Indexed In
- RefSeek
- Hamdard University
- EBSCO A-Z
- Publons
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 8, Issue 5
Non-Association of Visceral Adipose Macrophage Content, Body ,Mass Index and Inflammatory Markers in COVID-19
Steven H Su1*, Yael R Nobel1, Sepideh Besharati2, Armando del Portillo2, Michaela R Anderson3 and Daniel E Freedberg1*2Department of Pathology and Cell Biology, Columbia University Irving Medical Center, New York, NY 10032, USA
3Department of Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA
Received: 24-Aug-2023, Manuscript No. JIDD-23-22703; Editor assigned: 28-Aug-2023, Pre QC No. JIDD-23-22703 (PQ); Reviewed: 11-Oct-2023, QC No. JIDD-23-22703; Revised: 18-Sep-2023, Manuscript No. JIDD-23-22703 (R); Published: 25-Oct-2023, DOI: 10.35248/2576-389X.23.08.232
Abstract
Introduction: Adiposity, especially visceral adiposity with elevated Body Mass Index (BMI), is associated with a hyperinflammatory syndrome and poor outcomes in patients with COVID-19. In other diseases such as obesity, type 2 diabetes, and rheumatoid arthritis, systemic inflammation is driven directly by visceral adipose macrophages which release pro-inflammatory cytokines. Currently it is unknown whether visceral adipose tissue macrophage content may similarly explain the observation that COVID-19 patients with elevated BMI are at risk for a hyperinflammatory syndrome and death.
Methods: This was a retrospective study of hospitalized adults who died of COVID-19 between March 2020 and June 2020 and underwent autopsy. Visceral adipose tissue macrophage content was quantified by histological staining of visceral adipose tissue samples with CD68, using pericolic fat gathered at autopsy from each subject. Clinical data including inflammatory markers such as Erythrocyte Sedimentation Rate (ESR), C-Reactive Protein (CRP), Troponin, D-dimer, Interleukin-6 (IL-6), and ferritin as well as BMI were collected from electronic medical records.
Results: A total of 39 subjects were included in this study. There was no association between BMI and visceral adipose tissue macrophage content (Spearman R=0.025, p=0.88). Additionally, there was no association between adipose tissue macrophage content and any of the systemic markers of inflammation measured including ESR, CRP, Troponin, D-dimer, IL-6, and Ferritin (p>0.05 for all markers).
Conclusion: Unlike chronic diseases such as obesity, type 2 diabetes, and rheumatoid arthritis, elevated BMI is not associated with increased visceral adipose tissue macrophage content in patients who died of COVID-19. Additionally, among patients who died of COVID-19, visceral adipose tissue macrophage content is not associated with markers of systemic inflammation. These results suggest that the elevations in systemic markers of inflammation and the hyperinflammatory syndrome often observed during acute COVID-19-does not directly originate from visceral adipose macrophages as it seems to in chronic disease states.
Keywords
COVID-19; Type 2 diabetes; Rheumatoid arthritis; Erythrocyte Sedimentation Rate (ESR); C- Reactive Protein (CRP)
Introduction
Since the onset of the COVID-19 pandemic, numerous studies have demonstrated an association between increased Body Mass Index (BMI) and increased visceral adiposity with poor outcomes in patients with COVID-19 including increased likelihood of hospitalization, Intensive Care Unit (ICU) admission, invasive mechanical ventilation, and death [1-3]. Obesity, especially when associated with increased visceral adiposity, has been shown to increase systemic inflammation in a variety of illnesses including COVID-19 [1-8]. More specifically, previous work has demonstrated a positive association between visceral adiposity and serum biomarkers of systemic inflammation including C- Reactive Protein (CRP), Erythrocyte Sedimentation Rate (ESR), Interleukin-6 (IL-6), and Ferritin in a variety of diseases including COVID-19 [9-14].
