Indexed In
- Open J Gate
- Academic Keys
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- Google Scholar
- SHERPA ROMEO
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2024) Volume 12, Issue 4
Mobiderm® Autofit Garments for Reducing Lower Limb Lymphedema: A Single-Arm Study Exploring Underlying Mechanisms
Loic Vaillant1,2*, Valerie Tauveron2 and Maxime Courtehoux32Department of Dermatology, Chru Hospitals of Tours, Tours, France
3Department of Nuclear Medicine, Tours University Hospital, Tours, France
Received: 05-Jul-2024, Manuscript No. JVMS-24-26375; Editor assigned: 09-Jul-2024, Pre QC No. JVMS-24-26375 (PQ); Reviewed: 23-Jul-2024, QC No. JVMS-24-26375; Revised: 30-Jul-2024, Manuscript No. JVMS-24-26375 (R); Published: 06-Aug-2024, DOI: 10.35248/2329-6925.24.12.565
Abstract
Background: Lymphedema is a chronic, disabling condition that results from a dysfunctional lymphatic system. The Mobiderm® Autofit device is designed to apply pressure and mobilize lymph to reduce edema and prevent worsening. This study aimed to identify the mechanisms that underlie the limb volume improvements and skin changes that are seen when using the device.
Methods: In this single-center exploratory study, patients who had lower limb lymphedema (stage II/III) wore a thigh-high Mobiderm® Autofit device for 48 hours of intensive treatment. Measurements were obtained on Day 1 (D1) and Day 3 (D3) using lymphoscintigraphy, high-frequency ultrasound, a cutometer, and volume calculations.
Results: Nine patients (aged 28-72 years) were included. The mean volume of the treated limb fell from 9664.8 ± 2766.2 mL on D1 down to 9097.6 ± 2394.1 mL on D3 (p=0.0039). Lymphoscintigraphy showed that the number of visible lymph nodes increased slightly when the device was put on, which suggests that it facilitated the flow of lymph along the vessels towards the nodes. The radiotracer migrated up the leg more quickly on D3 than on D1, indicating improved lymph flow (although p>0.1). Ultrasound images showed that fewer patients had edema within the hypodermis on D3, which indicates lymphatic drainage from this tissue. The overall skin elasticity was lower on D3 (p=0.039); the net elasticity and viscoelasticity did not change significantly.
Conclusion: The Mobiderm® Autofit device can effectively reduce lymphedema over 48 hours. We provide preliminary evidence that the device induces changes in the lymphatic pathway.
Keywords
Edema distribution; High-frequency ultrasound; Intensive phase treatment; Lymphedema treatment; Limb volume; Lower limb lymphedema; Lymphoscintigraphy; Lymphatic vessels; Skin echogenicity; Mobilizing device
Introduction
Lymphedema is a chronic, disabling condition characterized by swelling that usually affects the limbs. It is caused by impaired lymphatic drainage, which leads to the accumulation of proteinrich fluid in the interstitial space. The disease has been estimated to affect 140-200 million people worldwide, and it affects more women than men [1,2].
Lymphatic system dysfunction usually results from an obstruction or damage to the lymphatic vessels and/or nodes (secondary lymphedema) this can be caused by a tumor, surgery, trauma, or inflammation. A particularly common cause is surgery for cancer, as lymph nodes are often removed. Lymphatic system dysfunction can also be caused by a hereditary genetic mutation (primary lymphedema). In both cases, the dysfunction leads to the accumulation of protein-rich fluid in the interstitial space. This results in a cycle of T-cell mediated inflammation and fibroadipose tissue deposition [3].
Lymphedema has a detrimental impact on people’s quality of life. Patients may experience discomfort, reduced mobility, and a poor body image [4]. Patients may also have skin changes, such as dermal thickening and induration, and develop psychological problems, such as depression and anxiety [5,6]. Several complications can occur, including recurrent bacterial skin infections (e.g., cellulitis) and in rare cases, angiosarcoma [7,8].
There is no cure for lymphoedema, but treatment can alleviate the condition and prevent worsening. Complex Decongestive Therapy (CDT) is the standard treatment, which includes compression therapy using multi-layer bandaging or compression garments, a massage technique known as Manual Lymphatic Drainage (MLD), therapeutic education, physical exercise, and skin care [9]. This treatment is conducted over several days, known as the intensive phase, and it aims to stimulate lymphatic circulation to reduce the volume of the affected body part. This is then followed by a maintenance phase, which aims to prevent another volume increase. Compression plays a key role in both phases, and it is believed to improve lymphatic flow by preventing backflow and enhancing the efficiency of the lymphatic vessel contractions; it may also encourage the movement of lymph through alternative, collateral pathways [10].
Multi-layer bandaging using low-stretch bandages has long been considered an essential element of the intensive phase treatment, and this is recommended in the French national health authority guidelines [11]. However, applying the bandages is time-consuming and the pressure applied to the limb may not be maintained [12]. To overcome these limitations, garments have been developed that can maintain a constant level of pressure and are easy to put on [12]. The Mobiderm® Autofit is one such garment, and studies have shown that it can effectively reduce limb volume during the intensive phase of treatment and prevent worsening during the maintenance phase [13,14]. As a result, it is now widely used to treat lymphedema.
An important feature of the Mobiderm® Autofit device is that the material contains small foam blocks. This creates zones of variable pressure on the underlying skin. It is thought that this displaces the edema and facilitates its removal. However, to date, no studies have formally assessed the mechanisms that underlie the device’s effectiveness in reducing limb volume.
