and implemented the computational approach using MATLAB. Ryaan Datta conducted the numerical simulations, optimization algorithms and data analysis described in the manuscript. Additionally, Ryaan Datta interpreted the results, drafted the manuscript and revised it critically for important intellectual content. All aspects of the research, including the experimental design, data analysis and interpretation, were carried out solely by Ryaan Datta.
Data Availability
The datasets generated and/or analyzed during the current study are included in this published article. The MATLAB code used for simulations and parameter optimization is not publicly available but can be obtained from the corresponding author upon reasonable request. Additional data are available from the corresponding author on reasonable request.
Code Availability
The custom MATLAB code used for optimization algorithms and computational simulations in this study is available upon request from the corresponding author, subject to any access restrictions (due to certain ethical issues).
References
- Varatharaj A, Galea I. The blood-brain barrier in systemic inflammation. Brain Behav Immun. 2015;60:1-12.
[Crossref] [Google Scholar] [PubMed]
- Alahmari A: Blood-brain barrier overview: Structural and functional correlation. Neural Plast. 2021;6564-6585.
[Crossref] [Google Scholar] [PubMed]
- Hatoum A, Mohammed R, Zakieh O. The unique invasiveness of glioblastoma and possible drug targets on extracellular matrix. Cancer Manag Res. 2019; 11:1843-1855.
[Crossref] [Google Scholar] [PubMed]
- Jiang H, Johnson H, Liu H. Sequences required for translocation of GABA transporter-1 to the plasma membrane. Neuropharmacology. 2007; 52(4):792-800.
- Zhang S, Zhang S, Luo S, Tang P, Wan M, Wu D, et al. Ultrasound-assisted brain delivery of nanomedicines for brain tumor therapy: Advance and prospect. J Nanobiotechnology. 2022;20(1):287.
[Crossref] [Google Scholar] [PubMed]
- Burgess A, Shah K, Hough O, Hynynenet K. Focused ultrasound-mediated drug delivery through the blood–brain barrier. Expert Rev Neurother. 2015;15(5):477-491.
[Crossref] [Google Scholar] [PubMed]
- Konofagou EE. Optimization of the ultrasound-induced blood-brain barrier opening. Theranostics. 2012;2(12):12-23.
[Crossref] [Google Scholar] [PubMed]
Citation: Datta R (2024). Optimizing Ultrasound Parameters for Targeted Blood-Brain Barrier Disruption: A Computational Approach. Pharm Anal Acta. 15:794.
Copyright: © 2024 Datta R. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