In obesity and diseases other than COVID-19 such as type 2 diabetes mellitus and rheumatoid arthritis, systemic inflammation is driven by visceral adipose macrophages through their direct release of inflammatory cytokines including Tissue Necrosis Factor-α (TNF-α) and IL-6 [13,15-18]. Interestingly there is also an established association between elevations in inflammatory serum biomarkers and the severity of COVID-19 illness yet there remains a question of whether adiposity and specifically visceral adipose macrophage content drives systemic inflammation in COVID-19 [14]. Given the established relationships between BMI and adipose tissue macrophage content and between adipose tissue macrophage content and systemic inflammation in diseases other than COVID-19, we sought to determine whether elevated BMI is associated with increased visceral adipose macrophage content and whether increased visceral adipose macrophage content is associated with increased serum biomarkers of inflammation in patients with COVID-19.
In this study, we quantified adipose tissue macrophage content in patients who died from COVID-19 through autopsy and also obtained relevant exam and laboratory values including BMI and serum inflammatory markers including ESR, CRP, Troponin, D-dimer, IL-6, and Ferritin to investigate the potential relationships between BMI and adipose macrophage content and between adipose macrophage content and systemic inflammation.
Materials and Methods
Patient population
Subjects included in this study tested positive for SARS-CoV-2 by nasopharyngeal PCR and subsequently died of COVID-19 between March 2020 and June 2020 at Columbia University Irving Medical Center. All subjects were age 18 or older and underwent autopsy at the request of next of kin. All protocols utilized in this study were approved by the Columbia University Institutional Review Board and by the Columbia University COVID-19 Biobank (CUB).
Quantification of visceral adipose tissue macrophage content
Paraffin-embedded tissue sections of colon from 45 COVID-19 autopsy cases were retrieved from the Columbia University Department of Pathology. The microscopic slides from each block were immunolabeled with an antibody against CD68 (Agilent, M087629-2) diluted at 1:50 in antibody diluent (Agilent, S080983- 2) per manufacturer’s instructions. Each slide was digitally scanned using a Leica SCN400 whole slide digital imaging system (Leica Biosystems, USA/Germany) at 20X magnification. Using the Aperio Image Scope program (Leica Biosystem Pathology Imaging Inc, Buffalo Grove, IL), images was analyzed. Four areas with pericolic adipose tissue were selected randomly. The numbers of positive CD68 macrophages were quantified within the selected areas at highest magnification. The median number of CD68- positive macrophages was computed as the visceral adipose macrophage content for each patient.
BMI, Laboratory values and Demographics
BMI, date of death, most recent ESR, CRP, Troponin, D-dimer, IL-6 and Ferritin serum laboratory values prior to death and demographics including age, sex, and race were obtained from electronic medical records for each subject. Our rationale for using the most recent laboratory values prior to death was that these values would be more likely to reflect visceral adipose tissue macrophage content compared to (e.g.) peak values for the same labs which may have been from weeks prior. In a secondary analysis, we also examined peak values of the same inflammatory markers during the hospitalization.
Statistical analysis
Spearman correlation and p-values were computed for continuous data. For analysis of serum inflammatory markers values (ESR, CRP, Troponin, D-dimer, IL-6 and Ferritin), patients were grouped into tertiles based on visceral adipose macrophage content and p-values were computed using a Kruskal-Wallis test to test for differences within each serum inflammatory marker based on tertiles of macrophage content. All statistical analysis was performed using RStudio software version 2021.09.0-351 running R version 4.1.1.
Results
Study population
Out of 45 patients who underwent autopsy, 6 were excluded because no BMI was recorded during the hospitalization or at autopsy leaving 39 patients for inclusion in the study. Clinical characteristics of these subjects are shown in Table 1. The median age was 72 with Interquartile Range (IQR) 67-76. The majority of subjects were male and Hispanic with a median BMI of 26.5 kg/m2 (IQR 24.7-29.8) and a median adipose macrophage count of 11.5 macrophages/High Power Field (HPF) (IQR 8.25-13.5).
| Characteristics | Total (n=39) |
|---|---|
| Age, Median (IQR) | 72(67-76) |
| Sex, n (%) | |
| Male | 26(66.7%) |
| Female | 13(33.3%) |
| Race/Ethnicity, n (%) | |
| Hispanic | 25(64.1%) |
| Black Non-Hispanic | 2(5.1%) |
| White | 1(2.6%) |
| Other | 11(28.2%) |
| BMI, Median (IQR) | 26.5(24.7-29.8) |
| Visceral adipose tissue macrophage | |
| Content, Median (IQR) | 11.5(8.25-13.5) |
Note: IQR (Interquartile Range)
Table 1: Demographic and obesity-related characteristics among 39 patients with COVID-19 who died and underwent autopsy.