In this study, patients with lymphedema were treated using the Mobiderm® Autofit device for the first 48 hours of their intensive phase treatment. The primary aim was to determine the effects of the device on the lymphatic system in order to understand how the reductions in limb volume occur. For this, we used lymphoscintigraphy, which is often regarded as the gold standard for evaluating the lymphatic system in lymphedema [15]. This technique can be used to show lymphatic drainage and dermal backflow, and it can also provide images of the lymph nodes and vessels. Our study also investigated the effects of the treatment on the patients’ skin, as we hypothesized that there would be softening and other changes to the skin resulting from the reduced lymphedema. This was assessed using high-frequency ultrasound, which produces images of the skin at a high resolution and can be used to determine dermal thickness, tissue echogenicity, and edema distribution [16]. We also used a cutometer to assess the skin elasticity and viscoelasticity, as previous studies have shown that these measures can be affected by lymphedema [17].
Materials and Methods
Study design
This study was an exploratory, open-label, single-arm, single-center clinical study. It was approved by the local Committee for the Protection of Persons (‘East III’, Nancy, France; December 6, 2019), and authorization was obtained from the French national drug safety agency (ANSM; January 8, 2020). The study was registered on ClinicalTrials.gov (ID: NCT04252690), and it was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practice guidelines (ICH-GCP), and French law. All participants provided informed consent prior to taking part in the study.
Participants
Patients were recruited between June 2020 and June 2022 at the University Hospital of Tours, France. Patients were included who were about to begin five days of intensive phase treatment for lower limb lymphedema at the hospital. Eligible patients were at least 18 years of age and had been diagnosed with primary or secondary lymphedema of the lower limb, which was at stage II or stage III according to the International Society of Lymphology (ISL) classification; they also had leg measurements that were within the range of the Mobiderm® Autofit garments [4]. Patients were excluded if they had a skin infection, acute inflammation, or an oozing dermatological disease that affected the lower limb. They were also excluded if they had advanced diabetic microangiopathy, stage III/IV limb arteritis, decompensated heart failure, an allergy or intolerance to components of the Mobiderm® Autofit device, or another medical condition that could interfere with the conduct of the trial. Patients were also excluded if they were pregnant, breastfeeding, participating in another clinical trial, incapable of understanding the information provided, or if they were women of childbearing age not using contraception.
Treatment
For the first 48 hours of the intensive phase treatment, the patients wore a Mobiderm® Autofit garment, a class I medical device designed to treat lymphedema. These garments are inelastic with adjustable hook-and-loop fasteners that can alter the amount of pressure applied to the limb. The garments incorporate the patented MOBIDERM® technology, which consists of foam blocks (15 × 15 mm on the leg; 5 × 5 mm on the foot) that are encased between two layers of non-woven material; this gives rise to adjacent zones of uneven pressure, which may mobilize the lymph fluid. For this study, thigh-high garments were used that cover the entire leg. The patients wore the device both day and night. The patients did not receive any other lymphedema treatment over the 48 hours.
Outcome measures
We assessed the limb volume, lymphatic system, and skin at inclusion, which was the first day of hospitalization (D1), and again on Day three (D3). All measures were obtained with the patients in the supine position.
Lower limb volume: The volume of the lower limb was calculated using the mathematical formula for the volume of a truncated cone. The calculation used the circumference of the limb at the level of the ankle, the patella tip, below the patella (10, 20, and 30 cm), and above the patella (10 and 20 cm).
Lymphoscintigraphy: The lymphatic system was examined using lymphoscintigraphy. This involved injecting a radioactive tracer (technetium-labeled nanocolloid) into the first interdigital space of each foot at a depth of around 2 mm-3 mm. As this was carried out on both D1 and D3, we reduced the dosage to 100 MBq (compared to the standard 185 MBq) to limit the patients’ exposure to radioactivity; this was not expected to affect the image quality. Dynamic acquisition began from the time of the injection and lasted for 40 minutes. For the first 20 minutes, the patients refrained from moving (to analyze the spontaneous lymphatic activity), and for the remaining 20 minutes, they were asked to regularly flex their feet as if they were walking. Static planar acquisition was performed at the end of the dynamic acquisition (40 minutes post-injection; T0). The Mobiderm® Autofit device was then placed on the limb and further acquisitions were performed at 2 hours (T1) and 4 hours (T2) post-injection with the patients wearing the device.
For the dynamic images, the radiotracer propagation speed was calculated using syngo. via software (Siemens) based on five regions of interest, and the images were inspected visually. The spontaneous lymphatic vascular activity (first 20 minutes) was categorized as normal, almost normal, abnormal, or absent. ‘Normal’ indicated that the tracer migrated through the lymphatic vessels at a normal speed (determined by comparison with the contralateral limb), ‘abnormal’ indicated slow tracer migration through lymphatic collaterals or without passing through the lymphatic vessels, and ‘absent’ indicated that there was no tracer migration. For the total duration of the dynamic acquisition (40 minutes), the velocity of the lymphatic vascular flow was categorized as normal, slow, or almost absent. We also recorded the time taken for the marker to reach the ankle, the middle/lower third of the lower limb, the middle/ upper third of the lower limb, and the knee. This was determined by analyzing the propagation speed of the radiotracer bolus as well as the amplitude and timing of the peak flow rate. Activity at the dermis was also analyzed, based on regions of interest at the limb surface, in order to detect dermal reflux.
For the static planar acquisitions, the images were inspected visually and the following were recorded: The number of lymph nodes, the intensity of the visualized lymph nodes (weak/average/strong), the presence of tortuous deep lymphatic vessels (yes/no), the presence of lymphatic collaterals (yes/no), the presence of dermal backflow (yes/no), and the presence of popliteal lymph nodes (yes/no).
High-frequency ultrasonography: High frequency ultrasonography (2020 Dermcup®, Atys Medical, Soucieu en Jarrest, France; center frequency: 25 MHz; axial resolution: 70 microns; lateral resolution: 200 microns) was used to examine the patients’ skin at the anterior ankle, lower leg, and thigh to a depth of 8 mm. The following measures were obtained: The echogenicity (hypoechoic, isoechoic, or hyperechoic), which was determined by comparison with the contralateral limb (assessed by a dermatologist); the dermal thickness, which was calculated using the ultrasonography software; and the distribution of edema, which was again determined by comparison with the contralateral limb (assessed by a dermatologist). For each patient, the D3 ultrasonography was carried out by a different dermatologist to on D1, and they were blinded with respect to the results of the first examination.
