Indexed In
- Online Access to Research in the Environment (OARE)
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Scimago
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2020) Volume 11, Issue 7
New Natural Method for the Elimination of Salmon Farms Parasite Copepods
Juan E. Trigo1* and Meritxell Mondéjar22Campus DoMar, Pza. Miralles - Local A7, University Campus, 36310, Vigo (Pontevedra), Spain
Received: 22-Jun-2020 Published: 20-Jul-2020, DOI: 10.35248/2155-9546.20.11.595
Abstract
The observations carried out during the spring of 2014 on different Atlantic salmon (Salmo salar Linnaeus, 1758) sea farms around the island of Frøya (Sor–Trøndelag), Norway, served to lay the foundations for a totally natural and environmentally friendly method for removal salmon parasitic copepods known as sea lice.
The method is based on two known data: the different stages of copepod larval development, some of which are free-living as part of plankton and the existence of marine invertebrates that feed on plankton and more specifically on zooplankton, that is, the animal part of the plankton that is where the copepod larvae are.
If we combine these two facts, we obtain the main idea of the method: introducing marine invertebrate filter feeders in special devices (patent pending) inside the cages of the marine farms, it will only be necessary to let these invertebrates carry out their natural function of feeding based on copepod larvae, that is, when they are in their most vulnerable phase and in which they have not yet caused harm to the salmon. In this way, sea lice can be eliminated without the use of chemicals, medicines or methods that can cause unnecessary stress to salmon on sea farms.
Keywords
Sea-lice elimination; Salmon farming; New method; Organic; Filter feeders
Introduction
The aquaculture production has increased at an average annual growth rate of 5.8%, from 44.3 million tonnes in 2005 to 73.8 million tonnes in 2014 (FAO). Only in Europe the aquaculture sector employs more than 100.000 people in rural and coastal areas with a production over of 2 mills of tons with a value over 10 bills. € (Data taken from FEAP which represent 26 organizations in 22 European Nations). Salmon farming is one of the fish cultures most practiced worldwide. The production of Atlantic salmon, reached approximately 2 million tons in 2014, with Norway as the largest producer, followed by Chile, the United Kingdom and Canada [1]. The sea louse (Lepeophtheirus salmonis [2]) has a circumpolar distribution in the northern hemisphere and is primarily a parasite of salmonids in the genera Salmo, Oncorhynchus and Salvelinus [3].
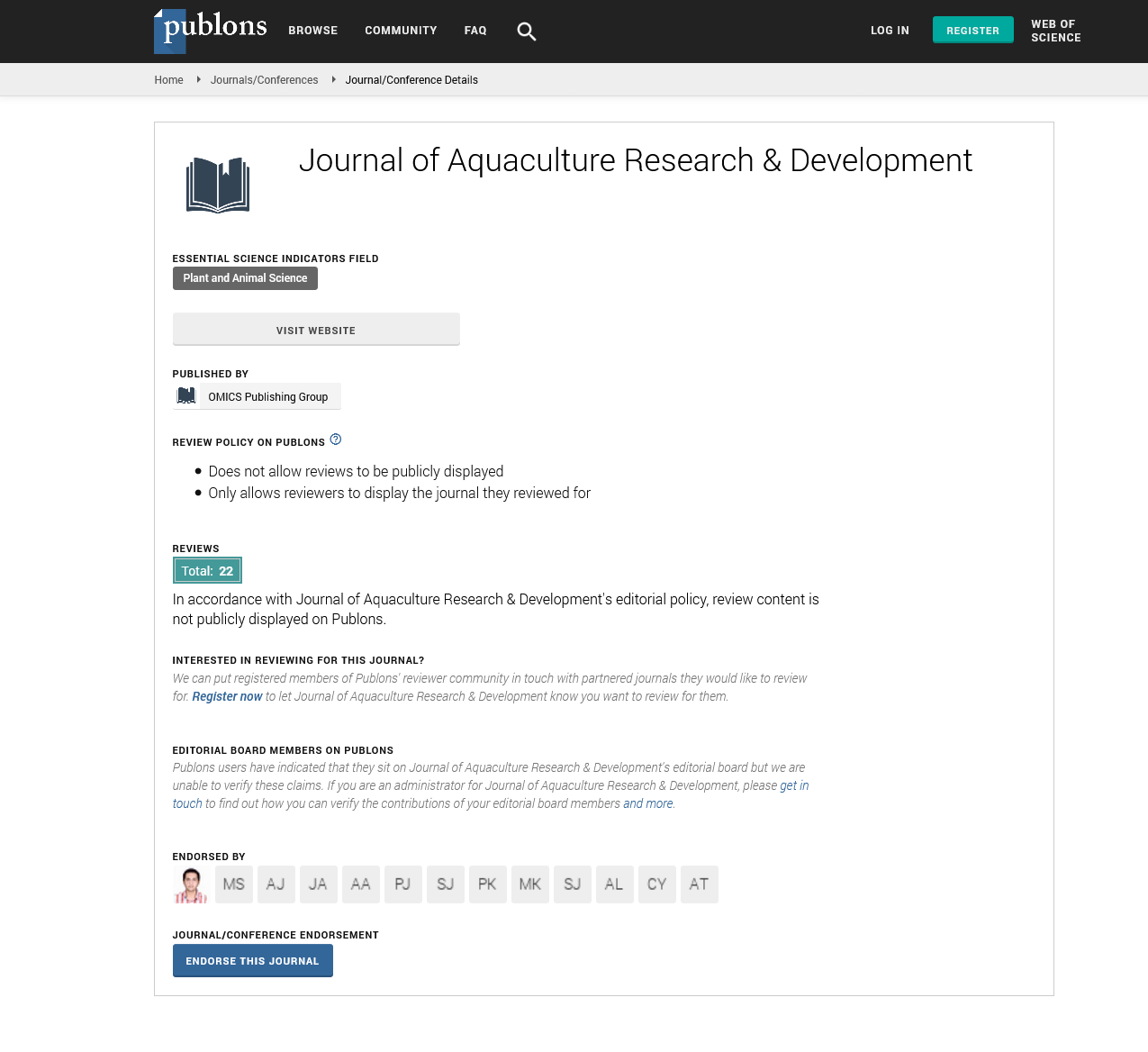
Copepods are crustaceans and reproduce by laying eggs. After hatching, they go through microscopic stages (nauplius I and II) living free in the sea (zooplankton) until they reach the stage of copepodite and chalimus (I and II), which is when it attacks salmon [4,5] (Figures 1 and 2).
