Indexed In
- RefSeek
- Hamdard University
- EBSCO A-Z
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Case Report - (2023) Volume 8, Issue 4
Neonatal Purpura Fulminans in Neonate with Severe Congenital Protein C Deficiency: Case Report
Avantika Chauhan1*, Stuti Shukla1, Tanvi Agrawal2, Shalini Tripathi1 and Mala Kumar12Department of Dermatology, King Georges Medical University, Chowk, Lucknow 226003, Uttar Pradesh, India
Received: 31-May-2023, Manuscript No. JOD-23-21548; Editor assigned: 02-Jun-2023, Pre QC No. JOD-23-21548 (PQ); Reviewed: 16-Jun-2023, QC No. JOD-23-21548; Revised: 31-Jul-2023, Manuscript No. JOD-23-21548 (R); Published: 07-Aug-2023, DOI: 10.35248/2684-1436.8.211
Abstract
Neonatal purpura fulminans is a rare life threatening condition caused by congenital or acquired causes. The condition is fatal so prompt diagnosis and management is important. It presents as acute disseminated intravascular coagulation and haemorrhagic skin necrosis and requires judicious use of anticoagulant therapy. Here we describe a case of neonatal purpura fulminans due to congenital protein C deficiency presenting late with haemorrhagic and necrotic skin lesions, anemic retinopathy and cystic encephalomalacia, managed with fresh frozen plasma transfusions and low molecular weight heparin.
Keywords
Neonatal; Purpura fulminans; Congenital; Protein C deficiency; Anticoagulant
Introduction
Neonatal purpura fulminans is a clinic-pathological entity of dermal microvascular thrombosis associated with Disseminated Intravascular Coagulation (DIC) and perivascular haemorrhage, occurring in the neonatal period. It presents clinically as acute DIC and haemorrhagic necrosis of skin. It is a rare entity and is fatal if not treated early and effectively. Therefore, it is required to take prompt actions with investigating and treating this condition. It may occur due to congenital and acquired causes, with congenital protein C deficiency being a rare cause with incidence of 1 in 4 million. We present a case of neonatal purpura fulminans managed with fresh frozen plasma and anticoagulation therapy.
Case Presentation
A seventeen day old male baby presented to our side with multiple ecchymotic necrosed lesions involving hand, feet, thigh and genital regions. He was second in birth order born at term out of an uneventful pregnancy by vaginal delivery at home to non-consanguineous parents. On admission on day 17 he was breastfeeding well and his vitals were stable. According to parents he was well till day 6 of life when he developed fever following which bluish black discoloration developed on the skin below the umbilicus which further extended to groin. For the next seven days lesions gradually progressed to involve whole body along with peeling and crusting. There was no family history of any thromboembolic disorder. Mother had no history of recurrent pregnancy loss, risk factors for sepsis or antenatal torch infection. Elder sibling had no similar complaints.
On examination (Figure 1) baby had ecchymotic lesion with irregular central haemorrhagic necrosis with surrounding erythema on the dorsum of right hand and both feet. Lesions present on the back of thigh, below the umbilicus, in the right iliac fossa and scrotum which had black eschar formation. During the course of hospital stay new ecchymotic lesions appeared in the oral cavity and lesions in the hand and feet also developed eschar. Routine hemogram, sepsis screen, blood culture, coagulation parameters Table 1 revealed anemia, leukocytosis, raised CRP, with features of consumptive coagulopathy (raised D-Dimer levels, presence of Fibrin Degradation Product (FDP), decreased fibrinogen).
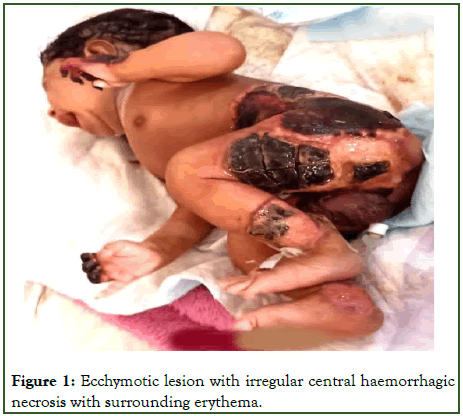
Figure 1: Ecchymotic lesion with irregular central haemorrhagic necrosis with surrounding erythema.