Cutometer: A Cutometer SEM 474 (Courage and Khazaka Electronic GmbH, Köln, Germany) was used to measure the firmness and elasticity of the patients’ skin at the anterior lower leg, 20 cm below the patella. This device generates negative pressure to draw the skin up into a probe before releasing it again. The penetration depth of the skin within the probe is assessed over time and presented as a curve. From this, several parameters are obtained that have been widely used in previous studies [18]. We focused on the overall skin elasticity (R2), which is calculated by dividing the delayed skin relaxation by the total skin extensibility (Ua/Uf); the net elasticity (R5), which is calculated by dividing the immediate skin retraction by the immediate skin deformation (Ur/ Ue); and the viscoelasticity (R6), which is calculated by dividing the delayed skin deformation by the immediate skin deformation (Uv/Ue).
Device safety and patient satisfaction
All adverse events were recorded for the 48 hours of treatment. Patient satisfaction was assessed using a self-report questionnaire, which was administered before the final examination on D3.
Statistical analysis
As this was an exploratory study, a sample size calculation was not required. We estimated that 10 patients would be sufficient for this initial trial.
For the quantitative data, we calculated the means, Standard Deviations (SD), medians, and inter-quartile ranges. For the qualitative data, we calculated the frequencies and percentages. Differences between D1 and D3 were analyzed using Wilcoxon tests.
Results
Patient characteristics
Twelve patients were recruited between June 8, 2020, and June 20, 2022. Of these, two were unable to participate in the study because of the COVID-19 pandemic, and one did not meet the eligibility criteria, leaving a total of nine patients. Data collection for the study ended on June 22, 2022.
There were seven female and two male patients with a mean (SD) age of 57.6 (16.1) years (range: 28-72) and a mean BMI of 28.7 (3.5) kg/m2 (Table 1). The lymphedema had been diagnosed at the mean age of 46.1 (14.1) years (0.5-29 years beforehand), and the disease was at stage II for six patients and stage III for three patients. Three patients had secondary lymphedema, which was caused by cancer of the uterus (endometrium or cervix); the remaining patients had primary lymphedema. The lymphedema affected the left leg for five patients, the right leg for three patients, and both legs for one patient (note that the patient’s left leg was treated in this study). Four patients had previously undergone CDT (3-31 times).
| Characteristic | At inclusion (n=9) |
|---|---|
| Age (years), mean ± SD (range) | 57.6 ± 16.1 (28-72) |
| Sex, female, n (%) | 7 (77.8) |
| Body mass index (kg/m2), mean ± SD (range) | 28.7 ± 3.5 (22.6-32.9) |
| Lymphedema type, n (%) | |
| Primary | 6 (66.7) |
| Secondary | 3 (33.3) |
| Lymphedema stage, n (%) | |
| Stage II | 6 (66.7) |
| Stage III | 3 (33.3) |
| Affected lower limb, n (%) | |
| Right | 3 (33.3) |
| Left | 5 (55.6) |
| Bilateral | 1 (11.1) |
| Affected leg volume (mL), mean ± SD (range) | 9664.8 ± 2766.2 (6412.4-15736.2) |
Table 1: Baseline demographic and clinical characteristics.
Treatment compliance and device pressure
All patients complied with wearing the device over the 48 hours. The pressure applied by the device was assessed at the ankle, and the mean (SD) values were 40 (15.8; n=5), 55 (22.9; n=6) and 34.9 (21.7; n=6) mmHg for D1, D2, and D3, respectively.
Outcome measures
Limb volume: On D1, the mean (SD) volume of the treated limb (with lymphedema) was 9664.8 (2766.2) mL, while that of the untreated limb was 6349.0 (671.3) mL, with the latter measure excluding the patient with bilateral lymphedema. The mean excess volume on D1 was 3664.4 (2567.3) mL (57.7%), ranging from 546.3 mL up to 9315.74 mL. On D3, the mean volume of the treated limb was 9097.6 (2394.1) mL, which was significantly lower than on D1 (mean decrease: 567.2 (499.5) mL; p=0.0039), with individual decreases ranging from -28.0 to -1729.1 mL. Overall, there was a mean 5.4% reduction in the volume of the affected limb and a 15.8% reduction in the excess volume (Figure 1).
Figure 1: Limb volume on day 1 and day 3. Note: The mean volumes are shown; the error bars show the standard deviation. Note: ( ) Day 1; (
) Day 1; ( ) Day 3.
) Day 3.
Lymphatic system activity: The lymphoscintigraphy images showed spontaneous movement of lymph for five patients (55.6%) on D1 and four patients (44.4%) on D3. This activity was categorized as abnormal, except for one patient who had almost normal activity on D1; note that this latter patient showed no spontaneous adctivity on D3, but dermal reflux was observed, which indicates that lymph had passed into the dermis. Data concerning the velocity of the lymphatic flow were available for seven patients. Of these, two displayed a normal flow velocity on D1 and one on D3; the remaining results were rated as ‘slow’ or ‘almost absent’.
We analyzed the time taken for the radiotracer to appear along the limb. However, data were not available for several patients (5 or 6 on D1, and 4 or 6 on D3, depending on the limb location). The results that were available showed an increase in the time taken, from the ankle up to the knee (Figure 2). The mean times were consistently shorter on D3, but this was not statistically significant for any of the four limb locations (difference at ankle: -2.8 (7.2) min, -18.5%; middle/lower third: -5.5 (4.7) min, -14.0%; middle/ upper third: -4.7 (6.4) min, -16.2%; knee: -5.7 (6.4) min, -18.6%; p>0.1 for all comparisons).
