Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- ResearchBible
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2020) Volume 11, Issue 2
Novel Strategy of Photodynamic Therapy Targeting RAS mRNA with G-Quadruplex Ligands for Cancer Treatment
Takeru Torii, Wataru Sugimoto, Keiko Kawauchi* and Daisuke Miyoshi*Received: 08-Jul-2020 Published: 29-Jul-2020
Abstract
The constitutive activation of RAS proteins is closely linked with the aggressiveness of cancer cells. Although RAS proteins are effective targets for cancer therapy, the development of drugs targeting RAS proteins has been unsuccessful so far due to the absence of a suitable active site for small molecule drugs. RAS mRNA that forms a Gquadruplex (G4) in the 5′-untranslated region (UTR) has been deemed as a druggable target. In this study, we demonstrated the advantages of photodynamic therapy (PDT) for the treatment of cancer using G4 ligands targeting RAS mRNA, including our previously reported ligand anionic phthalocyanine ZnAPC.
Keywords
G-quadruplex; RAS; RNA; Photodynamic therapy; Genotoxic stresses
Introduction
Photodynamic therapy (PDT) is a minimally invasive treatment based on the cytotoxic activities of photosensitizers that can be controlled by photo-irradiation [1,2].
The characteristics of photosensitizers used in PDT are as follows:
• Photo-irradiated photosensitizers exhibit high cytotoxicity via the generation of reactive oxygen species (ROS)
• The photosensitizers are activated by near-infrared light to penetrate deeper into target tissues; this characteristic is linked to low side effects in normal tissues, as the activation of endogenous chromophores is induced by a shorter wavelength
• The photosensitizers selectively accumulate in the cancerous tissue and exhibit low cytotoxicity in the absence of photoirradiation [1].
In this Research, we describe potential photosensitizers for a new strategy of PDT that involves the use of G4 ligands. Porphyrins and their analogs, phthalocyanines, are well-known G4 ligands that are primarily used as photosensitizers in PDT [3,4]. Given the fact that several derivatives of porphyrin and phthalocyanine cleave RAS mRNA G4 upon photo-irradiation [5-7], such compounds might be useful for specifically targeting RAS in the tumor tissue during PDT.
Materials and Methods
Cell culture and materials
MCF-7 human breast cancer cells were cultured as described in a previous report [7]. ZnAPC [zinc (II) phthalocyanine 3,4′,4′′,4′′′- tetrasulfonic acid tetrasodium salt] and Etoposide (VP16) were purchased from Frontier Scientific and Calbiochem, respectively.
Fluorescence microscopy
Immunostaining of Ser139-phosphorylated H2AX (henceforth used as γH2AX) was performed as described previously [8]. Images were obtained using a confocal microscope (A1R HD25, Nikon) and analyzed using ImageJ software (NIH).
Results and Discussion
Mutations in genes encoding the canonical RAS family proteins HRAS, KRAS, and NRAS often occur during tumor progression, resulting in the aberrant activation of RAS-signaling pathways [9]. G4–forming guanine-rich sequences found in the 5′-UTRs of KRAS and NRAS are different from the mutational hotspots [6,10]. Ligands targeting such G4–forming sequences transcribed in the mRNAs of RAS are expected to prevent RASdriven cancer progression via the inhibition of the transcription and translation of RAS genes. Since certain G4 ligands, such as porphyrins and phthalocyanines, can act as photosensitizers [3,4], the development of a novel PDT modality targeting RAS mRNA G4s with light-sensitive G4 ligands is possible; we termed this method as molecularly-targeted (mt) PDT [11].
The topologies of G4 are classified as antiparallel, parallel, and hybrid orientations [12]. Almost all RNA G4s adapt the parallel G4 topology while DNA G4 topology depends on the factors affecting its environment, such as cation species and their concentrations, and molecular crowding [13]. Moreover, the application of DNA G4 ligands might lead to genotoxic damage. Hence, targeting RNA G4 sequences instead of DNA G4s using photosensitizers will yield favorable results.