| 13/06 (DOL17) | 14/06 (DOL18) | 15/06 (DOL19) | 17/06 (DOL21) | 18/06 (DOL22) | 06/07 (DOL40) | 09/07 (DOL43) | Reference value | |
|---|---|---|---|---|---|---|---|---|
| Prothrombin time (sec) | 17.1 | 21 | 12.8 | 12.8 | 11-15 sec | |||
| INR | 1.28 | 1.4 | 0.95 | 0.98 | ||||
| APTT (sec) | 34.3 | 86 | 24.8 | 35.6 | 24-32 sec | |||
| Fibrinogen (mg/dl) | 131 | 111 | 150 mg/dl-400 mg/dl | |||||
| FDP | Negative | Positive | ||||||
| CRP (mg/dl) | 30.07 | 0 mg/dl-6 mg/dl | ||||||
| Hb (gm/dl) | 8.1 | 7.677 | 11.1 | 11.5 mg/dl-16.5 gm/dl | ||||
| TLC (cells/mm3) | 48500 | 32300 | 33500 | 5000-19000 cells/mm3 | ||||
| Platelet (cells/mm3) | 2.1 | 1.5 | 5.34 | 1.5 -4.5 lac cells/mm3 | ||||
| Protein C-functional assay | <10% | 70% to 130% | ||||||
| Protein S-functional assay | 67% | 65% to 100% | ||||||
| Anti-thrombin III functional assay | 107% | 80% to 120% | ||||||
| Note: INR: International Normalised Ratio, APTT: Activated Partial Thromboplastin Time, FDP: Fibrin Degradation Product, CRP: C Reactive Protein, Hb: Hemoglobin, TLC: Total Leucocyte Count | ||||||||
Table 1: Laboratory test values of various investigations.
He was managed with Fresh Frozen Plasma (FFP) transfusions and packed red cell transfusions with broad spectrum antibiotics. The baby was fed on mother’s own milk. Colour Doppler of upper and lower limb vessels was suggestive of biphasic flow in bilateral upper and lower limb arteries. There was no evidence of renal vein thrombosis. Cranial USG was suggestive of cystic encephalomalacia of right frontal horn. Fundus examination showed multiple pre retinal and intraretinal hemorrhage over posterior pole, suggestive of anaemic retinopathy. The clinical picture of this newborn with local deep necrotic skin lesions without features of generalized sepsis suggested a provisional diagnosis of purpura fulminans and sample for the prothrombotic disorders (protein C, protein S and antithrombin III levels) were sent. Protein C levels were <10 percent. Antithrombin III and protein S levels were within normal range. As baby had congenital protein C deficiency and protein C concentrate is not available, he was managed with therapeutic anticoagulation with FFP transfusions (at 15 ml/kg/dose) and Low Molecular Weight Heparin (LMWH) (Enoxaparin at 1.5 mg/kg/dose twice a day). LMWH was given for 20 days and INR was 0.95. After this treatment, there was some healing of lesions, plan was to replace LMWH with warfarin with overlap of 2 drugs for 5 days maintaining INR of >2. Also, baby was planned for DNA next generation sequencing panel but due to monetary issues it could not be done. Despite some improvement in the skin lesions parents did not wish for any further treatment and took the baby home against medical advice on day 45 of life.
Results and Discussion
Protein C is a vitamin K dependent anticoagulant that regulates thrombosis and is a predominantly autosomal dominant trait with heterozygous carriers. Homozygous mutations leading to autosomal recessive protein C deficiency is less common but causes more severe form of disease with onset of thrombotic manifestations at birth [1-3]. Neonates born to unrelated parents may have compound heterozygous gene mutations while homozygous state may be considered if history of consanguinity is present in parents [4].
In the present case neonate was clinically asymptomatic till six days and then he developed purpuric lesions. Although in other reports earlier presentation has been described varying from birth to 72 hours after birth [5-9].
Most of the studies reported rapid progression of lesion which is in contrast to our case where the lesions were gradually progressive over a period of seven days [10].
There was no history of consanguinity present in our case which was similar to another case reported from India, although most of the previous reports had history of consanguinity [10].
In our case there was no family history suggestive of similar complaints and elder sibling was healthy but few cases have been reported with affected older siblings who succumbed to death [6,8]. In both the cases with affected older sibling, history of consanguinity was present but there are reports in which there was consanguineous marriage with normal elder sibling [4,9].
Sharma, et al., reported a case in which mother had history of cortical vein thrombosis in previous pregnancy with low protein C level. Devi, et al., from India reported a case with normal maternal protein C levels with low normal levels of protein C antigen and decreased protein C activity in father [6].