Figure 2: Time taken for the tracer to be seen along the limb using lymphoscintigraphy. Note: The mean times are shown; the error bars show the standard deviation; ( ) Day 1; (
) Day 1; ( ) Day 3.
) Day 3.
Visible features of the lymphatic system: For the planar acquisitions, there were just two patients with visible lymph nodes at T0. This was found on D1 and again on D3. When the device was put on (T1 and T2), this increased to four patients on D1, but not on D3. For those with visible nodes at T0, the number of nodes was seen to increase from T0 to T1/T2, both on D1 and D3 (from 3 to 5 for one patient; from 5 to 6 for the other patient, on both days). The overall mean number of visible nodes increased both on D1 (0.89, 1.67, and 1.67 for T0, T1, and T2, respectively) and on D3 (0.89, 1.11, and 1.22 for T0, T1, and T2, respectively). However, these differences were not statistically significant (p>0.1 for all comparisons with T0). The intensity of the visible nodes was not found change for any of the patients. At T0, the nodes for one patient were rated to have a weak intensity, while those for the other patient were rated to have a strong intensity. For the two patients who had visible nodes for the first time at T1 (D1), the nodes were rated to have a weak intensity. Popliteal nodes were only seen for one patient, and this was only at T2 on D1.
Concerning the other visible features, tortuous deep lymphatic vessels and collaterals were seen for over half of the patients who had available data (Table 2). These features did not change for any of the patients. Dermal backflow was seen for over a third of the patients. There were two patients for whom this changed: one had backflow on D1 at T0 only; the other had backflow only for T1 and T2 (on both days).
| Day 1 | Day 3 | |||||
|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T0 | T1 | T2 | |
| Lymph nodes, N/total (%) | 2/9 (22.2) | 4/9 (44.4) | 4/9 (44.4) | 2/9 (22.2) | 2/9 (22.2) | 2/9 (22.2) |
| Popliteal lymph nodes, N/total (%) | 0/9 (0) | 0/9 (0) | 1/9 (11.1) | 0/9 (0) | 0/9 (0) | 0/9(0) |
| Dermal backflow, N/total (%) | 4/9 (44.4) | 4/9 (44.4) | 4/9 (44.4) | 3/9 (33.3) | 4/9 (44.4) | 4/9 (44.4) |
| Tortuous deep lymphatic vessels, N/total (%) | 5/6 (83.3) | 5/6 (83.3) | 5/6 (83.3) | 5/6 (83.3) | 5/6 (83.3) | 5/6 (83.3) |
| Collaterals, N/total (%) | 4/7 (57.1) | 4/7 (57.1) | 4/7 (57.1) | 4/7 (57.1) | 4/7 (57.1) | 4/7 (57.1) |
Note: The number and percentage of patients with each feature is shown. T0 is 40 minutes after the tracer injection (no device); T1 is 2 hours after the injection (wearing the Mobiderm® Autofit device); T2 is 4 hours after the injection (wearing the Mobiderm® Autofit device).
Table 2: Lymphatic system features visualized using lymphoscintigraphy.
Dermal thickness: On D1, the mean (SD) dermal thickness at the ankle, lower leg, and thigh was 2.45 (1.09), 2.61 (0.91), and 2.08 (0.63) mm, respectively. On D3, the mean dermal thickness decreased at the ankle to 2.21 (0.71) mm, but it increased slightly at the lower leg to 2.90 (1.58) mm and remained unchanged at the thigh (2.06 (0.66) mm; p values: 0.32, 0.55, and 1.0, respectively).
Scatter plots were created to show the percentage change in dermal thickness against the limb volume decrease Figure 3. These showed that the patients who had a limb volume reduction of 5% or more had reduced dermal thickness at the ankle; however, this was not the case at the lower leg or the thigh.
Figure 3: Scatter plots of the change in dermal thickness against the limb volume decrease. Note: The change in dermal thickness is from day 1 to day 3.
Dermal echogenicity: On D1, the ultrasound scans were hypoechoic for most patients at the ankle (7 patients; 77.8%), lower leg (9 patients; 100%), and thigh (6 patients; 66.7%). The remaining scans were isoechoic, apart from one hyperechoic scan of a patient’s thigh, which could be attributed to the absence of edema at this location. On D3, the echogenicity increased for two patients (22.2%) at the ankle, five patients (55.6%) at the lower leg, and three patients (33.3%) at the thigh; a decrease was only seen for two patients (from isoechoic image at ankle and hyperechoic image at thigh). As a result, there was a smaller proportion of patients with hypoechoic images on D3 see Figure 4. Note that the remaining images on D3 were all isoechoic, with one exception at the thigh (hyperechoic).
Figure 4: Skin echogenicity and edema location based on high-frequency ultrasound. A) The percentage of patients with hypoechoic images. Note that the remaining images were all isoechoic, except for one image at the thigh (hyperechoic) on both day 1 and day 3; B) The percentage of patients with edema in the hypodermis and dermis. Note that the edema for the remaining patients was seen in the dermis alone (superficial dermis, deep dermis, or both). Note: ( ) Day 1; (
) Day 1; ( ) Day 3.
) Day 3.
Edema distribution: On D1, the edema was seen in both the hypodermis and dermis for most patients (ankle: 6/9; lower leg: 6/9; thigh: 4/8). For the remaining images, the edema was seen within the dermis, mainly in both the superficial dermis (papillary layer) and the deep dermis (reticular layer) (ankle: 2/9, lower leg: 2/9, thigh: 4/8), but occasionally in the superficial dermis alone (ankle: 1/9, lower leg: 1/9). On D3, this distribution did not change for most of the patients, but the changes that did occur mainly involved less edema in the hypodermis. This was found for two patients at the ankle, two patients at the lower leg, and two patients at the thigh, although there was one patient (11.1%) who developed edema in the hypodermis of the lower leg (in addition to the superficial and deep dermis; (Figure 4). The remaining changes involved the dermal layers that were affected (superficial dermis+deep dermis to superficial dermis alone: thigh: 2/8, lower leg: 1/9; superficial dermis alone to deep dermis+superficial dermis: lower leg: 1/9).