Xodo et al. have shown that the cationic alkyl-substituted porphyrins including tri-meso(N-methyl-4-pyridyl)-meso(Ntetradecyl- 4-pyridyl), commonly named as TMPyP4-C14, which is a derivative of a well-known G4 stabilizing agent tetra-meso(Nmethyl- 4-pyridyl) (TMPyP4), can be the candidate photosensitizer targeting KRAS and NRAS mRNAs [5,6,14]. TMPyP4 leads to DNA damage response and exhibits cytotoxicity even in the absence of photo-irradiation [15]. This indicates that TMPyP4 is not suitable as a photosensitizer in PDT. In contrast, TMPyP4-C14 has low cytotoxicity in the absence of photo-irradiation and its cellular uptake is significantly higher than that of TMPyP4 [5,6,14]. When photoirradiated, TMPyP4-C14 cleaves the G4s in mRNAs transcribed from KRAS and NRAS genes via the generation of ROS. It is suggested that photo-irradiated TMPyP4-C14 prevents cancer growth through the activation of apoptotic pathways, as well as the suppression of RAS-driven aggression of cancer cells via ROS production (Figure 1).
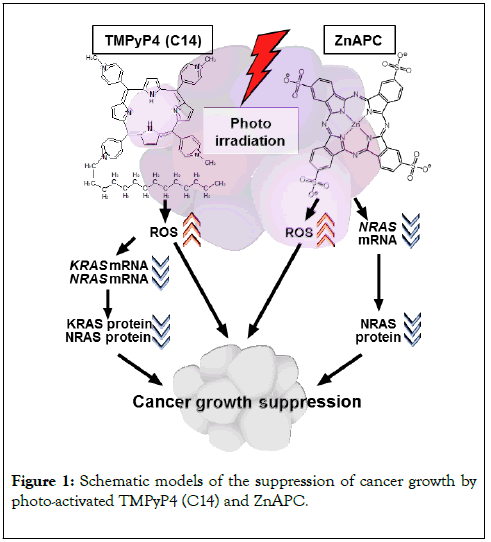
Figure 1. Schematic models of the suppression of cancer growth by photo-activated TMPyP4 (C14) and ZnAPC.
We have demonstrated the possibility of anionic an phthalocyanine coordinating Zn2+, ZnAPC, being a prominent photosensitizer targeting NRAS mRNA [7]. To confirm the low cytotoxicity of ZnAPC in the absence of photo-irradiation, we examined a DNA damage marker γH2AX by immunostaining. The levels of γH2AX expression could confirm whether ZnAPC leads to a DNA damage response in cancer cells (Figure 2). The amount of γH2AX increased in response to a DNA damaging agent VP16 used as a positive control. However, no such results were observed with the ZnAPC treatment. This result shows that ZnAPC does not exhibit a genotoxic effect unlike other G4 stabilizing agents, such as TMPyP4. Moreover, a crucial feature of ZnAPC indicates that the cleavage mechanism for the NRAS mRNA-G4 sequence is not dependent on oxygen [7]. Taken together, these results support the notion that in the presence of photo-irradiation, ZnAPC can prevent cancer growth via the suppression of NRAS expression. This effect can be observed even under hypoxic conditions or when the cancer cells acquire resistance against ROS, which is frequently observed (Figure 2).

Figure 2. ZnAPC does not induce DNA damage in the absence of photo-irradiation. MCF-7 cells were treated with ZnAPC (10 μM) and VP16 (100 μM) as a positive control for 24 h. Confocal image of cells stained with an antibody against the DNA damage marker γH2AX (red) and DAPI for observing DNA (blue). Scale bar, 20 μm.
Conclusion
The mtPDT targeting RAS mRNA with the aforementioned photosensitive G4 ligands will exhibit stronger cytotoxic effects against cancer cells than the effects exerted by conventional PDT. Furthermore, the efficiency of the procedure will be observed for the cancer types that cannot be cured by the conventional PDT.
