Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- ResearchBible
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2021) Volume 0, Issue 0
Occurrence and Evaluation of Antimicrobial Susceptibility of Staphylococcus aureus Isolated from Chicken Eggs, Eastern Ethiopia
Jelalu Kemal1*, Wakene Beji2 and Gebregeorgis Tesfamariam22Department of Botany and Microbiology, Jigjiga University, Jigjiga, Ethiopia
Received: 26-Feb-2021 Published: 19-Apr-2021, DOI: 10.35248/2155-9597.21.s9.003
Abstract
Staphylococcus aureus is responsible for a variety of infections in humans and animals that particularly causes staphylococcal food poisoning when it present in foods. This study was aimed to isolate Staphylococcus aureus present on the shell surfaces and in the contents of chicken eggs, and determine antimicrobial susceptibility patterns. A total of 335 egg samples were obtained from open market (n=174) and poultry farm (n=161), eastern Ethiopia. A sterile cotton swab was used to sample the surface of eggs. After sterilizing the shells, the egg contents were sampled. Identification of S. aureus was done based on culture characteristics, Gram staining and biochemical tests. The isolates were subjected to antimicrobial susceptibility testing using disc diffusion method. Out of the total 335 eggs sample examined, 93 (27.8%) samples yielded S. aureus. Out of these, 28 (17.4%) were from poultry farm while 65 (37.4%) were obtained from open market. Similarly, 63 (18.8%) were from the shell while 30 (8.9%) were from the content. The occurrence of S. aureus in the egg shell collected from open market was significantly higher than egg shell obtained from poultry farm (P=0.021). The level of S. aureus in egg contents was also significantly higher in the open market (P=0.003). All 76 S. aureus isolates were resistant to at least one of the antimicrobials tested with overall 3.9%-92.0% level of resistance pattern showing higher resistant to penicillin (92%), ampicillin (89.5%) and amoxicillin (55.3%). A lower level of resistance was observed to chloramphenicol, gentamycin and ciprofloxacin with complete susceptibility to vancomycin. Multiple drug resistance to more than two antimicrobial agents was detected in 86.8% of the total S. aureus isolates. The study showed high level of S. aureus with considerable antimicrobial resistant pattern. Further study is needed to better define bacterial resistance to antimicrobial agents with emphasis on surveillance of multiple drug resistant.
Keywords
Antimicrobial susceptibility; Egg shell; Egg content; S. aureus; Eastern Ethiopia
Introduction
Staphylococci are the most common and leading cause of foodborne outbreaks worldwide [1] and constitute a major public health burden and represent a significant cost in many countries [2]. Reports demonstrate that Staphylococcus aureus is responsible for a variety of infections in humans and animals [3]. In humans it is responsible for a number of conditions ranging from super?cial skin infections to life-threatening diseases, such as endocarditis and hemolytic pneumonia [4]. The presence of the pathogen in food is one of the most common causes of staphylococcal food poisoning and toxic shock syndrome worldwide [5]. In animals, S. aureus cause a disease responsible for signi?cant ?nancial losses to dairy farmers called mastitis [6]. Some studies conducted in Ethiopia found the occurrence of S. aureus from animal derivative food chain such as 35.8% in Adama, 12% in Jimma, 24% in Bishoftu, 29% in Borena, 58.6% in Assela and 28.7% in Addis Abeba [7-12].
Misuse of antimicrobials in food animals can generate genomic selective pressures to enable microbes to adapt and acquire resistance that has become an increasing public health issue worldwide [13,14]. Antimicrobial resistance, especially of pathogenic bacteria, has been partly attributed to the misuse of antimicrobial agents in medicine and agriculture [14]. They have been used in both human and veterinary medical practices that are widely used by the poultry industry to enhance growth and feed efficiency [15]. Incorporation of these agents into the poultry feed in poultry production poses the emergence or variability of some resistant bacteria either through genetic or nongenetic mechanisms [16]. This and the husbandry practice used in the poultry industry made poultry a major reservoir of antimicrobial resistant pathogen.