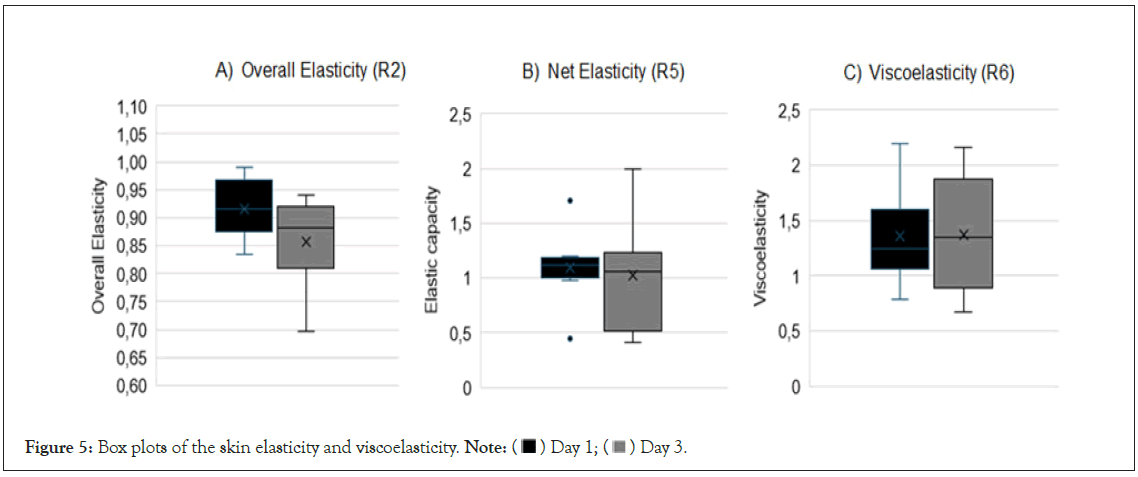
Skin firmness and elasticity: The mean (SD) overall skin elasticity decreased significantly from D1 to D3 (from 0.92 (0.06) to 0.86 (0.08), p=0.039), but there was no significant change in the net elasticity (from 1.10 (0.32) to 1.02 (0.51), p=0.46) or the viscoelasticity (from 1.36 (0.42) to 1.37 (0.52), p=0.95). These results are shown in Figure 5.
Figure 5: Box plots of the skin elasticity and viscoelasticity. Note: ( ) Day 1; (
) Day 1; ( ) Day 3.
) Day 3.
Patient satisfaction
All patients reported that the device was either comfortable (8 patients) or very comfortable (1 patient) both day and night. The patients also all reported that the pressure was bearable (rather bearable: 1 patient; totally bearable: 8 patients), which is in line with their full compliance over the 48 hours. Most of the patients put on and took off the device themselves (N=5), and three of the remaining four patients felt that they would be able to do so. The patients all felt that their lymphedema had improved following the treatment (strong improvement: 2 patients; slight improvement: 7 patients) and that their skin had become more supple (strong improvement: 1 patient; moderate improvement: 4 patients; slight improvement: 4 patients). Most patients also reported that the discomfort caused by the lymphedema (e.g., heaviness, numbness) had improved (strong improvement: 1 patient; moderate improvement: 5 patients). At the end of the study, all patients decided to keep the device for the maintenance phase of their treatment, and five of the patients used it for the rest of the intensive phase (the others used classical bandages).
Safety results
No adverse events were reported, and the device was found to be well tolerated by all patients.
Discussion
This study showed that the Mobiderm® Autofit device successfully reduced limb volume in patients with lymphedema over the first 48 hours of their treatment. As this intensive phase usually includes various interventions, such as MLD and physical exercise, it is striking that improvements were still seen in our patients who were treated using the Mobiderm® Autofit device alone. The significant reduction in limb volume can therefore be attributed to the device as there were no other interventions. Although previous studies have shown limb volume reductions when using Mobiderm® garments and bandages, this is the first time that improvements have shown for a Mobiderm® garment without any other treatment [13,19,20]. The effectiveness is likely to result from the continual pressure applied by the device and may be enhanced by the foam blocks, which could facilitate the flow of lymph by creating a ‘massage effect’. We explored the underlying mechanisms by obtaining various measures of the patients’ lymphatic system and skin.
We first examined the patients’ lymphatic system using lymphoscintigraphy. The dynamic images showed that spontaneous activity could be seen in the lymphatic vessels for around half of the patients, but that this was mostly abnormal. This is in line with the patients’ dysfunctional lymphatic system. After 48 hours of wearing the Mobiderm® Autofit device, we found that the tracer migrated up the leg to the knee in a shorter space of time. Although this difference was not statistically significant, the pattern of results indicates that the device led to an improved flow of lymph through the lymphatic vascular system. We hypothesize that the pressure applied by the device and the presence of foam blocks leads to an increase in the speed of the radiotracer propagation and its movement through the lymphatic vascular system, thus improving the circulation of lymph. The seemingly contradictory results for certain patients who no longer showed normal spontaneous activity or flow speed on D3 may relate to the fact that the device had just been taken off for the measurements (this was not the case on D1). For instance, it is possible that dermal backflow may occur when the device is taken off, as was seen in one such patient.