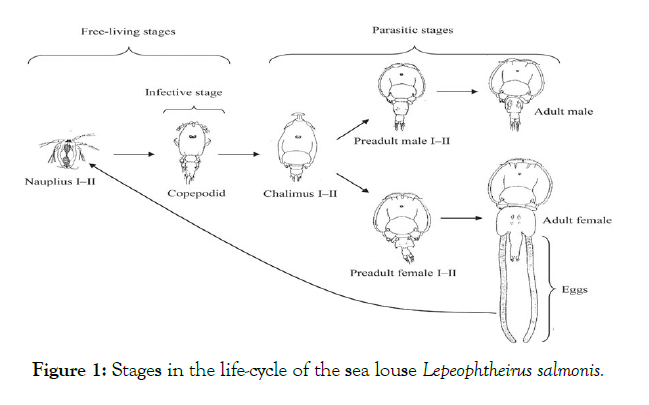
Figure 1:Stages in the life-cycle of the sea louse Lepeophtheirus salmonis.

Figure 2: Left: 1. Mature female with egg strings. 2. Mature female without egg strings. 3. Immature louse
Stages in the life-cycle of the sea louse Lepeophtheirus salmonis
The life cycle of sea lice comprises 8 different stages. After hatching, a nauplius emerges that develops in two stages and between 5 and 15 days later, depending on the environmental conditions, they become copepodites, which are already infective and are characterized by being able to hook on their host. From this stage is when they no longer separate from it, although even certain phases can still move freely on their skin.
The host suffers physiological consequences due to their interactions with L. salmonis that depend largely on the number and state of development of copepods. The infections lead to severe erosion of the epidermis and dermis exposure, in severe cases, skeletal muscle [6]. Most common and significant is the subclinical symptoms, including physiological stress, and changes in blood glucose or electrolytes, haematocrit reduction and swimming ability [7].
Since the beginning of the 1970s, sea lice have been treated with formaldehyde baths of questionable effect [8], but immediately afterwards they were treated with all kinds of chemicals like organophosphates: Metrifonate and Dichlorvos, Macrocyclic lactone ivermectin [9-11] in the mid-1980s and natural pyrethrins in the late 1980s [12]. Subsequently, inhibitors of chitin synthesis such as Diflubenzuron and Teflubenzuron, hydrogen peroxide baths [13-15] were used, in addition to Synthetic pyrethroids such as Cypermethrin and Deltamethrin and Macrocyclic Lactone Emamectin Benzoate [16,17] all in the 1990s.
The vast majority of chemicals have caused that parasites develop resistance to them within 10-15 years [18-20], so these chemicals have had to become increasingly lethal and dangerous in order to be effectively. This has led to serious environmental consequences, as the fauna in areas close to marine farms have been severely affected [21].
Not only chemicals were used but, for example, the first cleaner fish tests, in particular wrasses, were carried out in 1988 [22], but it was not until 2012 that lump fish was tested [23]. Although it is theoretically unthinkable that sea lice can develop resistance to cleaner fish, in 2018 a mutation of the parasites was detected on the island of Frøya consisting in the variation of their color (Fishfarmingexpert, 2018). They are usually brown more or less intense, but the mutation has caused their color to be transparent, which prevents the cleaner fish from seeing them and are the ones with the highest survival rates (Figures 3-5).
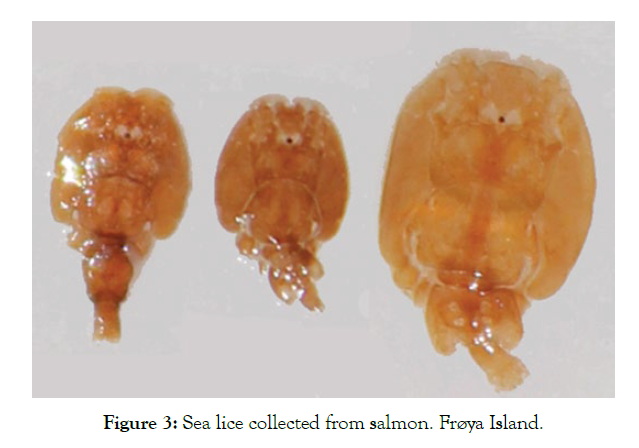
Figure 3: Sea lice collected from salmon. Frøya Island.

Figure 4: Color mutation (taken from Fish farming expert).

Figure 5: Cleaner fish. Ctenolabrus rupestris. Goldsinny wrasse.
During the months of April, May and June, 2014, one of the authors had the opportunity to carry out an internship on the Island of Frøya, one of the most important enclaves for breeding Atlantic salmon in Norway. Different works were carried out in the marine farms of Ørnøy, Rataran and Kattholmen of the company Salmar Farming As and in them it was possible to know and observe the problems that sea lice cause in the fattened salmon on those marine farms (Figures 6-8). Back in Spain and with the observations made in the marine farms, a new method was devised and designed and an action plan was conceived for the implementation of this method that should meet the following fundamental requirements:

Figure 6: : Salmar farming AS marine salmon farms from Ørnøy.

Figure 7: Salmar farming AS marine salmon farms from Rataran.

Figure 8: Salmar farming AS marine salmon farms from Kattholmen.
1. Be different from those already used: its a method of removal or reduction of planktonic larval stages of ectoparasite copepods using marine invertebrate filter feeders as biological barrier. There are previous tests carried out only with one bivalve species [24-28], mainly mussels, oysters and scallops, or several species [29-33], but in this case it is intended to go much further, using invertebrates belonging to different marine invertebrate phyla, with high filtration and digestion capacities and roundly rejecting mussels and oysters.
2. Be respectful with the environment: on the one hand, using exclusively native invertebrate filter feeders from the place where the method is started, and on the other hand, having the possibility to breed different species of invertebrates in hatcheries in order not to alter natural stocks.
3. More economical than current methods: it can be started immediately with only special devices (patent pending) without mechanical parts, practically maintenance-free and in which the invertebrate filter feeders would be placed.
4. More efficient than those used so far: parasites would be eliminated in their non-infective phase and regardless of their color mutations 24 hours a day and 365 days a year.
5. Easy to test on sea farms: only a few devices into the sea farm cages without taking up a lot of space and without preventing routine work.
For this, it was done with the participation of the University of Vigo (Department of ecology and animal biology), its research center Station of Marine Sciences of Toralla (ECIMAT) and also of the Galician Institute for Aquaculture Training (IGaFA) from Arousa Island.
Material and Methods
Work protocols were established that consisted of the following: firstly, laboratory determination of the method's viability, the species to be tested and the temperature ranges to which they should adapt and work:
• Preliminary tests of 16 species of indigenous invertebrate filter feeders were done separately. They belonged to different invertebrate phyla with a wide range of distribution all around Europe. They were tested in 5, 10 and 100L aquariums during 24 hours.
