Indexed In
- Genamics JournalSeek
- JournalTOCs
- CiteFactor
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 12, Issue 3
Microstructural Characterization of Mild Steel Used in Oil and Gas Pipeline
Engr Shamsher Khan*, Aamir Iqbal, Muhammad Alamzaib Khan, Shakeel Ahmad and Tanzeela SajjadReceived: 12-Jan-2023, Manuscript No. JAME-23-19574; Editor assigned: 16-Jan-2023, Pre QC No. JAME-23-19574 (PQ); Reviewed: 30-Jan-2023, QC No. JAME-23-19574; Revised: 13-Apr-2023, Manuscript No. JAME-23-19574 (R); Published: 20-Apr-2023, DOI: 10.35248/2168-9873.23.12.475
Abstract
Result associated with microstructural and strength analyses of the material collected from the oil field are presented. The effects of varying microstructure and hardness by heat treatment process were presented. The microstructural characterization and surface analysis were carried out by Scanning Electron Microscope (SEM), Energy Dispersive Spectroscopy (EDS), X-Ray Diffraction (XRD), and Rockwell for hardness analysis. The energy dispersive spectroscopy EDS indicated that the samples were in the range of low carbon steel (AISI 1008) i.e. highly ductile and soft. XRD analysis showed that iron carbide phase was formed during service condition which is brittle in nature. Scanning electron microscopy (SEM) of the surface examination denotes that the material was greatly affected by erosion leading to crack initiation and propagation.
Keywords
Low carbon steel; Corrosion; Microstructure; Hardness; Microscope
Introduction
The mild or low carbon steel is very acceptable material due to its huge applications in the industries. It provides good deformability due to its low yield strength, minimum cost, simplicity in fabrication and manufacturing, good strength, toughness and wild ability. Materials are being processed to convert it from high stable state to lower stability by performing engineering processes in order to withstand harsh environment. Metals are unstable at ordinary environment and certain environment condition gives opportunities for these materials to combine chemically with elements to get stable state or lower energy state. Mild steel is one of the most usable materials in various production and chemical industries. However due to its high susceptibility to corrosion it deteriorate very rapidly as compared to other metals. Different strengthening mechanism can be applied to enhance the grain refinement in order to increase the strength and toughness both simultaneously. Mild steel or low carbon steel consist a small fraction of carbon that gives better plastic deformation behavior in sealing and leakage prevention. Mild or Low carbon steel are universally used for structural applications, food production industries, automotive and aircraft industries; however, its poor corrosion resistance at ambient atmosphere condition is a matter of serious concern [1,2]. All ferrous materials consist mainly of iron with small proportions of other alloying elements. These alloying elements have added to obtain specific characteristics such as corrosion resistance, strength and wear resistance to enhance the mechanical behavior of the resultant alloy. Most common steel alloying elements are aluminum, vanadium, nickel, manganese, molybdenum, chromium, tungsten, boron and phosphorous.
Based on the carbon contents steel is divided into three major categories, low carbon or mild steel (wt.% 0.04-0.30), medium carbon steel (wt.% 0.31-0.6) and high carbon steel (wt.% 0.61-2.40). Increasing the carbon contents results in increasing the hardness of the materials and decrease in the ductility as well as corrosion resistance. High carbon steel can be used where there is hardness and strength is the first priority as in transporting, construction and relative motion between the parts, whereas mild steel can be appreciated in low cost and high ductile application environment. Despite the fact the low carbon or mild steel has high susceptibility to corrosion, is used in several application in which corrosion phenomena is a key factor [3,4]. Many researches have been conducted to analyze the corrosion phenomenon of mild steel used in various industries. It has been investigated that the corrosion first initiate at grain boundaries and spread to the bulk of the grains [5]. Statically evaluation of the material indicates that the inhibition of the materials has greatly affected the corrosion process [6].
Metallurgical factors are greatly affecting the corrosion rate. In practice the characterization of the material are determining the influence of grain sizes structure and orientation on corrosion behavior. In oil and gas industries due to high temperature and pressure the erosion-corrosion phenomena are greatly investigated. It is also investigated that sulfide inclusion in pure iron has also a marked tendency to react in corrosive environment. Moreover, crystal structure and orientation also influence on corrosion rate. The crystal structure assumed to be perfect in three dimensional spaces, but in facts, there is variation in the arrangement of crystal which is considered as crystalline defects caused an exchange of electron and leads to electrochemical corrosion.