Disassociates BMI and adipose tissue macrophage content in patients who died of COVID-19
To quantify visceral adipose tissue macrophage content, pericolic visceral adipose tissue was obtained from each subject at autopsy (Figure 1). Tissue sections were stained with both Hematoxylin and Eosin as well as with CD68, a macrophage marker. Representative images of staining are shown in Figure 1A-1B. To examine the potential association between BMI and adipose tissue macrophage content, we plotted BMI versus adipose tissue macrophage content (Figure 1C). Upon visual inspection there appeared to be no clearly discernable relationship between the two and this apparent lack of association was supported by a Spearman correlation coefficient near zero with a non-significant p-value (R=0.025, p=0.88), suggesting the absence of a monotonic association between visceral adipose macrophage content and BMI in patients who died of COVID-19.
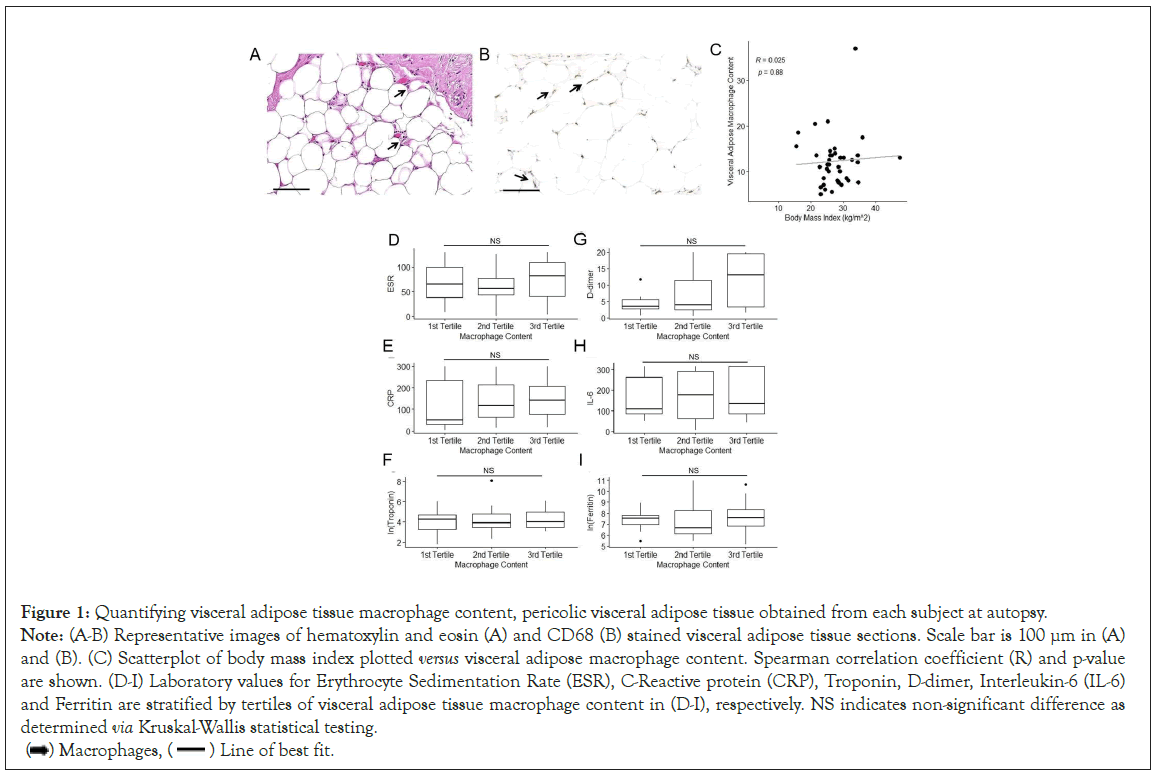
Figure 1: Quantifying visceral adipose tissue macrophage content, pericolic visceral adipose tissue obtained from each subject at autopsy.