The reservoir of resistant bacteria in food animals, poultry and poultry products including eggs for consumption implies a potential risk for transfer of resistant bacteria, or resistant genes to humans. High level of antimicrobial resistance proportion in S. aureus isolates has been documented by several authors from countries such as Brazil, United States, Lebanese, Portugal and Gujarat [9].
In Ethiopia antimicrobials can be obtained without prescription [2]. For our best, the extent of S. aureus contamination of eggs sold at retail outlets and farms, and the antimicrobial profile of the S. aureus isolates has not been adequately studied. And there is very limited information available and none from eastern Ethiopia at all. The study was designed to investigate the occurrence and antimicrobial resistance patterns of S. aureus isolated from chicken eggs collected from poultry farm and the nearby retail outlets.
Materials and Methods
Study design, sample collection and transportation
A total of 335 chicken eggs from Haramaya University poultry farm (n=161) and local chicken eggs directly from the farmers who sold their eggs in the local market (n=174) in Haramaya district were undertaken from December 2017 to April 2018. Haramaya University poultry farm practiced intensive management system with exotic breed chicken. The farm aimed to supply live chicken, egg and three month-old chick to the surrounding farms, farmers and private producers. The farm supply antibiotics and other feed additives aimed to stimulate egg production, enhance growth performance and for growing healthier chicks. Some of these antibiotics and additives include egg stimulant (Medion, Bandung, Indonesia), Oxytetracycline 20% power (Chengdu Qiankun Veterinary Pharmaceutical Co.,Ltd., China), Trisulpha Forte (Jordan Vet and Agr. Med. Ind., Co., Amman, Jordan), Amprolium 20% powder (Chengdu Qiankun Veterinary Pharmaceutical Co., Ltd., China), Aminovit (Medion, Bandung, Indonesia), Laprovet (Tours Cedex 2, France) and Vita Chicks (Medion, Bandung, Indonesia). The eggs were clean, with no cracks or visible defects in the shells. Ten eggs from the market (one egg from one egg seller) and 10 eggs from Haramaya University caged white leghorn birds were collected once per week using a simple random sampling technique. Eggs were collected individually in sterile plastic bags and transported on ice in an ice box for analysis in the microbiology laboratory of the College of Veterinary Medicine at Haramaya University within few hours of collection.
Sample processing
The sterile plastic bags containing sampled eggs were opened with scissors and were processed immediately. Sterile cotton swabs dipped into sterile buffered peptone water (BPW) were used to swab the entire surface area of the egg shell. The swabs were then incubated separately in a test tube that contained 10 mL BPW (Oxoid Ltd, Hampshire, UK; Lab M Ltd., Quest Park, UK). The same eggs from which the shell samples were collected were used for interior (egg content) sampling. The egg's surface was sterilized by immersing the egg in 70% alcohol for 2 minutes; the egg was then air dried in a sterile chamber for 10 minutes, and then cracked with a sterile scalpel blade. The egg contents were homogenized in stomacher bags containing 225 mL sterile BPW for 1 minute in a stomacher and incubated at 37°C for 18-24 h.
The samples were then transferred onto blood agar plates containing 5% sheep blood (Oxoid Ltd, Hampshire, UK; Lab M Ltd., Quest Park, UK) and then incubated under aerobic conditions at 37°C for 24-48 h, depending on the rate of growth of the bacteria. An initial bacteriological characterization was performed by evaluating the morphology of the colonies and the presence and type of haemolysis. S. aureus identification was done based on Gram staining, morphology, and traditional biochemical tests, including catalase, coagulase and mannitol fermentation tests as described [25,26].