It is also indicated that the corrosion process has a great dependency on geotechnical characteristics of site, the presence of electrochemical corrodant and time period of the materials [7]. The effect of inhibition of the materials to increase the corrosion resistance can be enhancing by applying suitable coating parameter time and temperature [8]. Moreover; Hassane Lgaz, et al., has explained the effect of different mixed inhabitant on the corrosion process of the mild steel, which leads to high increase polarization resistance [9]. It is also concluded that the materials hardened by heat treated land recrystallization leads to increase the corrosion resistance [10]. The effect of zinc coating were studied and it is concluded that zinc coating is a promising method of silane coating which is better inhabitant among the several inhabitant [11]. It is investigated that the presence of several ferrous ions in the material can changed the corrosion acceleration by one or more aspects [12,13]. The successful use of carbon steel line pipe relies on appropriate design allowances and corrosion controls. Carbon steel line pipe used in oil/gas production and transmission, is manufactured in accordance with American Petroleum Institute (API) specification 5 L, does not have a closely specified elemental composition and microstructure. Consequently, it is fabricated to a set of mechanical requirements such as yield strength, tensile strength and fracture toughness. This can allow for significant variations in the elemental composition and microstructure, which can also influence corrosion performance. Although the specification emphasizes material strength and toughness, concentration limits of some elements (i.e., carbon, manganese, phosphorus and sulfur) are also specified to ensure wild ability, formability and corrosion resistance. However, the levels of alloying elements such as nickel, chromium and niobium, which may be added to the steel, are not specified. Furthermore, permitted levels of carbon, manganese, phosphorus and sulfur, which are specified for each grade, may be different for seamless, welded and cold worked pipe. Similarly, the compositional and microstructural properties can vary significantly between pipes of the same grade from different manufacturers, and these variations may lead to substantial differences in the corrosion resistance of steel line pipe [14].
Materials and Methods
Materials
Soft iron/low carbon steel specimens were collected from Mari gas oil field in the form of ring gasket as shown in Figure 1. The material in the ring gasket form which use in oil and gas production pipeline between the flanges groves in order to seal the surface to prevent leakage. Mostly the ring gaskets are formed from soft material to flow in the groves easily when joint the two flanges by nut and bolt.

Figure 1: Corroded and uncorroded ring gasket collected from Mari gas oil field.
Experimental setup
Samples are prepared from both corroded and uncorroded materials for SEM analysis. The total numbers of samples (corroded and uncorroded) were 16, in which 8 samples are corroded and, 8 samples of uncorroded material. A special cutting machine was used to cut the sample of specified dimension carefully, so that not overheats to damage the gran size and shape as shown in Figure 2a. All the samples were mounted in backlit as shown in Figure 2b, and polished with 9- micron diamond paste and nylon cloth. As the samples prepared free from scratches and obtained a mirror like surface, then carefully break the mounted and etched in two different nital solutions. The two nital solutions were based on the concentration of Nitric Acid (HNO3) in Ethanol (C2H5O). the two etchants containing 8% nitric acid in ethanol and second 3% nitric acid in ethanol. The polished samples were etched in two ways in order to get good and visible grains i.e. some samples were etched for 15, 30, 45, 60 and 90 seconds in nital containing 3% nitric acid while others were etched for a fixed interval of 60s in nital containing 8% nitric acid in ethanol.
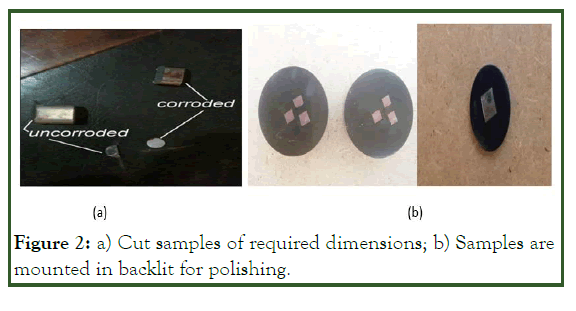
Figure 2: a) Cut samples of required dimensions; b) Samples are mounted in backlit for polishing.
Result and Discussion
SEM analysis
A SEM analyzer was used to investigate the microstructure with a JEM-5910 (JEOL) analyzer. The SEM analysis of the uncorroded sample in 3% HNO3 in ethanol are obtained at 200 X. Samples etched for 30 sec revealed the granular structure as shown in Figure 3, the same solution and procedure is applied for corroded sample gives the granular structure as shown in Figure 4. The samples over etched for longer than 30 sec in 8% nital solution. But as the sample etched in the accurate nital solution and time i.e. 8% and 30 sec or 3% and 60 sec, a more visible structure obtained. According to the lever rule low carbon steel (AISI 1008) contains 97.4% ferrite (α) and 2.6% cementite (FeC3). Only ferrite could be observed due to its relatively higher concentration in the examined sample which had two phase BCC crystal structure as shown in Figure 5. Moreover; it is clearly observed from Figure 5, that the pearlitic area is increases strongly after corrosion, this is due to the inclusion of carbon exist in crude oil under high temperature and pressure. As the carbon is absorbed under high temperature and pressure a chemical segregation effect is initiated in which a specific phases precipitated on the grain boundaries. The phase containing high percentage of iron sulfide FeS as observed in Figure 6.