Acknowledgments
We would like to thank Dr. Hisae Tateishi-Karimata and Mr. Chikara Maeda for discussion and Editage (www.editage.com) for English language editing. This work was supported by JSPS KAKENHI Grant Numbers JP18K19153, JP19J21096, 20K21259 and 17H06351(Grant-in-Aid for Scientific Research on Innovative Areas “Chemistry for Multimolecular Crowding Biosystems])”, the research grant from Research Grant of the Princess Takamatsu Cancer Research Fund, and the Asahi Glass Foundation, Japan.
REFERENCES
- Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nature rev Cancer. 2003;3(5):380-7.
- Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, et al. Photodynamic therapy of cancer: an update. CA: a cancer J clin. 2011;61(4):250-81.
- Yaku H, Murashima T, Miyoshi D, Sugimoto N. Specific binding of anionic porphyrin and phthalocyanine to the G-quadruplex with a variety of in vitro and in vivo applications. Mol (Basel, Switzerland). 2012; 17:10586-613.
- O'Connor AE, Gallagher WM, Byrne AT. Porphyrin and nonporphyrin photosensitizers in oncology: preclinical and clinical advances in photodynamic therapy. Photochemphotobiol. 2009;85(5):1053-74.
- Rapozzi V, Zorzet S, Zacchigna M, Della Pietra E, Cogoi S, Xodo LE. Anticancer activity of cationic porphyrins in melanoma tumour-bearing mice and mechanistic in vitro studies. Mol cancer. 2014;b13:75.
- Ferino A, Nicoletto G, D'Este F, Zorzet S, Lago S, Richter SN, et al. Photodynamic Therapy for ras-Driven Cancers: Targeting G-Quadruplex RNA Structures with Bifunctional Alkyl-Modified Porphyrins. J MedChem. 2020;63(3):1245-60.
- Kawauchi K, Sugimoto W, Yasui T, Murata K, Itoh K, Takagi K, et al. An anionic phthalocyanine decreases NRAS expression by breaking down its RNA G-quadruplex. Nature Communicat. 2018;9(1):2271.
- Itoh K, Ebata T, Hirata H, Torii T, Sugimoto W, Onodera K, et al. DMPK is a New Candidate Mediator of Tumor Suppressor p53-Dependent Cell Death. Mol (Basel, Switzerland). 2019;24(17).
- Hobbs GA, Der CJ, Rossman KL. RAS isoforms and mutations in cancer at a glance. JCell Sci. 2016;129(7):1287-92.
- Kumari S, Bugaut A, Huppert JL, Balasubramanian S. An RNA G-quadruplex in the 5' UTR of the NRAS proto-oncogene modulates translation. Nat ChemBiol. 2007;3(4):218-21.
- Bugaut A, Rodriguez R, Kumari S, Hsu ST, Balasubramanian S. Small molecule-mediated inhibition of translation by targeting a native RNA G-quadruplex. Org BiomolChem. 2010;8(12):2771-6.
- Ma Y, Iida K, Nagasawa K. Topologies of G-quadruplex: Biological functions and regulation by ligands. BiochemBiophylResCommunicat. 2020.
- Xiao CD, Ishizuka T, Xu Y. Antiparallel RNA G-quadruplex Formed by Human Telomere RNA Containing 8-Bromoguanosine. ScientRep. 2017;7(1):6695.
- Faudale M, Cogoi S, Xodo LE. Photoactivated cationic alkyl-substituted porphyrin binding to g4-RNA in the 5'-UTR of KRAS oncogene represses translation. ChemCommunicat (Cambridge, England). 2012;48(6):874-6.
- Fujimori J, Matsuo T, Shimose S, Kubo T, Ishikawa M, Yasunaga Y, et al. Antitumor effects of telomerase inhibitor TMPyP4 in osteosarcoma cell lines. J OrthopaedRes. 2011;29(11):1707-11.
Citation: Torii T, Sugimoto W, Kawauchi K, Miyoshi D (2020) Novel Strategy of Photodynamic Therapy Targeting RAS mRNA with GQuadruplex Ligands for Cancer Treatment. J Data Mining Genomics Proteomics.11:226. DOI:10.35248/2153-0602.20.11.226.
Copyright: © 2020 Torii T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