Antimicrobial susceptibility test
The S. aureus isolates were tested for antimicrobial susceptibility by the Kirby-Bauer disc agar diffusion method on Mueller-Hinton medium (Oxoid Ltd, Hampshire, UK; Lab M Ltd., Quest Park, UK), according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI) [2]. The antimicrobial discs (Oxoid Ltd., Cambridge, UK) were selected in line with the recommendation of CLSI; ampicillin (10 µg/disc), amoxicillin (20 µg/disc), chloramphenicol (30 µg/disc), penicillin G (10 IU/disc), tetracycline (30 µg/disc), gentamicin (10 µg/disc), cefoxitin (30 µg/disc), erythromycin (15 µg/disc), streptomycin (10 µg/disc), kanamycin (30 µg/disc), ciprofloxacin (5 µg/disc), and trimethoprim-sulfamethoxazole (SXT, 25 µg), vancomycin (30 µg/disc). The antimicrobials used were selected from the currently available and commonly used chemotherapeutic agents for the treatment of S. aureus infection in humans and animals in Ethiopia including Haramaya University farms. S. aureus ATCC 29213 and S. aureus ATCC 25923 were used as quality control during the test. The results were read and interpreted based on the diameter of the zone of inhibition. The strains were designated as resistant (R), intermediate resistant (I), or susceptible (S). Multiple drug resistant (MDR) were recorded for isolates showing resistance to more than two antimicrobials [8].
Data management and analysis
The data were entered into Excel databases and analyze using STATA version 11.0 statistical software package programs. Descriptive statistics such as percentages and frequency distribution was used to describe the nature and the characteristics of the data. Comparison between sample source and sample type was done by Chi-square (χ2). Logistic regression was used to reveal the strength of the association of the potential risk factors with positivity of sample. In this line, the degree of association between risk factors and the prevalence of S. aureus was analyzed using test odds ratio (OR). In all the analysis, the level of significance was set at 5% and at the 95% confidence interval.
Results
Occurrence of S. aureus spp. in raw chicken egg shell and egg contents
Out of the 335 eggs sample examined for bacteriological status obtained from farms and open market, 93 (27.8%) samples yielded S. aureus. The levels of S. aureus varied among the sampling sites and types. Out of the 93 (27.8%) positive egg samples, 63 (18.8%) were from the shell while 30 (8.9%) were from the content. Of these 93 positive samples, 28 (17.4%) were from poultry farm while 65 (37.4%) were obtained from open market (retail outlets). The occurrence of S. aureus in the egg shell collected from local open market was significantly higher than the level of S. aureus in egg shell obtained from poultry farm (CI=0.2904-0.9078; p=0.021). The level of S. aureus in egg contents from the open market was also significantly higher than the level of S. aureus in egg contents from the poultry farm (CI=0.0962-0.6085; p=0.003). Similarly, the level of S. aureus in open market was significantly higher than the level of S. aureus in eggs obtained from the poultry farm (CI=0.2591-0.7585; p=0.003).
Antimicrobial susceptibility testing
In the antimicrobial resistance trials, out of 93 S. aureus isolates, 76 (81.7%) were subjected to antimicrobial resistance test. All the isolates showed antimicrobial resistance properties to at least one of the antimicrobials tested. The percentage of isolates susceptible, intermediate, and resistant to each antimicrobial agent is outlined. Overall, S. aureus isolates revealed 3.9-92.0% level of resistance pattern to the antimicrobials tested. A large proportion of the isolates were resistant to penicillin (92%), ampicillin (89.5%), amoxicillin (55.3%) and erythromycin (51.3%). A lower level of resistance was observed to chloramphenicol, gentamycin and ciprofloxacin with resistance level of 3.9% each. All the S. aureus isolates were susceptible to vancomycin (100%).
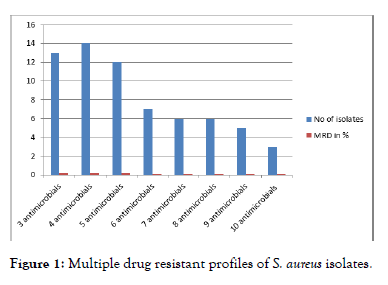
The level of multiple resistance patterns in S. aureus isolates is given. Multiple drug resistance to more than two antimicrobial agents was detected in 66 (86.8%) of the total 76 S. aureus isolates. Three isolates (4.5%) were resistant to ten antimicrobials tested. Fourteen isolates were resistant to 4 antimicrobials tested. Multiple drug resistance was defined as resistance exhibited to more than two antimicrobials tested. Among the S. aureus isolates, 19.7%, 21.2%, and 18.2% were expressed resistance to three, four, and five antimicrobials, respectively (Figure 1).