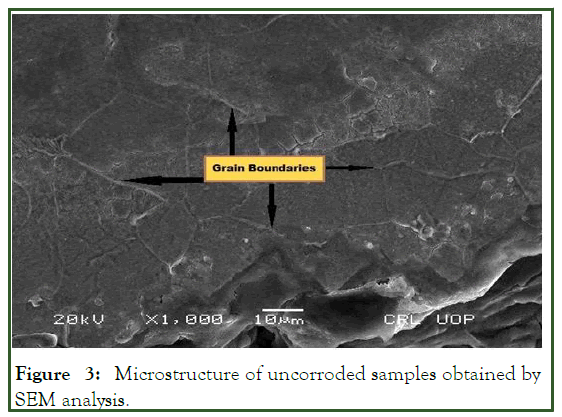
Figure 3: Microstructure of uncorroded samples obtained by SEM analysis.
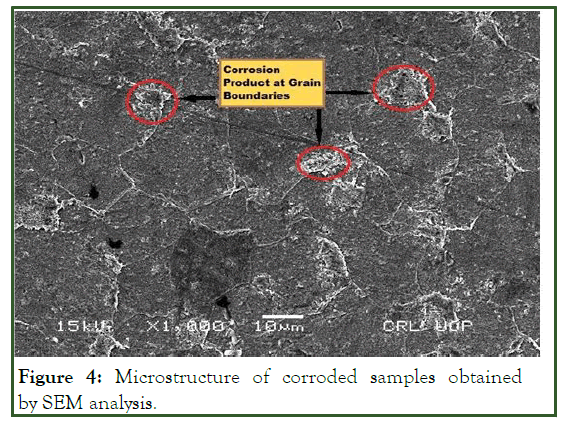
Figure 4: Microstructure of corroded samples obtained by SEM analysis.
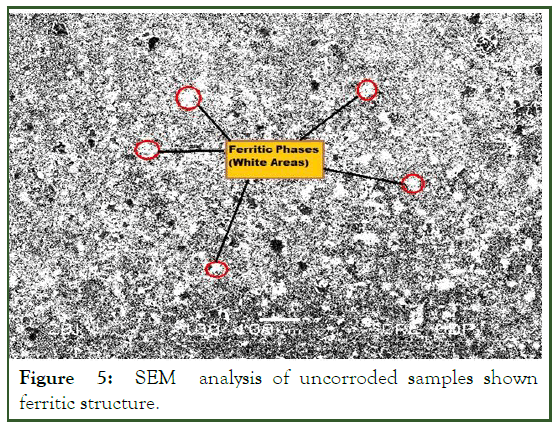
Figure 5: SEM analysis of uncorroded samples shown ferritic structure.
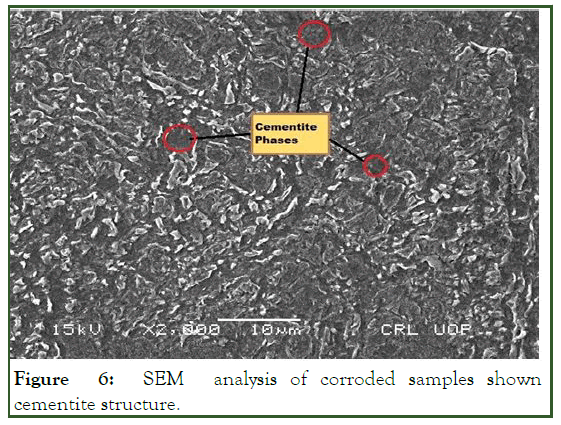
Figure 6: SEM analysis of corroded samples shown cementite structure.
The attack is usually related to the segregation of specific phase formed on grain boundaries (Figure 7). Corrosion then occurred by preferential attack on the grain boundaries, makes the grain boundaries weaker. As the grain boundaries is affected by corrosion and make it weaker so there is high temperature and pressure and crack is initiated and propagated towards the bulk of the grain as indicated in Figure 8.
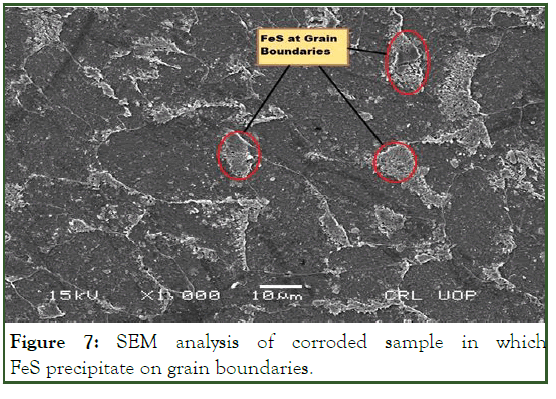
Figure 7: SEM analysis of corroded sample in which FeS precipitate on grain boundaries.