Note: (A-B) Representative images of hematoxylin and eosin (A) and CD68 (B) stained visceral adipose tissue sections. Scale bar is 100 μm in (A) and (B). (C) Scatterplot of body mass index plotted versus visceral adipose macrophage content. Spearman correlation coefficient (R) and p-value are shown. (D-I) Laboratory values for Erythrocyte Sedimentation Rate (ESR), C-Reactive protein (CRP), Troponin, D-dimer, Interleukin-6 (IL-6) and Ferritin are stratified by tertiles of visceral adipose tissue macrophage content in (D-I), respectively. NS indicates non-significant difference as determined via Kruskal-Wallis statistical testing.
 Line of best fit.
Line of best fit.
Disassociates Adipose tissue macrophage content and systemic inflammatory markers associated in patients who died of COVID-19
To investigate the potential association between visceral adipose tissue macrophage content and systemic inflammation in COVID-19, the most recent serum ESR, CRP, Troponin, D-dimer, IL-6 and Ferritin values prior to death were obtained for each study subject. In some instances, patients did not have all 6 of these lab values recorded in the electronic medical record and in these cases the remaining available lab values were used. The number of subjects for which each lab value was available is shown in Table 2 while the median days prior to death for which each of these lab values was measured is shown in Table 3. Overall, 58.3% of lab values were within two days of death.
| Inflammatory markers | Visceral adipose macrophage content median (IQR) |
p-value | |||
|---|---|---|---|---|---|
| Overall | 1st Tertile | 2nd Tertile | 3rd Tertile | ||
| ESR (n=34) | 69(47-101.5) | 65(38-100) | 56.5(42.7-76.8) | 81(40.5-109) | 0.69 |
| CRP (n=35) | 99.7(40.6-214) | 51.3(28.4-236) | 118(63.1-214) | 142(78.7-207) | 0.7 |
| Troponin (n=37) | 63(31-112) | 69(27-107) | 51.5(31-118) | 55(31.5-154) | 0.92 |
| D-dimer (n=33) | 3.9(2.6-13) | 3.4(2.7-5.5) | 3.9(2.3-11.3) | 13(3.2 -19.6) | 0.2 |
| IL-6 (n=31) | 134.7(85.7-315) | 108(87.1-261) | 177.3(61.9-292) | 135(87-315) | 0.89 |
| Ferritin (n=34) | 7450(665-3,550) | 1880(1,050-2,430) | 806(461-4,090) | 1970(944-4,120) | 0.72 |
Note: ESR (Erythrocyte Sedimentation Rate), CRP (C-Reactive Protein), IL-6 (Interleukin-6); p-value computed with Kruskal-Wallis test.
Table 2: Serum inflammatory markers, stratified by visceral adipose macrophage content.
| Serum inflammatory marker | Median days prior to death (IQR) |
|---|---|
| ESR | 2(1-6.5) |
| CRP | 2(1-5) |
| Troponin | 3(1-9) |
| D-dimer | 1(0-5) |
| IL-6 | (1-7) |
| Ferritin | 1(1-5) |
Note: IQR (Interquartile Range), ESR (Erythrocyte Sedimentation Rate), CRP (C-Reactive Protein), IL-6 (Interleukin-6).
Table 3: Timing of serum inflammatory markers relative to death.
Subjects were then stratified into tertiles based on their visceral adipose tissue macrophage content. For each marker of systemic inflammation (ESR, CRP, Troponin, D-dimer, IL-6, and Ferritin) the median and IQR were computed within each tertile as well as all subjects together. This data is shown in Table 2 and Figure 1D-1I. For all 6 markers of inflammation, there was no significant differences between tertiles (Kruskal-Wallis p-values>0.05 for all, Table 2). Additionally, when comparing visceral adipose macrophage content to each marker of systemic inflammation, there were no correlations (Spearman coefficients all>0.17 with p>0.05). When this analysis was repeated using peak laboratory values obtained at any time during the index hospitalization, the results were substantively unchanged (data not shown).