• The ones that filtrated the most were tested again on 5-10L aquariums during 4 hours,
• Afterwards, the ones with most filtering capacity were tested again on 100L tanks during 4 hours.
• Lastly some testing was done too in a 120 m3 pool during 24 hours.
The trials were done using Artemia sp. as a model and a substitute of sea lice. The artemia nauplii are a food widely used in aquaculture and easy to grow. In addition, its size (450-550μm) is almost equal to that of the nauplii of Caligus sp. and Lepeophtheirus salmonis (500μm) which is the target larval stage of this method.
The preparation carried before the experiments were common for all scales of the experiments. Filter feeders were collected on the Ría de Vigo and Ría de Arousa in Nort-West Spain. Then, other filtering animals from the surface of the invertebrates were eliminated to avoid interferences. This epifauna could filtrate instead of the invertebrates that were being tested. Next, the animals were acclimated during 4 days to laboratory conditions (13 °C, 35% salinity and filtered water). There were also acclimated to experimental temperature during 2 days. The experiments that served of first screening were done in batches of 2, 10 and 100 individuals. Control and duplicate or triplicate of the tests was done. The temperatures were 5, 10, 15 and 20° ± 1°C and the nauplii concentration ranged from 2000 to 7000 nauplii/L. These first tests were done in order to design the following trials.
For the 10 L and 100 L experiments the starting nauplii concentration was approximately 1000 Nauplii/L. 15 × 102 Isochrysis galbana cells/mL were added to stimulate filtration and to better reproduce natural conditions. This was tested in the previous trials. The experimental replicates were randomly select and control replicates without invertebrate filter feeders were done.
On the 10L trials (S2) the filter feeders were distributed in experimental groups of 100g. There were no significant differences between the weight of the replicates (ANOVA; p>0.05; p=0.57). The temperatures were 14.1°C ± 0.24°C, 9.3°C ± 0.12°C, 5.6°C ± 0.21°C. Filter feeders were in 15 L aquaria with 10 L micro-filtered sea water in closed circuit with strong aeration during 4 hours. 20 ml samples were taken at the beginning and every hour at 3 points. They were then fixated with lugol and counted on an inverted microscope.
At the 100L trials (S3) the experimental groups were of 1000 g. in special devices or directly in the tank. There were no significant differences between the weight of each replicate (ANOVA; p>0.05: p=0.79). The temperatures were 14.28°C ± 0.22°C, 10.29°C ± 0.46°C, 6.65°C ± 0.33°C. Filter feeders were in 150 L tanks filled with 100 L micro-filtered sea in closed circuit with strong aeration during 4 hours. 20 ml samples were taken at the beginning and every hour in 6 points. They were then fixated with lugol and counted on an inverted microscope. The special devices were used as a way to cage the filter feeders and also force water through it. This way the filtering capacity would be increased. These devices were also used in the 120 m3 pool trial to further test their attraction ability in larger water quantities.
Results
As referenced before, the preliminary tests were done to implement the following protocols; in consequence the results would not be discussed here. The obtained results of the 10L randomized experiments are synthesized in Table 1. We calculated the nauplii consumption percentage that is the difference between the initial and final number of nauplii in the 10L aquaria. If we exclude specie 4, 4 hours after the beginning of the experiment the other 3 species eliminated a minimum of the 80% of the nauplius. All species ate nauplius (ANOVA; p>0.05, p=0.068). All of them also ate less with lower temperatures (p=0.058).
| % Consumed | ||||
|---|---|---|---|---|
| Temperature | Species 1 | Species 2 | Species 3 | Species 4 |
| 15°C | 95.32 | 91.45 | 97.19 | 72.37 |
| 10°C | 91.49 | 92.22 | 98.41 | 30.87 |
| 5°C | 88.83 | 87.73 | 83.07 | 26.67 |
Table 1: Percentage of nauplii consumed by the different species used during the 4 hours of the experiment at 10 L.
The obtained global results of the 100L randomized experiments are synthesized in Table 2. We calculated the nauplii consumption percentage that is the difference between the initial and final number of nauplii in the 100L tanks. We observed that the species eat less; this could be caused by methodological issues or by the amount of feed available and the duration of the experiment. The 4 species ate nauplius and ate less at lower temperatures, as it was expected. 4 hours pass of the beginning of the experiment the 3 species eliminated a minimum of the 30% of the nauplius.
| % Consumed | ||||||
|---|---|---|---|---|---|---|
| Temperature | Device | No Device | ||||
| Species 1 | Species 2 | Species 3 | Species 1 | Species 2 | Species 3 | |
| 15°C | 68.67 | 69.19 | 29.98 | 69.19 | 72.37 | 55.94 |
| 10°C | 47.23 | 52.09 | 54.91 | 55.16 | 45.24 | 44.96 |
| 5°C | 59.45 | 34.40 | 48.67 | 44.81 | 35.33 | 33.48 |
Table 2: Percentage of nauplii consumed by the different species used during the 4 hours of the experiment at 100 L.
The detailed results for each temperature and treatment at the 100L can be seen in the following graphs (Figures 9-18). In the boxplot are represented the different values of nauplii/liter for every hour of the experiment. The results are expressed for every temperature and especies and with the device and without. Further comparison of the use or not of the device is illustrated in Figure 19. Next, expressed in graph (Figure 19) are the results for the test in the 120 m3 pool in 24 hours. This was done as a way to further prove the effectivity of the special device and to test the experiments in larger water volumes.
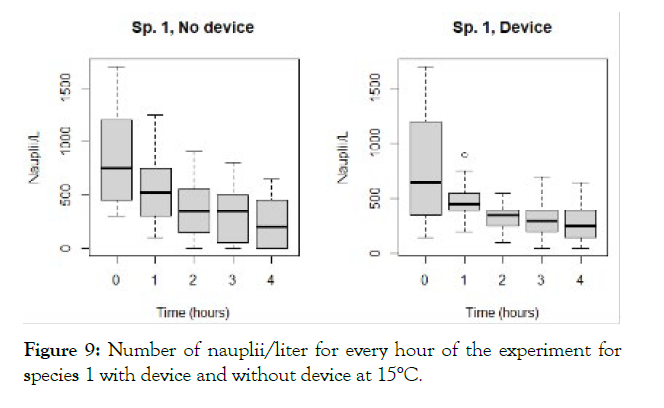
Figure 9: Number of nauplii/liter for every hour of the experiment for species 1 with device and without device at 15°C.