Figure 1: Multiple drug resistant profiles of S. aureus isolates.
Discussion
The presence of pathogenic bacteria in food, including table chicken eggs, may pose a serious health problem (food poisoning and foodborne infections) [2,3]. Even though eggs are foods with high value of nutrition, they are an excellent source of nourishment for many pathogens. Bacteria can contaminate the egg and chickens are infected in diverse means such as during its development in the reproductive system, directly after hatching, during storage and transport, or even while prepared for consumption [1]. Among the most widespread foodborne infections directly connected with egg consumption are due to toxins related to S. aureus infections. Our study revealed high level of S. aureus contamination of chicken table eggs with accounting for 27.8% of isolates. In a study, 19.8% of the total Staphylococcus species isolated from table eggs descended from different source were S. aureus. In another study done on chicken table eggs, 18 (17.4%) S. aureus strains were reported [3].
The available literature shows that while these bacteria are isolated from eggs with varying frequency depending on geographical location, they can pose a serious threat to consumer health by inducing food poisoning. In France, for instance, a fairly high percentage (11%) of cases of food poisoning in 1999-2000 resulted from eating eggs and egg products contaminated with staphylococci [2]. Analysis of the epidemiological situation of food poisoning and foodborne infections in Poland showed that 25% of food poisoning cases induced by S. aureus in humans in 2009 were caused by consumption of table eggs [9]. He demonstrated S. aureus was the second most numerous Staphylococcus species isolated from eggs.
In our study, although most of the S. aureus strains were isolated from shells 18.8% (n=63), considerable number of the pathogen, 8.9% (n=30), were isolated from the contents that varied with significantly. Pyzik and Marek reported fairly high rate of S. aureus isolates on the shells of the eggs (55.5%) than the contents (27.8%) from Poland. In contrary to our study reported less isolates of S. aureus on the eggs shells (10.4%) than the contents (35.2%) that were collected from large and small scale poultry farms and eggs purchased from supermarkets [1]. These variations might be due to different sampling techniques, areas, time, storage practice, production practices difference among farms or countries and the low isolation rate of culture methods compared to more sensitive immunological and molecular methods.
Higher numbers of S. aureus isolates were detected in egg samples collected from open market 65 (37.4) than eggs from Haramaya University poultry farm 28 (17.4). There was a significant difference between the sample sources and sample type with the occurrence of S. aureus. This variation might be due to differences in biosecurity or sanitation practices among the two egg sample collection sources and type of the sample. Eggs collected from hens kept in cage system have been less likely exposed to pathogens than those kept in litter system and retail outlets or open markets. According to fewer Staphylococcus species were isolated from eggs collected at large scale poultry farms than eggs obtained from supermarkets. There are several points that contribute for the contamination of eggs with microorganisms through reaching to the consumers such as the environment, storage condition, transport and handling practices of the egg by egg handlers [1].
Another important point that has a serious threat to consumer health with a global concern is antimicrobial resistance of S. aureus strains isolated from eggs. In this study, all the isolates showed antimicrobial resistance properties to at least one of the antimicrobials tested. A large proportion of the isolates were resistant to penicillin (92%), ampicillin (89.5%), and amoxicillin (55.3%) followed by erythromycin (51.3%). All (100%) of the S. aureus isolates showed susceptibility to the vancomycin among the total 13 antimicrobials tested. A higher level of susceptibility was also observed to chloramphenicol, ciprofloxacin and gentamycin with the same proportion of (93.4%) each. Higher susceptibility of the isolates was also detected in trimethoprim-sulfamethoxazole (90.8%), cefoxitin (89.5%) and kanamycin (75%). A relatively high percentage of the S. aureus isolates were also found to be resistant to streptomycin (35.5%) and tetracycline (34.2%). He reported similar finding that all isolates of S. aureus were susceptible to vancomycin followed by chloramphenicol (97.5%). Comparatively, highest level of resistance was detected in their study in penicillin (95%) and ampicillin (92.5%). Similarly, displayed 100% susceptibility to vancomycin and cefoxitin with a higher rate to chloramphenicol.