The lymphoscintigraphy images enabled us to examine the features of the lymphatic system that became visible through the tracer. The lymph nodes, which collect lymph from multiple afferent vessels, are one of the most distinctive features. When they are visible, this indicates that the radiotracer has been successfully transported along the lymphatic vessels as far as the node. We found that there were two patients with visible lymph nodes prior to wearing the device, and that this number increased to four when the device was put on (D1 only). In addition, the mean number of visible lymph nodes increased when the patients wore the device. These results therefore indicate that the Mobiderm® Autofit device facilitates the flow of lymph along the vessels towards the lymph nodes. It is possible that this is particularly effective for patients who initially have some lymphatic vascular activity. In support of this, a previous study found that visible lymph nodes (through lymphoscintigraphy) could predict the effectiveness of CDT for patients with breastcancer related lymphedema [21]. However, many of our patients did not have any visible lymph nodes, and yet they showed improvement in terms of their limb volume. We argue that this may be because only a small amount of radiotracer was injected, which had served to limit the patients’ exposure to radioactivity (as lymphoscintigraphy was carried out twice within three days), and so this may have reduced the visibility of the lymph nodes. In support of this possibility, a previous study found that additional injections of a radiotracer greatly increased the number of patients (with lower limb lymphedema) who had visible lymph nodes [22]. Specifically, it was found that lymph nodes became visible for 40 patients out of a total of 43 who initially had no visible lymph nodes. It is therefore possible that an additional injection would have increased the number of patients with visible lymph nodes in our study.
Other important features of the lymphatic system were also seen in the lymphoscintigraphy images. These included tortuous vessels, which were seen for most of the patients (83%) and are in line with the dysfunctional lymphatic system. Collaterals were also seen for over half of the patients (57%), which indicate that alternative lymphatic pathways were being used. These features did not change for any of the patients over the course of the study, which suggests that the device did not affect these features over the short term. Another feature was the presence of dermal backflow, which has been described as a diagnostic finding in patients with lymphedema [23]. This feature indicates that the lymphatic valves are dysfunctional or that there is an obstruction, which leads to the lymph flowing backwards along the normally unidirectional vessels. This feature was seen for four of the patients (44%) on D1; we speculate that for the remaining patients this feature may have become visible with an additional tracer injection, as described above for the lymph nodes. When the device was put on, we found that the backflow was no longer apparent for just one patient, which may indicate that the device improved dermal lymphatic flow for this patient.
We examined the patients’ skin using high-frequency ultrasound, which provides high resolution images. It has previously been shown that patients with lymphedema have increased dermal thickness and that this can improve following treatment [17,24]. It has also been found that the decrease in limb volume correlates significantly with the reduction in dermal thickness, although this association is not strong (r=0.37) [17]. In our study, we did not find a significant reduction in the dermal thickness, which could potentially be attributed to the timescale of our study, over just 48 hours. However, we did find that the patients with a larger reduction in limb volume all showed decreased dermal thickness at the ankle. This therefore suggests that improvements in dermal thickness may begin at the level of the ankle when patients are treated for lower limb lymphedema.
Ultrasound images were also used to examine the echogenicity of the skin, which has previously been shown to be low (hypoechoic) in patients with lymphedema [16,25,26]. In line with this, we found that all of the patients had hypoechoic images at the lower leg, and most also had hypoechoic images at the thigh and ankle. Following the 48 hours of treatment, we found fewer hypoechoic images at all tested locations, particularly the lower leg, where the proportion of hypoechoic images fell from 100% down to just 44%. These findings can be attributed to a reduction in the patients’ edema, which relates to the tissue echogenicity, and is in line with the reduction in limb volume following the Mobiderm® Autofit treatment [25]. However, it is important to note that these echogenicity results are limited to the patients’ dermis and the upper part of the hypodermis, because the depth of our highfrequency ultrasound scans was limited to 8 mm.
We examined the distribution of the edema at the skin for each patient using the ultrasound scans. We found that most patients had edema within the hypodermis as well as the dermis on D1. This is line with previous work, which has shown that most excess lymph is located within the subcutaneous tissues (hypodermis) [27]. However, for several patients, we found that the edema was mainly located within the dermis rather than the hypodermis, although this may be due to the depth of our scans; specifically, deeper scans are likely to have revealed edema within the deeper subcutaneous layers. On D3, we found that the distribution of edema remained largely unchanged, although for several patients the edema was no longer seen within the hypodermis. This result indicates that the excess fluid had been drained away from these tissues in these patients. This would imply that the device can improve the drainage of lymph from the hypodermis, at least in certain patients. Concerning the dermis, we found that the edema occasionally shifted between the dermal layers, although there was no consistent pattern. This result is in accordance with the dermal thickness remaining largely unchanged. However, it is possible that differences would have emerged over a longer period.
We used a cutometer to assess the patients’ skin elasticity and viscoelasticity. Previous studies have shown that patients with lymphedema have low skin elasticity [5,28], and that this worsens with the progression of the disease [5]. There is evidence that this is due to a decrease in the elastic fibers within the dermis and hypodermis [5]. Measures of viscoelasticity, which relate to the displacement of interstitial fluid within the dermis, have also been found to be poorer in patients with grade III lymphedema, but this can improve following treatment [17,29]. In our study, we found that the measures of net elasticity (R5) and viscoelasticity (R6) did not change significantly from D1 to D3, thus indicating that short-term treatment using the Mobiderm® Autofit device did not affect these skin parameters. We also found that the overall skin elasticity (R2) decreased slightly. This was previously found in a study on patients with lymphedema who underwent five days of intensive CDT; although an increase in the final deformation was also found, resulting from the reduced edema [17]. The authors argued that the elasticity did not improve because the patients had severe lymphedema with ‘irreversible destruction of elastic fibers’. It is possible that this may also be the case for the patients in our study, and it could account for our findings. However, it is also possible that the results relate to the short duration of our study.
Although the Mobiderm® Autofit device was originally designed for the maintenance phase of lymphedema treatment, our results show that it can also be effective for the intensive phase. This involves adjusting the garment to tighten it, so that it applies the pressure required for the intensive phase. In our study, this was about 40 mmHg, which contrasts with the maintenance phase pressure of around 20 mmHg. Despite the increased pressure, the patients nevertheless reported that the garment was comfortable and that the pressure was tolerable. They were also able to wear the device for the full 24 hours, both day and night.