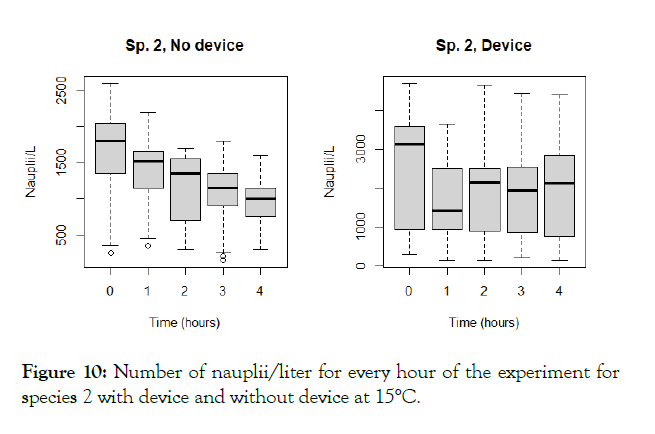
Figure 10: Number of nauplii/liter for every hour of the experiment for species 2 with device and without device at 15°C.
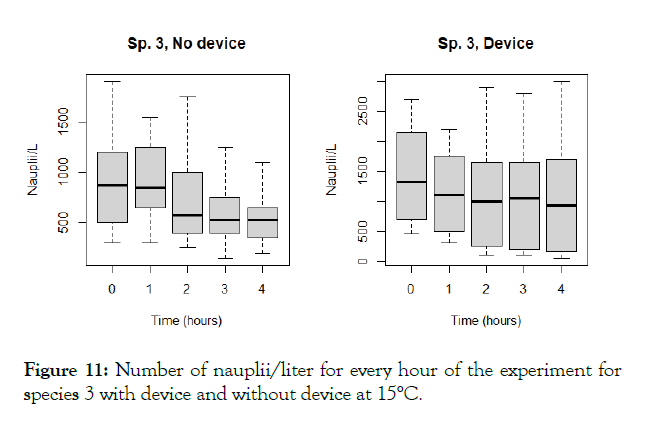
Figure 11: Number of nauplii/liter for every hour of the experiment for species 3 with device and without device at 15°C.
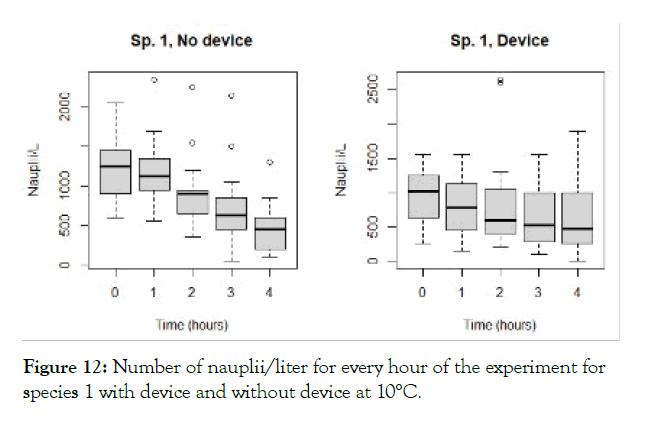
Figure 12: Number of nauplii/liter for every hour of the experiment for species 1 with device and without device at 10°C.
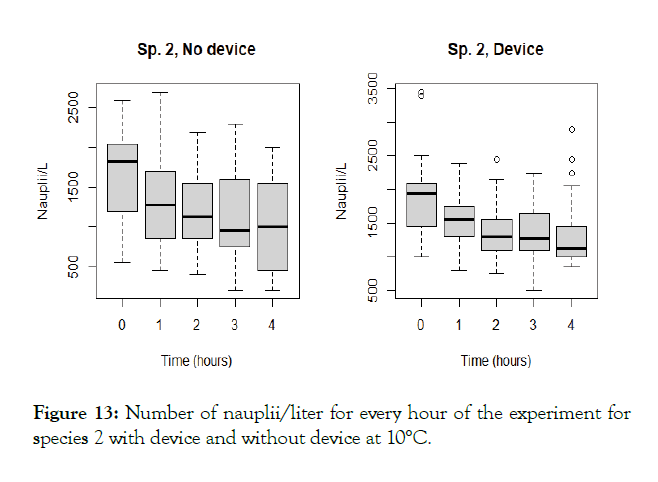
Figure 13: Number of nauplii/liter for every hour of the experiment for species 2 with device and without device at 10°C.
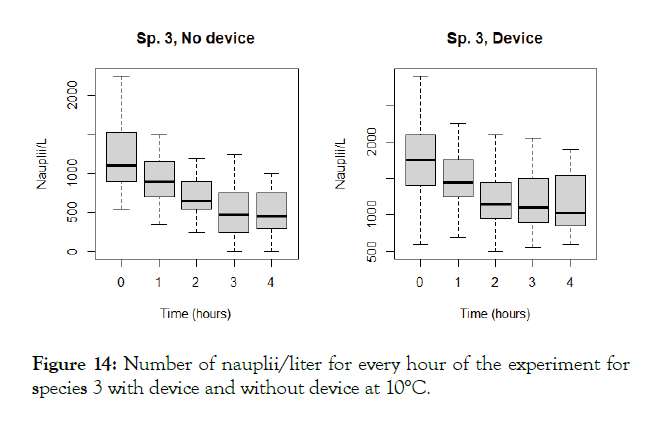
Figure 14: Number of nauplii/liter for every hour of the experiment for species 3 with device and without device at 10°C.
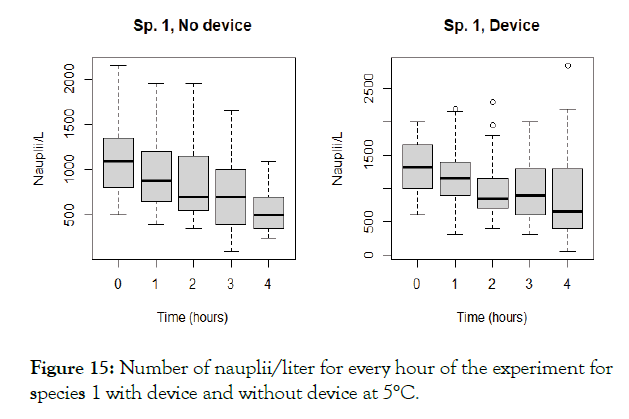
Figure 15: Number of nauplii/liter for every hour of the experiment for species 1 with device and without device at 5°C.