Ophthalmologic manifestations are common in babies of neonatal purpura fulminans with most of babies having leukocoria or bilateral vitreous haemorrhage due to vitreoretinal disorder [6-10]. Monagle, et al., followed a baby till 12 years (10 years after liver transplantation) and his only permanent deficit was blindness which occurred in utero. In our case features of anemic retinopathy were present [5].
Cranial ultrasonography was suggestive cystic encephalomalacia of right frontal horn which was similar to case reported by Tawab, et al., with baby having features of bilateral cystic lesions suggestive of periventricular leukomalacia. This baby was born preterm at 35 weeks gestation and was kept on conventional ventilation and CPAP while our baby did not require resuscitative or ventilatory support. In case reported by Saeidi, et al., baby had 2 episodes of seizures within 20 hours of birth and brain MRI was suggestive of acute ischemic lesion. Including us, all the cases were managed with FFP transfusions, LMWH and warfarin therapy as protein C concentrate was not readily available at many centres.
Conclusion
The findings of our case emphasize that neonatal purpura fulminans should be suspected in severe necrotic skin lesions in an otherwise well looking newborn. It may have delayed presentation but needs to be managed aggressively with fresh frozen plasma and anticoagulation therapy.
Ethical Approval and Consent to Participate
Approval from ethics committee was not required for this case report.
Consent for Publication
Consent has been taken from parents for publication of case report.
Availability of Supporting Data
No supporting data required.
Competing Interests
There are no competing interests.
Funding
No funding was required.
Author's Contribution
Dr. Avantika Chauhan and Dr. Stuti Shukla were responsible for drafting of the article. Dr. Shalini Tripathi and Dr. Tanvi Agrawal were responsible for revising it critically. Dr. Mala Kumar and Dr. Shalini Tripathi performed final approval of the version to be published.
Acknowledgements
We would like to acknowledge the nursing stagg at our institution.
References
- Price VE, Ledingham DL, Krumpel A, Chan AK. Diagnosis and management of neonatal purpura fulminans. Semin Fetal Neonatal Med. 2011;16(6):318-322.
[Crossref] [Google Scholar] [PubMed]
- Goldenberg NA, Manco-Johnson MJ. Protein C deficiency. Haemophilia. 2008;14(6):1214-1221.
[Crossref] [Google Scholar] [PubMed]
- Millar DS, Johansen B, Berntorp E, Minford A, Bolton-Maggs P, Wensley R, et al. Molecular genetic analysis of severe protein C deficiency. Hum Genet. 2000;106(6):646-653.
[Crossref] [Google Scholar] [PubMed]
- Tawab A. A rare case of protein C mutation causing neonatal purpura fulminans. Int J Contemp Pediatr. 2021;8(10):1753.
- Monagle K, Ignjatovic V, Hardikar W, Newall F, Monagle P. Long term follow-up of homozygote protein C deficiency after multimodal therapy. J Pediatr Hematol Oncol. 2014;36(7):e452- e455.
[Crossref] [Google Scholar] [PubMed]
- Kawankar N. A novel protein C mutation causing neonatal purpura fulminans. Indian Pediatr. 2016;53(11):1019-1021.
[Crossref] [Google Scholar] [PubMed]
- Sharma S, Anbazhagan J, Plakkal N. Neonatal purpura fulminans due to protein C deficiency. Arch Dis Child Fetal Neonatal Ed. 2015;100(5):F453.
[Crossref] [Google Scholar] [PubMed]
- Jafarri SA, AlAttas KM, Bajawi SM, Ahsan MK, Al-Sheikh AM, Buraik MA, et al. Neonatal purpura fulminans in newborn with severe congenital protein C deficiency: Case report. J Dermatol Surg. 2017;21(2):104-106.
- Saeidi R, Gharaee R, Nobakht Z. Neonatal purpura fulminans. Iran J Neonatol. 2014;5(1):34-37.
- Sen K, Roy A. Management of neonatal purpura fulminans with severe protein C deficiency. Indian Pediatr. 2006;43(6):542-545.
[Google Scholar] [PubMed]
Citation: Chauhan A, Shukla S, Agrawal T, Tripathi S, Kumar M (2023) Neonatal Purpura Fulminans in Neonate with Severe Congenital Protein C Deficiency: Case Report. J Dermatitis. 8:211.
Copyright: © 2023 Chauhan A, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.