Discussion
Adiposity, especially visceral adiposity with increased BMI, has been associated with elevations in systemic inflammation in a variety of diseases including COVID-19 [4-8]. In diseases other than COVID-19 such as type 2 diabetes mellitus and rheumatoid arthritis, systemic inflammation is in part driven by adipose tissue macrophages through the release of inflammatory cytokines; in these disease states, the degree of inflammation has been shown to be proportional to visceral adipose tissue macrophage content [13,15-18]. In this study of patients who died of COVID-19, however, we found no association between BMI and visceral adipose macrophage content and also no association between visceral adipose macrophage content and markers of systemic inflammation. To our knowledge, this is the first study to directly examine adipose tissue macrophage content and systemic inflammation in COVID-19.
Our results are interesting given that adiposity and increased BMI are associated with poor outcomes and increased peak levels of markers of systemic inflammation in COVID-19 [1-3,7,8,14]. Indeed, one of the striking clinical features of the early pandemic was the hyper inflammatory state that often accompanied severe COVID-19 and led to death, especially in very obese patients. One explanation for the discrepancy between our findings and the previously observed associations between adiposity and BMI with increased systemic inflammation in COVID-19 is that the clinical observations come from living patients whereas our current study is limited to patients at autopsy [7].
Additionally, our patients were older and had a slightly lower median BMI compared to patients in previous studies that showed strong associations between systemic inflammation and obesity in COVID-19 [7]. It is possible that obesity (and visceral adipose tissue macrophage content) is crucial to inflammation in young but not old patients with COVID-19.
Another potentially surprising finding is that, unlike in patients with type 2 diabetes, among patients who died of COVID-19, there was no association between BMI and visceral adipose tissue macrophage content. This suggests the possibility that visceral adipose tissue and visceral adipose tissue macrophages may not serve as the primary source of systemic inflammation in severe COVID-19. Speculatively, our results suggest that systemic inflammation may be driven by an element other than visceral adipose tissue macrophages. This idea is supported by the lack of increase in IL-6 in patients with increased adipose macrophage content (Figure 1H and Table 2) since IL-6 together with TNF-α have previously been identified as the key inflammatory cytokines released by adipose tissue macrophages in driving systemic inflammation in non-COVID-19 conditions [4,15-18]. It is possible that the source of systemic inflammation lies outside of adipose tissue, for example in the lungs where the primary pathology of COVID-19 infection occurs. Alternatively, the lack of association between visceral adipose tissue macrophage content and BMI maybe attributable to the relatively low median BMI of our study population.
Our study has several limitations including the inability to study clinical outcomes and lack of standardization in the timing of when laboratory values were collected. Despite this latter limitation, 58% of labs were collected within 48 hours before death. Our cohort had a median age greater than 65 and was predominantly normal weight or overweight (median BMI 26.5, IQR 24.7 kg/m2- 29.8 kg/m2). Younger and more obese patients may have stronger associations between obesity, visceral adipose tissue macrophage content, and systemic inflammation. Another limitation is that our study population was predominantly male and Hispanic, which may affect generalizability.
Conclusion
In summary, we found no association between BMI and visceral adipose tissue macrophage content among patients who died of COVID-19. Additionally, we found no significant association between visceral adipose tissue macrophage content and systemic markers of inflammation (specifically with ESR, CRP, Troponin, D-dimer, IL-6, and Ferritin). To our knowledge, this is the first study to directly examine visceral adipose tissue and systemic inflammation among patients who died of COVID-19. Adiposity is an established risk factor for the hyper inflammatory syndrome associated with COVID-19, especially among younger patients, and further studies are needed to elucidate what mechanisms may drive this. However, studies looking for the origins of systemic inflammation may have to look elsewhere than visceral adipose tissue macrophages.
References
- Huang Y, Lu Y, Huang YM. Obesity in patients with COVID-19: A systematic review and meta-analysis. Metabolism. 2020;113:154378.