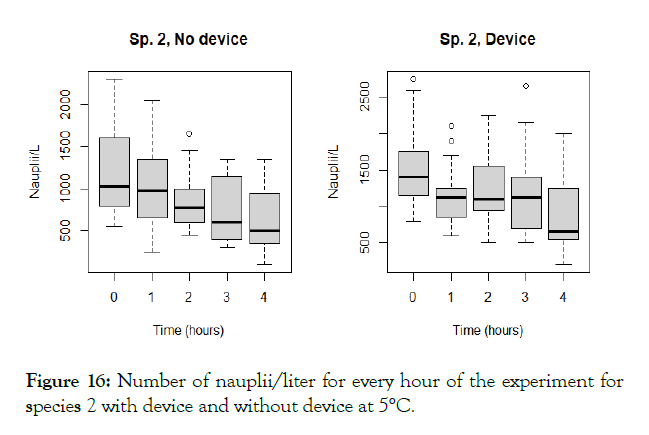
Figure 16: Number of nauplii/liter for every hour of the experiment for species 2 with device and without device at 5°C.
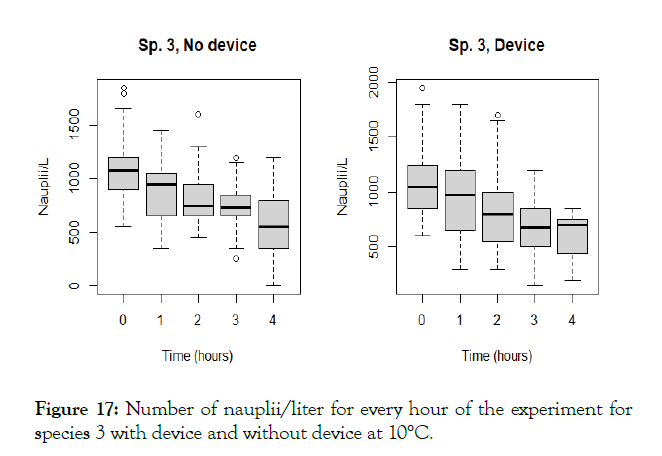
Figure 17: Number of nauplii/liter for every hour of the experiment for species 3 with device and without device at 10°C.
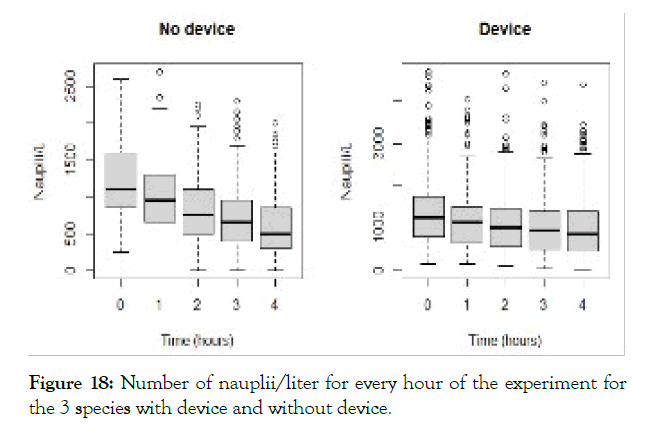
Figure 18: Number of nauplii/liter for every hour of the experiment for the 3 species with device and without device.
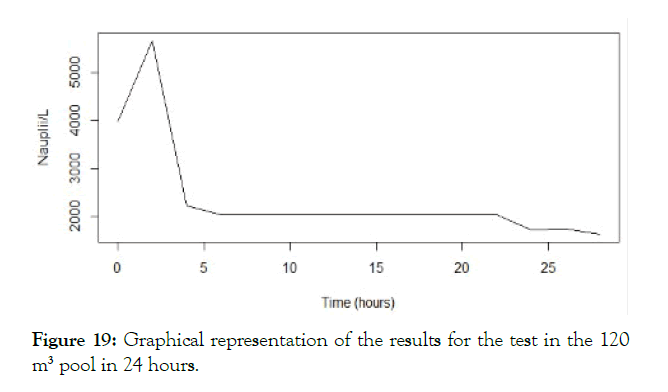
Figure 19: Graphical representation of the results for the test in the 120 m3 pool in 24 hours.
Discussion
Different authors have tried to estimate the costs of controlling sea lice [34-37]. The first of these proposes figures of just over EUR 300 million per year worldwide, at a cost of EUR 0.2 per kilo produced and makes no reference to the cost of cleaner fish. The second ones make a direct calculation, but taking into account the cost of certain treatments only in Norway and estimate it between 0.21 euros/kilo and 0.47 euros/kilo. They said that for wrasse treatment, the only cost incurred was the cost of purchasing wrasse. The third parties estimate an expenditure of GBP 700 million, at a cost of EUR 0.4 per kilo and the last ones said that only in Norway, EUR 0.66 per kilo, but none of them mention costs for cleaner fish (Figure 20).
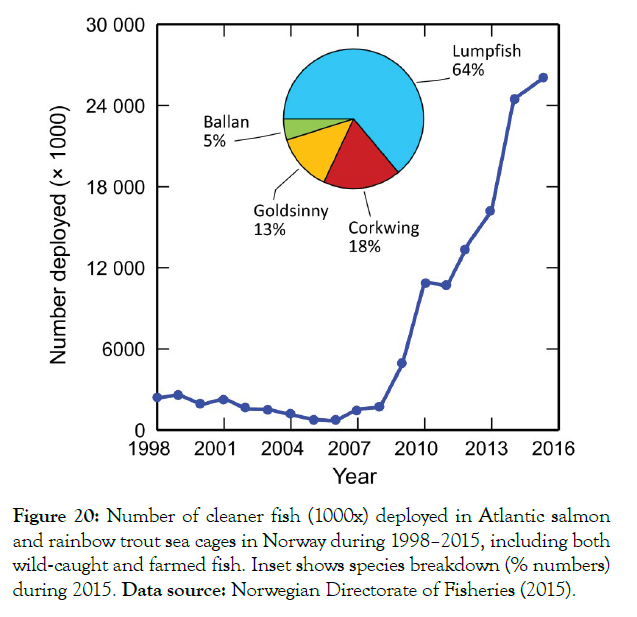
Figure 20: Number of cleaner fish (1000x) deployed in Atlantic salmon and rainbow trout sea cages in Norway during 1998–2015, including both wild‐caught and farmed fish. Inset shows species breakdown (% numbers) during 2015. Data source: Norwegian Directorate of Fisheries (2015).
The number of cleaner fish taken from the wild and farmed in fish farms used in 2019 in Norway was 60,565,000 with a market value of NOK 1,304,954,000. Those farmed were a total of 39,017,000 with a market value of NOK 851,100,000, or an average of 2.18 euros per unit and the numbers are increasing every year (http:// www.fiskeridir.no/English/Aquaculture/Statistics/Cleanerfish-Lumpfish-and-Wrasse). In the case of our method, the average calculation per invertebrate seed specimen can range from 0.005 to 0.25 euros per unit, almost 10 times less than the cleaner fish.