Comparably, found all the S. aureus isolates tested in their study were susceptible to chloramphenicol and gentamicin. Among the isolates of S. aureus, the most frequently observed resistance patterns was to amoxicillin, penicillin G, erythromycin, and tetracycline which is in line with the current study. Vancomycin and gentamicin susceptibility of the isolates we found were in agreement with the reports in some other studies of different countries. The higher resistance frequency against beta-lactams, penicillin, ampicillin and amoxicillin, among the isolates from chicken eggs could be owing to the extensive and uncontrolled use of these group antibiotics in agriculture sector.
He had reported cefoxitin and trimethoprim- sulfamethoxazole susceptibility rate to 93 (94.9%) and 82 (83.7%) tested S. aureus isolates respectively which is comparable to our finding [2]. Our current study finding is distantly related to the finding who found 69.4% of S. aureus isolates susceptibility to tetracycline. In some other studies S. aureus were obtained resistance to erythromycin (1.7%–100%), tetracycline (5%–84%), ciprofloxacin (0%–42%) and vancomycin (9%–46%) in different percentages. High penicillin resistance S. aureus isolates was also identified in other parts of the world. In addition, considerable numbers of the isolates were found to be intermediately resistant to amoxicillin, chloramphenicol ciprofloxacin, gentamycin, kanamycin, penicillin, streptomycin and tetracycline. This susceptibility and resistance difference among the isolates may be due to the widely and indiscriminate use of antibiotics for therapeutic and prophylactic purposes at poultry farms in different countries.
Multidrug resistance to more than two antimicrobial agents was detected in 66 (86.8%) of the total 76 S. aureus isolates. The resistance pattern most frequently observed in the isolates was resistance to ampicillin in combination with penicillin, erythromycin and amoxicillin. Among the S. aureus isolates, 21.2%, 19.7%, 18.2% and 9.9% expressed resistance to four, three, five and six antimicrobials, respectively, and resistance to seven, eight, nine and ten antimicrobials occurred at a frequency of 9.9%, 9.9%, 7.6% and 4.5% respectively. 13.2% of the isolates showed resistance to two antimicrobials while no isolates displayed resistance to single antimicrobial.
Similar finding was reported with multiple drug resistance on 89.3% of the total isolates tested. Barena and Fetene and Chao reported nearly similar rate of multi-drug resistant S. aureus (80%) and (79%) with the present investigation respectively. The current finding was slightly higher who reported 60%-70% of the isolates were observed with multiple drug resistance. Such high incidence of multidrug resistance may apparently have occurred due to indiscriminate use of antimicrobial agents which enhances the development of drug resistance. The multiple drug resistance observed in the current study might also be mediated by genetic mobile elements such as plasmids, transposons, and integrons as seen in other studies.
Conclusion
The current findings obtained indicate that considerably high percentage of the chicken eggs tested was contaminated with S. aureus. Egg shells had significantly higher level of S. aureus compared to egg contents. A significant variation of infection was also recorded in open market than Haramaya University poultry farm. Detection of such high prevalence of S. aureus in this study indicates a potential risk for causing food poisoning. The present study has demonstrated the existence of alarming level of resistance of S. aureus to commonly used antimicrobial agents in veterinary and human practices such as ampicillin, amoxicillin, penicillin and erythromycin in chicken eggs obtained from poultry farm and open market. The vast majority of S. aureus isolates showed multiple drugs resistance for three to nine antimicrobials tested. The presence of high prevalence of S. aureus and multiple drug resistance isolates is alarming because this could have a significant public health consequence if these microorganisms are transmitted to humans through food chain. Therefore, additional research is required with continuous surveillance and monitoring of pathogens to better define bacterial resistance to antimicrobial agents with emphasis on surveillance of multiple drug resistant S. aureus isolates.