[Crossref] [Google Scholar] [PubMed]
- Petersen A, Bressem K, Albrecht J. The role of visceral adiposity in the severity of COVID-19: Highlights from a unicenter cross-sectional pilot study in Germany. Metabolism. 2020;110:154317.
[Crossref] [Google Scholar] [PubMed]
- Anderson MR, Geleris J, Anderson DR. Body mass index and risk for intubation or death in SARS-CoV-2 infection: A retrospective cohort study. Ann Intern Med. 2020;173(10):782-790.
[Crossref] [Google Scholar] [PubMed]
- Elks CM, Francis J. Central adiposity, systemic inflammation, and the metabolic syndrome. Curr Hypertens Rep. 2010;12(2):99-104.
[Crossref] [Google Scholar] [PubMed]
- Deng T, Lyon CJ, Bergin S, Caligiuri MA, Hsueh WA. Obesity, inflammation, and cancer. Annu Rev Pathol. 2016;11:421-449.
[Crossref] [Google Scholar] [PubMed]
- Karczewski J, Sledzinska E, Baturo A. Obesity and inflammation. Eur Cytokine Netw. 2018;29(3):83-94.
[Crossref] [Google Scholar] [PubMed]
- Foulkes AS, Selvaggi C, Shinnick D. Understanding the link between obesity and severe COVID-19 outcomes: Causal mediation by systemic inflammatory response. J Clin Endocrinol Metab. 2021;107(2):698-707.
[Crossref] [Google Scholar] [PubMed]
- McNeill JN, Lau ES, Paniagua SM. The role of obesity in inflammatory markers in COVID-19 patients. Obes Res Clin Pract. 2021;15(1):96-99.
[Crossref] [Google Scholar] [PubMed]
- Chiappetta S, Sharma AM, Bottino V, Stier C. COVID-19 and the role of chronic inflammation in patients with obesity. Int J Obes (Lond). 2020;44(8):1790-1792.
[Crossref] [Google Scholar] [PubMed]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860-867.
[Crossref] [Google Scholar] [PubMed]
- Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282(22):2131-2135.
[Crossref] [Google Scholar] [PubMed]
- Lapice E, Maione S, Patti L. Abdominal adiposity is associated with elevated C-reactive protein independent of BMI in healthy nonobese people. Diabetes Care. 2009;32(9):1734-1736.
[Crossref] [Google Scholar] [PubMed]
- Kunz HE, Hart CR, Gries KJ. Adipose tissue macrophage populations and inflammation are associated with systemic inflammation and insulin resistance in obesity. Am J Physiol Endocrinol Metab. 2021;321(1):105-121.
[Crossref] [Google Scholar] [PubMed]
- Zeng F, Huang Y, Guo Y. Association of inflammatory markers with the severity of COVID-19: A meta-analysis. Int J Infect Dis. 2020;96:467-474.
[Crossref] [Google Scholar] [PubMed]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796-1808.
[Crossref] [Google Scholar] [PubMed]
- Ferrante AW. Macrophages, fat, and the emergence of immunometabolism. J Clin Invest. 2013;123(12):4992-4993.
[Crossref] [Google Scholar] [PubMed]
- Harford KA, Reynolds CM, McGillicuddy FC, Roche HM. Fats, inflammation and insulin resistance: insights to the role of macrophage and T-cell accumulation in adipose tissue. P Nutr Soc. 2011;70(4):408-417.
[Crossref] [Google Scholar] [PubMed]
- Giles JT, Ferrante AW, BSroderick R. Adipose tissue macrophages in Rheumatoid Arthritis: Prevalence, disease-related indicators, and associations with cardio metabolic risk factors. Arthritis Care Res (Hoboken).2018;70(2):175-184.
[Crossref] [Google Scholar] [PubMed]
Citation: Su SH, Nobel YR, Besharati S, del Portillo A, Anderson MR, Freedberg DE (2023) Non-Association of Visceral Adipose Macrophage Content, Body, Mass Index and Inflammatory Markers in COVID-19. J Infect Dis Diagn. 8:232.
Copyright: © 2023 Su SH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