One of the most important parts of the method is the special device (patent pending). This device, is a simple structure, easy to handle and without mechanic parts or components, allows sea lice free life larval stages (Nauplius I and II) to enter inside, where the filter feeders are located, being ingested and with no possibility of escape. In addition to protecting them from salmon attack, allows filter feeders to develop very quickly and easily.
One of the most important characteristics of the device is that it allows the oxygenation of the water in which it is immersed, so it is not a problem to increase the bioload inside the cages. The only maintenance required for the device would be external cleaning which could be carried out with pressurized water. If the fouling is excessive, it can be removed for mechanical cleaning.
With regard to filter feeders, once or twice a month they should be reviewed to eliminate possible casualties, specimens suspected of having some pathology or too large specimens and replace them with new ones.
This method is also useful having in mind the raising interest in IMTA. Apart from the bio-mitigation derived from extraction of salmon lice and other pathogens, filter feeders capture organic particulates. Multitrophic aquaculture increases profitability and reduces risks through crop diversification and alternative sources of income. Also, there is a perception of sustainable production by the public [38,39].
Although the method has been tested at laboratory level only, the data obtained suggest that the potential of the idea is impressive. The following is a list of the advantages it may have over the methods currently used for the removal of parasitic copepods from salmon (Figure 21).
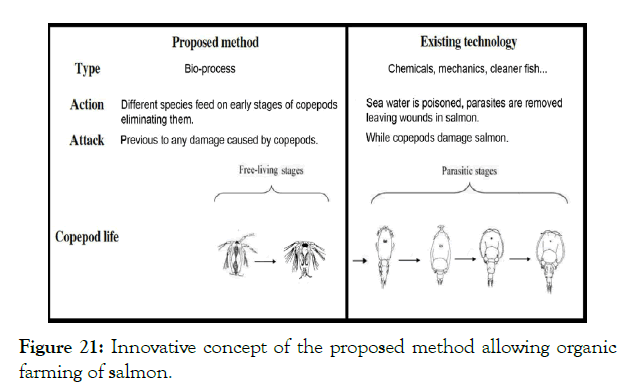
Figure 21: Innovative concept of the proposed method allowing organic farming of salmon.
Use of chemicals
Existing methods: The use of chemical treatment against sea lice in salmon farming has resulted in reduced effect and resistance against the different groups of compounds.
Proposed method: Our method is based on the exclusive use of different marine native filter feeders to create a completely natural, organic and biological barrier, safe for the environment. Sea lice cannot develop resistance against it.
Use of mechanical, laser, hot water removal methods
Existing methods: When parasites are eliminated, they leave wounds that are sources of entry for pathogens and infectious diseases.
Proposed method: Parasites are eliminated in their free life phases, never attacking the salmon.
Nearby sea farms
Existing methods: When other nearby sea farms starts a cleaning cycle, they must agree with each other to use the same or different treatments to get rid of sea lice.
Proposed method: Each sea farm can begin the treatment when necessary, without having to know what will make others. Use of wrasses and lump fish are listed below in Table 3.
| Existing methods | Proposed method |
|---|---|
| Production costs of cleaner juvenile fish are very high and the larvae feed based on food Artemia larvae or commercial diets are very expensive. | We use species that are very easy to breed in a hatchery, and feed only with natural plankton. It has very low costs of production and growth in a very small space. |
| The built hatcheries are not able to produce the necessary cleaner fishes, and even fishing from the natural environment more lumps and wrasses would not reach the necessary required quantities to supply the farms without putting in serious risk natural stocks. | The necessary filter feeder species can be extracted from the environment or raised in hatcheries without great difficulty and in a very small space. |
| Wrasses or lumpfish eat sea-lice, but in pre-adult or adult stages, when they have caused damages to the salmons. | Our method is based on eliminate sea lice in non parasitic earliest stages: nauplii I and II. Targets are the sea-lice first larval stages. |
| Wounds that parasites cause to salmon are a focus of disease and pathogens. | We prevent larvae from reaching the pre-adult and adult stages. Incidences produced by wounds, disappear. |
| Parasites of salmon appear to be a direct vehicle in viruses, bacteria and other pathogens transmission that cause diseases of high mortality among salmons. | Reducing the incidence of parasites in cages, the impacts of dangerous diseases would also be reduced. |
| Parasitic copepods are very specific and focus their attacks on fish, usually on salmon, but they can also do it with other species and among them, with the cleaner fish and may end up causing their death. | Invertebrate filter feeders are never attacked by the fish parasites. |
| Lumps: Their characteristic suction discs enable the fish to utilize only the surface of the cage and not the water column. Fish density is, therefore, measured in areal units rather than volume ones, making lumpfish a surface-demanding species. These features have challenged producers to find technical solutions for the design of culture cages offering higher areal use rather than conventional cages. There is also need for grading equipment adapted to lumpfish. | Filter feeders do not need any specific surface because they will be already inside specific devices. |
| Cleaner fish are subject to pathologies (normally common to those of salmon such as Aeromonas salmonicida, Vibrio anguillarum, V. ordalii and V. splendidus and viral haemorrhagic septicaemia in wrasse) that have to be treated to avoid mortality with vaccines and specific chemicals for them that increase in large measure their production costs. | Invertebrate filter feeders do not need to be treated. Animals with pathologic problems are much more profitable to remove them and replace the losses with news. |
| The female cleaner fish spawning are several tens of thousands of eggs. | Filter feeders will lay hundreds of thousands of eggs or even millions of them. Many invertebrate species, in addition, are hermaphrodites. |
| Lumpfish reach the market size between 5 and 9 months. |
Seeds of some species of filter feeders are ready from 3 weeks in hatchery. |
| Ballan wrasse may be ready for delivery to salmon farms between 9-18 months after hatching. | Seeds of some species of filter feeders are ready from 3 weeks in hatchery. |
| Captive farmed wrasse and lump, in addition to specific Artemia-based feed and specific dry feed, need to be vaccinated to prevent outbreaks of serious diseases for them and for salmon. | Juvenile invertebrate filter feeders feed on plankton or, in any case, on microalgae that are very easy to obtain and cultivate. No vaccination is necessary as their diseases are not transmitted to the salmon and if there is a serious problem, they are replaced by new ones. |
| Cleaner fish need dry feed in winter because parasites are virtually eliminated by water temperature. | Filter feeders reduce their metabolism, remaining practically in hibernation and it is not necessary to resort to alternative feeding. |
| Cleaner fish can suffer predation from salmon. They need mimicking kelp forests to survive. These should be removed from the cages every few days to be cleaned of fouling and in general disinfected with sodium hypochlorite. | Filter feeders will be placed inside a special device (patent pending) that favors its growth, oxygenates the water and acts as a trap for parasites. It can be simply cleaned with a pressurized water system. |
| In summer months, water temperature increases and dissolved oxygen levels decrease dramatically in salmon farms due to salmon and cleaner fish bioload. | Filter feeders will be placed inside a special device (patent pending) that favors its growth and oxygenates the water. |
| Cleaner fish, once they die are crushed and used simply as protein. | Some of the filter feeders used are edible and highly appreciated, so they have a high commercial value, and once used on marine farms, a very profitable production derived for human consumption can be obtained from them. Of the non-edible ones, the by-products that can be obtained from them are often of very high added value, such as astaxanthins, chitosans, chitins and others that can be reused, for example, to make the salmon dry feed composition. |
| At least, 40 percent of cleaner fish disappear or die. They disappear without a trace eaten or slaughtered with the salmon. | Filter feeders will be placed inside a special device (patent pending) and they will be never in contact with the salmon. |
| In 2018 a mutation of sea lice appears in which their natural brown coloration becomes transparent. Cleaner fish cannot see them and they survive longer and reproduce more easily than brown ones. | Filter feeders don't care about the color of the larvae. All, without differentiation, are ingested. |
Table 3: Use of wrasses and lump fish.