An advantage of the Mobiderm® Autofit device is that most patients felt able to put the device on and take it off by themselves. This translates into substantial time savings for hospital staff, who typically apply multilayer bandaging for the intensive phase of treatment [30]. The patients also all chose to keep the device for the maintenance phase of their treatment, thus highlighting their willingness to use the device; five of the patients also chose to continue using the garment for the rest of their intensive phase treatment, instead of changing to bandages. A further advantage relates to the adjustable straps, which means that patients can tighten and loosen the device when required.
The main limitation of this study is that the patients were only followed up for 48 hours, and so any changes beyond this would have been missed. Another limitation relates to the heterogeneity of the patients as they differed greatly in terms of their age (28-72 years) and the duration of the disease (0.5-29 years); there were also patients who were at stage II of the disease while others were at stage III. These factors may have affected the measurements and whether they changed from D1 to D3. For example, skin elasticity is known to be poorer in older adults and so it may change less following treatment for lymphedema [5]. There may also be less improvement for patients with more advanced (stage III) lymphedema. Finally, there were multiple statistical comparisons, which would have increased the risk of a type I error.
Despite these limitations and our small sample size, the results show that the Mobiderm® Autofit device can reduce limb volume within just two days, even without any other treatment (e.g., bandages, stockings, pressotherapy, MLD). The results also indicate that certain mechanisms may underlie the device’s effectiveness, which can be examined further in future studies. For example, the lymphoscintigraphy results indicate that the device may increase the speed of the lymphatic flow and improve movement towards the lymph nodes. In addition, the ultrasound measurements indicate that certain improvements may begin at the level of the ankle, where the edema may be greater due to gravity. We hypothesize that the reduction of edema within the dermis and hypodermis (reflected by less hypoechogenicity) mainly results from the passage of lymph through the superficial (cutaneous) lymphatic system rather than the deep lymphatic system. If confirmed, this would indicate that the Mobiderm device promotes the movement of lymph through the usual lymphatic pathways.
In the future, it would be helpful to run randomized controlled studies with larger sample sizes to confirm and expand upon these findings. It would also be helpful to examine the role of the deep lymphatic vessels, which were not the focus of the present study. In addition, it would be of interest to compare treatment using the Mobiderm® Autofit device with another device, such as multilayer bandaging, and to determine how the presence of foam blocks affects the results
Conclusion
This study shows that the Mobiderm® Autofit device is effective for reducing limb volume in patients with lymphedema after 48 hours of treatment. The imaging results indicate that the device improves the drainage of edema from the subcutaneous tissue and facilitates the flow of lymph towards the lymph nodes. Although skin improvements were not seen, this could be attributed to the short time span of the study.
Acknowledgement
We thank Jessica Foxton, PhD, from Newmed Publishing, Paris, for medical writing assistance.
Funding
This study was funded by Thuasne, France.
Conflicts of Interest
None to report
References
- Cemal Y, Pusic A, Mehrara BJ. Preventative measures for lymphedema: Separating fact from fiction. J Am Coll Surg. 2011;213(4):543-551.
[Crossref] [Google Scholar] [PubMed]
- Dean SM, Valenti E, Hock K, Leffler J, Compston A, Abraham WT. The clinical characteristics of lower extremity lymphedema in 440 patients. J Vasc Surg Venous Lymphat Disord. 2020;8(5):851-859.
[Crossref] [Google Scholar] [PubMed]
- Sung C, Wang S, Hsu J, Yu R, Wong AK. Current understanding of pathological mechanisms of lymphedema. Adv Wound Care (New Rochelle). 2022;11(7):361-373.
[Crossref] [Google Scholar] [PubMed]
- Lymphoedema Framework. Best practice for the management of lymphoedema. International consensus. London: MET Ltd. 2006.
- Sano M, Hirakawa S, Yamanaka Y, Naruse E, Inuzuka K, Saito T, et al. Development of a noninvasive skin evaluation method for lower limb lymphedema. Lymphat Res Biol. 2020;18(1):7-15.
[Crossref] [Google Scholar] [PubMed]
- Kalemikerakis I, Evaggelakou A, Kavga A, Vastardi M, Konstantinidis T, Govina O. Diagnosis, treatment and quality of life in patients with cancer-related lymphedema. J BUON. 2021;26(5):1735-1741.
[Google Scholar] [PubMed]
- Grada AA, Phillips TJ. Lymphedema: Pathophysiology and clinical manifestations. J Am Acad Dermatol. 2017;77(6):1009-1020.
[Crossref] [Google Scholar] [PubMed]
- Co M, Lee A, Kwong A. Cutaneous angiosarcoma secondary to lymphoedema or radiation therapy-A systematic review. Clin Oncol (R Coll Radiol). 2019;31(4):225-231.
[Crossref] [Google Scholar] [PubMed]
- Brandão ML, Soares HP, Andrade MD, Faria AL, Pires RS. Efficacy of complex decongestive therapy for lymphedema of the lower limbs: A systematic review. J Vasc Bras. 2020;19:e20190074.
[Crossref] [Google Scholar] [PubMed]
- Cohen SR, Payne DK, Tunkel RS. Lymphedema: Strategies for management. Cancer. 2001;92(S4):980-987.
[Crossref] [Google Scholar] [PubMed]
- Vignes S, Albuisson J, Champion L, Constans J, Tauveron V, Malloizel J, et al. Primary lymphedema French National Diagnosis and Care Protocol (PNDS; Protocole National de Diagnostic et de Soins). Orphanet J Rare Dis. 2021;16(1):18.
[Crossref] [Google Scholar] [PubMed]
- Mosti G, Cavezzi A, Partsch H, Urso S, Campana F. Adjustable velcro compression devices are more effective than inelastic bandages in reducing venous edema in the initial treatment phase: A randomized controlled trial. Eur J Vasc Endovasc Surg. 2015;50(3):368-374.