REFERENCES
- Khedr MMS, El-Seedy FR, Shafei SM, Shell WSA, El-Mahdy SS, Sadek MA. Preparation and evaluation of combined inactivated vaccine against salmonellosis and colibacillosis in chicken. Glob Vet. 2015;14(2):205-210.
- Mahdavi M, Jalali M, Safaei HG, Shamloo E. Microbial quality and prevalence of Salmonella and Listeria in eggs. Int J Environ Health Eng. 2013;1(6):16-20.
- Hata E, Katsuda K, Kobayashi H, Nishimori K, Uchida I, Higashide M, et al. Bacteriological characteristics of Staphylococcus aureus isolates from humans and bulk milk. J Dairy Sci. 2008;91(3):564–569.
- Pyzik E, Marek A, Hauschild T. Characterisation of Staphylococcus aureus and Staphylococcus aureus–like strains isolated from table eggs. Bull Vet Inst Pulawy. 2014;58(1):57-63.
- Fitzgerald JR. Livestock-associated Staphylococcus aureus: origin, evolution and public health threat. Trends Microbiol. 2012;20(6):192–198.
- Mekonnen H, Tesfaye A. Prevalence and etiology of mastitis and related management factors in market oriented smallholder dairy farms in Adama, Ethiopia. Revue Méd Vét. 2010;161(12):574-557.
- Haimanot T, Alemseged A, Getenet B, Solomon G. Microbial flora and food borne pathogens on minced meat and their susceptibility to antimicrobial agents. Ethiop J Health Sci. 2010;20(3)-137-143.
- Mekonnen A, Mahindra P, Moses NK. Isolation and Identification of Staphylococcus Species from Ethiopia Cottage Cheese (Ayib) in Debre Zeit, Ethiopia. Vet Res. 2011;4(1):13-17.
- Bedane A, Kasim G, Yohannis T, Habtamu T, Asseged B, Demelash B. Study on Prevalence and Risk Factors of Bovine Mastitis in Borana Pastoral and Agro-Pastoral Settings of Yabello District, Borana Zone, Southern Ethiopia. American-Eurasian J Agric Environ Sci. 2012;12(10):1274-1281.
- Birhanu A, Diriba L, Iyob I. Study of bovine mastitis in Asella government dairy farm of Oromia Regional state, South Eastern Ethiopia. Int J Curr Res Aca Rev. 2013;1(2):134-145.
- Zeryehun T, Aya T, Bayecha R. Study on prevalence, bacterial pathogens and associated risk factors of bovine mastitis in small holder dairy farms in and around addis ababa, Ethiopia. J Anim Plant Sci. 2013;23(1):50.
- Kohinur B, Tanvir AR, Margia H, Akil H, Kabirul H, Shaik Nahid H, et al. Isolation, identification and antimicrobial resistance pattern of Salmonella spp. from chicken eggs, intestines and environmental samples. Bangladesh Pham J. 2010;13(1):23-27.
- Michael CA, Dominey-Howes D, Labbate M. The antibiotic resistance crisis: Causes, consequences and management. Front Public Health. 2014;2(2):145.
- Landers TF, Cohen B, Witlum TE, Larson EL. A review of antibiotic use in food animals: Perspective, policy and potential. Public Health Rep. 2012;127(1):4-22.
- Ivanov I. Disinfection of eggs contaminated with some fungal moulds. Trakia J Sci. 2008;6(1):98-101.
- Crump JA, Griffin M, Angulo FJ. Bacterial contamination of animal feed and its relationship to human foodborne illness. Clin Infect Dis. 2002;35(2):859-865.
Citation: Kemal J, Beji W, Tesfamariam G (2021) Occurrence and Evaluation of Antimicrobial Susceptibility of Staphylococcus aureus Isolated from Chicken Eggs, Eastern Ethiopia. J Bacteriol Parasitol. S9: 003.
Copyright: © 2021 Kemal J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.