Installation of refuges mimicking kelp forests in salmon cages holding cleaner fish is essential for reduction of cleaner fish stress and predation from salmon (Figure 22).

Figure 22: Installation of refuges mimicking kelp forests in salmon cages.
Potential benefits of the method for the salmon industry
1. Higher value of salmons. Chemical treatments, antibiotics or vaccines will be avoided= Organic salmon.
2. Drugs, antibiotics and chemicals costs will be greatly reduced.
3. Concerns about the effects of antibiotic-resistant bacteria on human health not exist.
4. Salmon farming would be friendly with other wild fisheries, such as crustaceans, molluscs and fishes in the same area.
5. Increased protection against diseases. Sea lice are intermediate hosts of different diseases. If sea lice are removed, diseases disappear or, at least, reduced to a minimum.
6. Salmon farming would be friendly with wild salmon populations.
7. Higher value of fresh salmons: more specimens without external wounds or damages.
8. Salmon mortality will be reduced, so profits will be higher.
9. The high costs of building superstructures, closed systems and general research in areas of dubious effectiveness or very low profitability results, could be avoided.
10. Salmon industry image will be friendly.
Other benefits of our method would be:
There are around 559 species of sea-lice in 37 genera all around the world, including approximately 162 Lepeophtheirus and 268 Caligus species and both have free swimming (planktonic) and parasitic life stages. It works with any kind of copepod. We use a “cocktail” of species that have proven be the most effective in laboratory tests and are used only native species (European species in Europe, American species in America), so we can apply the method anywhere in the world and whatever the species of fish or crustacean in any sea farm.
There are algae cultures for human consumption in China, Korea and Japan mainly (Undaria pinnatifida) that are attacked by parasitic copepods causing problems such as those known as pin-holes. Some of them also have larval stages of free life. The method, could also serve to eliminate some great commercial importance seaweed parasites.
With slight modifications, it is also suitable for freshwater species. It’s complementary to any other type of action such as the use of wrasses and lumpfish at the same time (Figure 23).
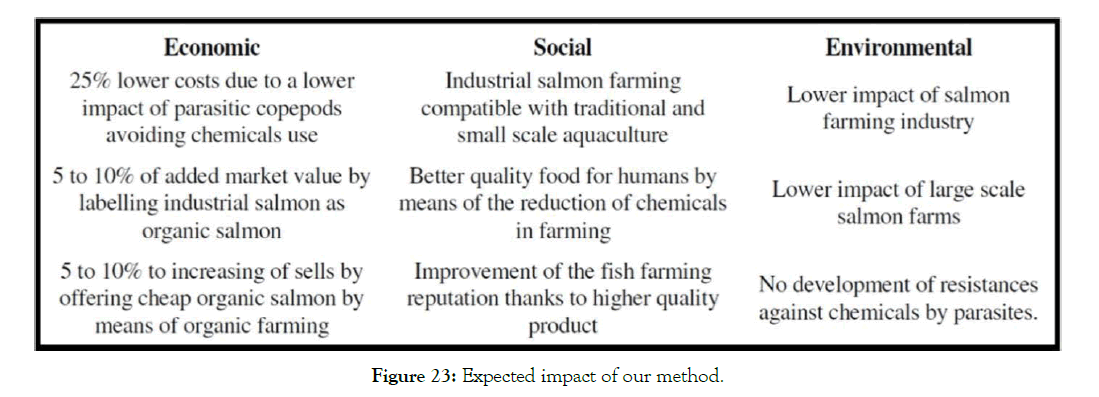
Figure 23: Expected impact of our method.
Others proposed method
The proposed method has been chosen for funding in the first phase of the Horizon 2020, SME Instrument call in 2016. In the second phase, grants of between 0.5 and 2.5 million euros are available for the development of a business plan. We need a partner from the salmon industry that has problems with parasitic copepods who wants to implement this new method with us.
Conclusion
The main idea of the method: introducing marine invertebrate filter feeders in special devices (patent pending) inside the cages of the marine farms, it will only be necessary to let these invertebrates carry out their natural function of feeding based on copepod larvae, that is, when they are in their most vulnerable phase and in which they have not yet caused harm to the salmon. In this way, sea lice can be eliminated without the use of chemicals, medicines or methods that can cause unnecessary stress to salmon on sea farms.
Acknowledgements
• We would like to thank Professor Jesús S. Troncoso of the University of Vigo (Department of Ecology and Animal Biology) and the Estación de Ciencias Marinas de Toralla (ECIMAT) for their support in carrying out the laboratory tests.
• We also wish to thank the collaboration of the Galician Institute for Aquaculture Training (IGaFA) and specifically Professor Jose L. Rodriguez Villanueva for his support in the tests carried out in the outdoor pool of this center.
• Finally to the engineering company SEISTAG Innovación, S.L. for the development for the presentation of the project to the EU call, Horizon 2020.
REFERENCES
- Aaen SM, Helgesen KO, Bakke MJ, Kaur K, Horsberg TE. Drug Resistance in Sea Lice: A Threat to Salmonid Aquaculture. Trends in Parasitology. 2015; 31: 72-81.
- Abolofia J, Asche, F, Wilen JE. The Cost of Lice: Quantifying the Impacts of Parasitic Sea Lice on Farmed Salmon. Marine Resource Economics. 2017; 32: 329-349.
- ANON. Nytt fra Havbruk. Oslo, Norway: Forskningsrådet (Norwegian Research Council); Lovende resultat med rognkjeks (Promising results with lumpfish). 2012; 1.