[Crossref] [Google Scholar] [PubMed]
- Mazur S, Szczęśniak D, Tchórzewska-Korba H. Effectiveness of Mobiderm Autofit in the intensive phase of breast cancer-related lymphedema treatment: A case series. Lymphat Res Biol. 2023;21(6):608-613.
[Crossref] [Google Scholar] [PubMed]
- Mestre S, Calais C, Gaillard G, Nou M, Pasqualini M, Ben Amor C, et al. Interest of an auto-adjustable nighttime compression sleeve (MOBIDERM® Autofit) in maintenance phase of upper limb lymphedema: The MARILYN pilot RCT. Support Care Cancer. 2017;25(8):2455-2462.
[Crossref] [Google Scholar] [PubMed]
- Villa G, Campisi CC, Ryan M, Boccardo F, Di Summa P, Frascio M, et al. Procedural recommendations for lymphoscintigraphy in the diagnosis of peripheral lymphedema: The Genoa protocol. Nucl Med Mol Imaging. 2019;53(1):47-56.
[Crossref] [Google Scholar] [PubMed]
- Naouri M, Samimi M, Atlan M, Perrodeau E, Vallin C, Zakine G, et al. High‐resolution cutaneous ultrasonography to differentiate lipoedema from lymphoedema. Br J Dermatol. 2010;163(2):296-301.
[Crossref] [Google Scholar] [PubMed]
- Hacard F, Machet L, Caille A, Tauveron V, Georgescou G, Rapeneau I, et al. Measurement of skin thickness and skin elasticity to evaluate the effectiveness of intensive decongestive treatment in patients with lymphoedema: a prospective study. Skin Res Technol. 2014;20(3):274-281.
[Crossref] [Google Scholar] [PubMed]
- Everett JS, Sommers MS. Skin viscoelasticity: Physiologic mechanisms, measurement issues, and application to nursing science. Biol Res Nurs. 2013;15(3):338-346.
[Crossref] [Google Scholar] [PubMed]
- Cho SC, Kwak SG, Cho HK. Effectiveness of Mobiderm® bandages in the treatment of cancer-related secondary lymphedema: A pilot study. Medicine (Baltimore). 2022;101(35): e30198.
[Crossref] [Google Scholar] [PubMed]
- Dhar A, Srivastava A, Pandey RM, Shrestha P, Villet S, Gogia AR, et al. Safety and efficacy of a Mobiderm compression bandage during intensive phase of decongestive therapy in patients with breast cancer-related lymphedema: A randomized controlled trial. Lymphat Res Biol. 2023;21(1):52-59.
[Crossref] [Google Scholar] [PubMed]
- Kim YH, Hwang JH, Bae JH, Choi JY. Predictive value of lymphoscintigraphy in patients with breast cancer-related lymphedema undergoing complex decongestive therapy. Breast Cancer Res Treat. 2019;173:735-741.
[Crossref] [Google Scholar] [PubMed]
- Bourgeois P, Leduc O. Value of one additional injection at the root of the limb in the lymphoscintigraphic evaluation and management of primary and secondary lower-limb lymphedemas. PLoS One. 2021;16(7):e0253900.
[Crossref] [Google Scholar] [PubMed]
- Oh A, Kajita H, Imanishi N, Sakuma H, Takatsume Y, Okabe K, et al. Three-dimensional analysis of dermal backflow in cancer-related lymphedema using photoacoustic lymphangiography. Arch Plast Surg. 2022;49(1):99-107.
[Crossref] [Google Scholar] [PubMed]
- Yildiz ED, Bakar Y, Hizal M. The effect of complex decongestive physiotherapy applied with different compression pressures on skin and subcutaneous tissue thickness in individuals with breast cancer-related lymphedema: A double-blinded randomized comparison trial. Support Care Cancer. 2023;31(7):383.
[Crossref] [Google Scholar] [PubMed]
- Gniadecka M. Localization of dermal edema in lipodermatosclerosis, lymphedema, and cardiac insufficiency: High-frequency ultrasound examination of intradermal echogenicity. J Am Acad Dermatol. 1996;35(1):37-41.
[Crossref] [Google Scholar] [PubMed]
- Iker E, Mayfield CK, Gould DJ, Patel KM. Characterizing lower extremity lymphedema and lipedema with cutaneous ultrasonography and an objective computer-assisted measurement of dermal echogenicity. Lymphat Res Biol. 2019;17(5):525-530.
[Crossref] [Google Scholar] [PubMed]
- Olszewski WL, Jain P, Ambujam G, Zaleska M, Cakala M. Where do lymph and tissue fluid accumulate in lymphedema of the lower limbs caused by obliteration of lymphatic collectors?. Lymphology. 2009;42(3):105-111.
[Google Scholar] [PubMed]
- Killaars RC, Penha TL, Heuts EM, Hulst RRVD, Piatkowski AA. Biomechanical properties of the skin in patients with breast cancer-related lymphedema compared to healthy individuals. Lymphat Res Biol. 2015;13(3):215-221.
[Crossref] [Google Scholar] [PubMed]
- Auriol F, Vaillant L, Lopes CP, Machet L, Diridollou S, Berson M, et al. Study of cutaneous extensibility in lymphoedema of the lower limbs. Br J Dermatol. 1994;131(2):265-269.
[Crossref] [Google Scholar] [PubMed]
- O'Donnell TF, Allison GM, Iafrati MD. A systematic review of guidelines for lymphedema and the need for contemporary intersocietal guidelines for the management of lymphedema. J Vasc Surg Venous Lymphat Disord. 2020;8(4):676-684.
[Crossref] [Google Scholar] [PubMed]
Citation: Vaillant L, Tauveron V, Courtehoux M (2024) Mobiderm® Autofit Garments for Reducing Lower Limb Lymphedema: A Single-Arm Study Exploring Underlying Mechanisms. J Vasc Surg. 12:565.
Copyright: © 2024 Vaillant L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.