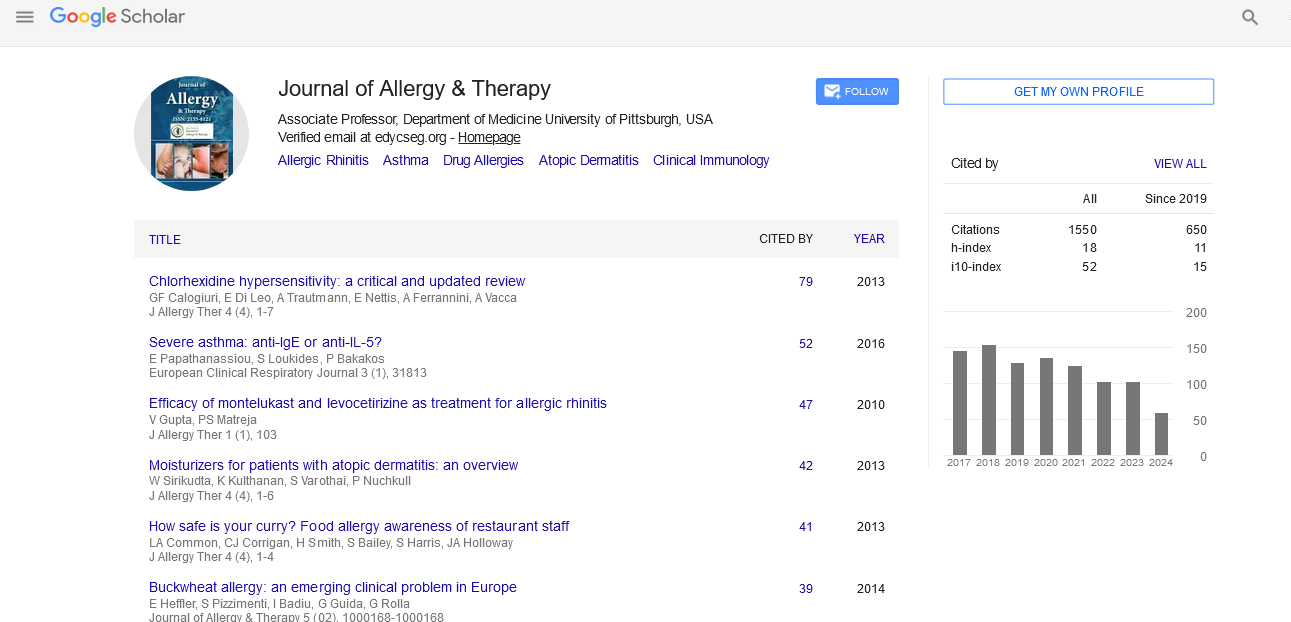
PMC/PubMed Indexed Articles
Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Perspective - (2023) Volume 14, Issue 1
Nasal and Bronchial Mucosa Inflammation in Children with Allergic Rhinitis
Andrade Huang*Received: 02-Jan-2023, Manuscript No. JAT-23-19903 ; Editor assigned: 05-Jan-2023, Pre QC No. JAT-23-19903 (PQ); Reviewed: 19-Jan-2023, QC No. JAT-23-19903 ; Revised: 26-Jan-2023, Manuscript No. JAT-23-19903 (R); Published: 03-Feb-2023, DOI: 10.35248/2155-6121.23.14.326
Description
The most prevalent chronic respiratory illnesses in children, affecting the upper and lower airways respectively, are Allergic Rhinitis (AR) and Allergic Asthma (AA). The respiratory epithelium of the upper and lower airways shares the same mucociliary clearance mechanisms.
There is a well-established connection between AA and AR, and many people have both allergy conditions. Given their similar epidemiology, pathophysiology, and treatment responses, Rowe- Jones proposed that they may be grouped together as a single disease; nevertheless, they are actually one disorder that manifests itself to varying degrees in the upper or lower airways. The most recent revision of the Allergic Rhinitis and Its Impact on Asthma (ARIA) guidelines recognised and underlined the connection between these two diseases.
Inflammation of the nasal and bronchial mucosa is a key factor in the pathophysiology of AA and AR. Th2 lymphocytes, eosinophils, basophils, and mast cells, which are comparable inflammatory cells, infiltrate both upper and lower airways. As a result, these illnesses have a same collection of cytokines (such as IL-4, IL-5, IL-13, and GM-CSF), chemical mediators of inflammation, and adhesion molecules. The severity of the disease and inflammation are further increased by increased expression of inflammatory proteins.
Although the mucosa of AA and AR exhibits a similar degree of Th2 cell-driven inflammation, the remodelling (disease-caused tissue structural changes) is much more widespread in the lower airway, indicating that the development of either condition cannot be solely attributed to the inflammation. 8 Studies on asthmatic infants appear to support this theory, as the bronchial biopsies display indications of structural alterations, aberrant cell activation, and thickening of the Reticular Basement Membrane (RBM), suggesting the disease's remodelling is a presenting symptom. The epithelium secretes growth factors that activate the mesenchymal cell unit, which in turn causes RBM thickening, subepithelial fibrosis, and Airway Smooth Muscle (ASM) hyperplasia. This signalling pathway between the epithelium and mesenchymal trophic unit may be responsible for the dissociation between airway remodelling and inflammation.
High quantities of inflammatory cytokines and growth factors (GM-CSF, IL-1, IL-5, IL-6, IL-8, FGF, eotaxin, and VEGF) are produced by hypertrophic ASM cells, which encourages cell development, vascularization, and an escalating inflammatory response. The data on remodelling in AR are sparse, and the research on nasal biopsies reveal mixed results: some found that the basement membrane was thicker than normal, while others found no differences from healthy controls. These findings suggested that either the nasal epithelium's inflammation is less severe than that of the lower airways or that the nasal mucosa is better able to withstand damage from the environment.
Studies on asthmatic kids revealed that lower airways remodelling is significantly more extensive than that of the upper airways and that the structural alterations of the lung tissue were caused by injury and frequently came before the inflammation. In order to determine if the expression pattern of the examined proteins would be disease-specific, we also compared the AA and AR patients. In contrast to periostin, which was much less expressed in AA than AR, CHI3L1/ YKL-40, IL-5, and VEGF all showed significantly higher expression in AA. Additionally, IL-5 levels were much higher in asthma than AR. 43 There isn't much research comparing periostin, VEGF, and CHI3L1/YKL-40 in AA and AR. It's interesting to note that each of these inflammatory proteins contributes to the remodelling and fibrosis of the airways.
Tissue scarring is a result of CHI3L1/ability YKL-40's to both enhance fibroproliferative healing and inhibit damage. IL-5- deficient animals exhibited reduced peribronchial fibrosis and peribronchial smooth muscle layer, whereas anti-IL-5 therapy reduced reticular basement membrane thickness in human subjects via controlling the matrix composition. VEGF affects surfactant synthesis and bronchial angiogenesis, whereas periostin alters the extracellular matrix via interacting with other proteins. Therefore, we propose that the different expression of these proteins in AA and AR may be brought on by various remodelling mechanisms: in the lower airways, the epithelium activates the mesenchymal cell unit, which is in charge of thickening the reticular basement membrane, subepithelial fibrosis, and airway smooth muscle hyperplasia, ultimately leading to an increase in inflammatory responsess.
Citation: Huang A (2023) Nasal and Bronchial Mucosa Inflammation in Children with Allergic Rhinitis. J Allergy Ther. 14:326.
Copyright: © 2023 Huang A. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.