- Bartsch A, Robinson SM, Liutkus M, Ang KP, Webb J, Pearce CM. Filtration of sea louse, Lepeophtheirus salmonis, copepodids by the blue mussel, Mytilus edulis, and the Atlantic sea scallop, Placopecten magellanicus, under different flow, light and copepodid-density regimes. Journal of Fish Diseases. 2013; 36: 361–370.
- Brandal PO, Egidius E. Preliminary report on oral treatment against salmon lice, Lepeophtheirus salmonis, with Neguvon. Aquaculture. 1977; 10: 177–178.
- Branson EJ, Ronsberg SS, Ritchie G. Efficacy of teflubenzuron (Calicide(R)) for the treatment of sea lice, Lepeophtheirus salmonis (Kroyer 1838), infestations of farmed Atlantic salmon (Salmo salar L.) Aquaculture Research. 2000; 31: 861–867.
- Bjordal Å. Cleaning Symbiosis between Wrasses (Labridae) and Lice Infested Salmon (Salmo salar) in Mariculture. Copenhagen: International Council for the Exploration of the Sea, Committee on Mariculture. 1988.
- Bjordal Å. Rense fisk mot lakselus (Cleanerfish against sea lice) Norsk Fiskeoppdrett. 1988; 37.
- Brooker AJ, Papadopoulou A, Gutierrez C, Rey S, Davie A, Migaud H. Sustainable production and use of cleaner fish for the biological control of sea lice: recent advances and current challenges. Veterinary Record. 2018; 183: 383.
- Costello MJ. Ecology of sea lice parasitic on farmed and wild fish. Trends in Parasitology. 2006; 22: 475-483.
- Costello MJ. The global economic cost of sea lice to the salmonid farming industry. Journal of Fish Diseases. 2009; 32: 115–118.
- Costello MJ. Ecology of sea lice parasitic on farmed and wild fish. Trends in Parasitology. 2012; 22: 10.
- Cranford PJ, Ward JE, Shumway SE. Bivalve filter feeding: variability and limits of the aquaculture biofilter. In: Shumway, S.E. (Ed.), Shellfish Aquaculture and the Environment. Wiley-Blackwell, Hoboken, New Jersey. 2011; 81–124.
- Davenport D, Smith RJ, Packer M. Mussels Mytilus edulis: significant consumers and destroyers of mesozooplankton. Marine Ecology Progress Series. 2000; 198: 131–137.
- Denholm I, Devine GJ, Horsberg TE, Sevatdal S, Fallang A, Nolan DV. Analysis and management of resistance to chemotherapeutants in salmon lice, Lepeophtheirus salmonis (Copepoda: Caligidae) Pest Management Science. 2002; 58: 528–536.
- FAO. The State of World Fisheries and Aquaculture, 2020. Sustainability in action. Rome. 2020.
- FAO. The State of World Fisheries and Aquaculture, 2020. Sustainability in action. Nature and Resources. 2020; 35.
- Granada L, Sousa N, Lopes S, Lemos MF. Is integrated multitrophic aquaculture the solution to the sectors’ major challenges? – A review. Reviews in Aquaculture. 2016; 8: 283–300.
- Hamre LA, Eichner C, Caipang CM, Dalvin ST, Bron JE, Nilsen F. The Salmon Louse Lepeophtheirus salmonis (Copepoda: Caligidae) Life Cycle Has Only Two Chalimus Stages. PLoS ONE. 2013; 8: e73539.
- Horsberg TE, Hoy T. Tissue distribution of 14C-diflubenzuron in Atlantic salmon (Salmo salar). Acta Veterinaria Scandinavica. 1991; 32: 527–533.
- Igboeli OO, Burka JF, Fast MD. Lepeophtheirus salmonis: a persisting challenge for salmon aquaculture. Animal Frontiers. 2014; 4: 22–32.
- Jakobsen PJ, Holm JC. Lovende forsøk med nytt middel mot lakselus [Promising tests with a new agent against salmon lice] Norsk Fiskeoppdrett. 1990; 16–18.
- Johannessen A. Lakselus [Salmon lice] Fisken og Havet Ser B. 1974; 2: 21–31.
- Jones MW, Sommerville C, Wootten R. Reduced sensitivity of the salmon louse, Lepeophtheirus salmonis, to the organophosphate dichlorvos. Journal of Fish Diseases. 1992; 15: 197–202.
- Kamiyama T. Microzooplankton as a food source for the Pacific Oyster Crassostrea gigas: seasonal variation in gut contents and food availability. Fisheries Science. 2011; 77: 961–964.
- Lehane C, Davenport J. Ingestion of mesozooplankton by three species of bivalve; Mytilus edulis, Cerastoderma edule and Aequipecten opercularis. Journal of the Marine Biological Association of the United Kingdom. 2002; 82: 615–619.
- Liu Y, Bjelland H. Estimating costs of sea lice control strategy in Norway. Preventive Veterinary Medicine. 2014; 117: 469–477.
- Macdonald BA, Robinson SM, Barrington KA. Feeding activity of mussels (Mytilus edulis) held in the field at an integrated multi-trophic Aquaculture (IMTA) site (Salmo salar) and exposed to fish food in the laboratory. Aquaculture. 2011; 314: 244–251.
- Molloy S, Pietrak M, Bouchard D, Bricknell I. Ingestion of Lepeophtheirus salmonis by the blue mussel Mytilus edulis. Aquaculture. 2011; 311: 61-64.
- Palmer R, Rodger H, Drinan E, Dwyer C, Smith PR. Preliminary trials on the efficacy of ivermectin against parasitic copepods of Atlantic salmon. Bulletin of the European Association of Fish Pathologists. 1987; 7: 47–54.
- Pascoe PL, Parry HE, Hawkins AJ. Observations on the measurements and interpretation of clearance rate variations in suspension-feeding bivalve shellfish. Aquatic Biology. 2009; 6: 181–190.
- Powell A, Treasurer JW, Pooley CL, Garcia DE Leaniz C. Use of lumpfish for sea-lice control in salmon farming: Challenges and opportunities. Reviews in Aquaculture. 2017; 1-20.
- Rae GH. On the trail of the sea lice. Fish Farmer. 1979; 2: 22–25.
- Roth M. The availability and use of chemotherapeutic sea lice control products. Contributions to Zoology. 2000; 69: 109–118.
Citation: Trigo JE, Mondéjar M (2020) New Natural Method for the Elimination of Salmon Farms Parasite Copepods. J Aquac Res Development 11:595. doi: 10.35248/2155-9546.19.10.595
Copyright: © 2020 Trigo JE, et al. This is an open access article distributed under the term of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.