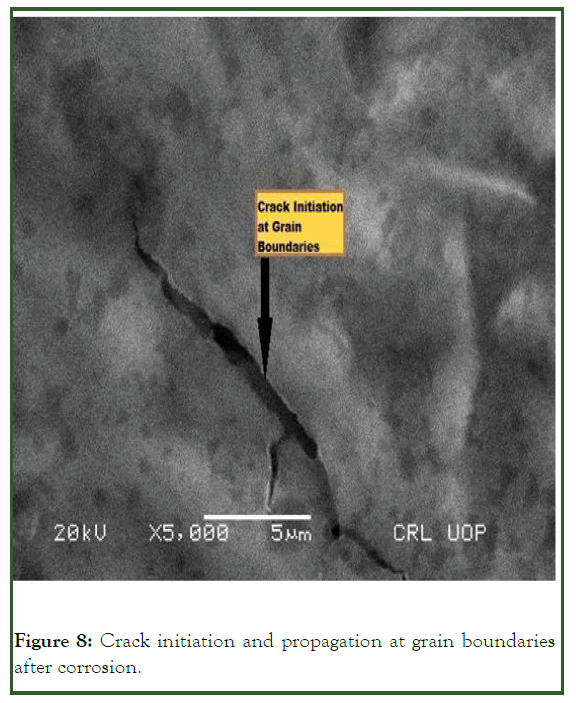
Figure 8: Crack initiation and propagation at grain boundaries after corrosion.
X-ray diffraction
The x-ray test was used to find out the corrosion product. The maximum corrosion product were contain iron sulfide shown in the Figure 9. The corrosion product contains α-FeOOH, γ- FeOOH and amorphous-like phase and the amorphous like phase contains more than 50% of the rust. The thickness of the rust is uneven showing wavy and undulating near the surface of the steel and rust the amorphous phase take place.
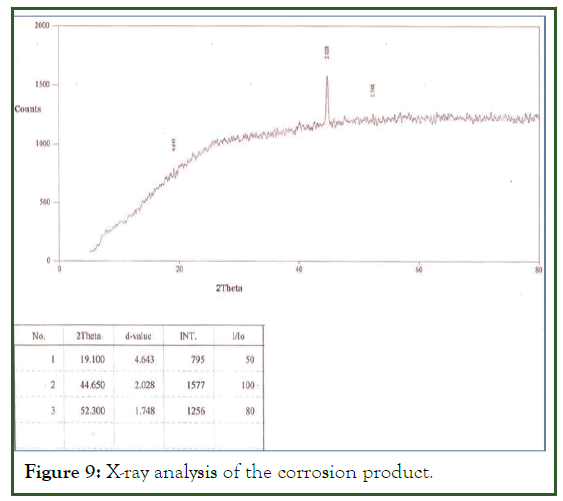
Figure 9: X-ray analysis of the corrosion product.
EDS analysis
Elemental composition of the material were determined by energy dispersive spectroscopic analysis of both the materials i.e. corroded and uncorroded were very similar as shown in Figures 10 and 11 respectively. The chemical composition is of the material is summarized in Table 1. Only a minor change was observed in oxygen. This is due to the carbon dioxide present in the crude oil which results of the chemical reaction of carbon with the water in the presence of high temperature and pressure.
| Material | Composition % |
|---|---|
| F | 89.8 |
| C | 0.96 |
| Mn | 0.68 |
| P | 0.18 |
| S | 0.01 |
Table 1: Chemical composition of the material.
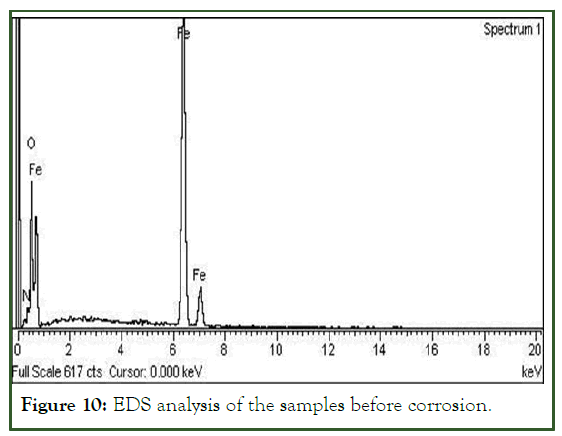
Figure 10: EDS analysis of the samples before corrosion.
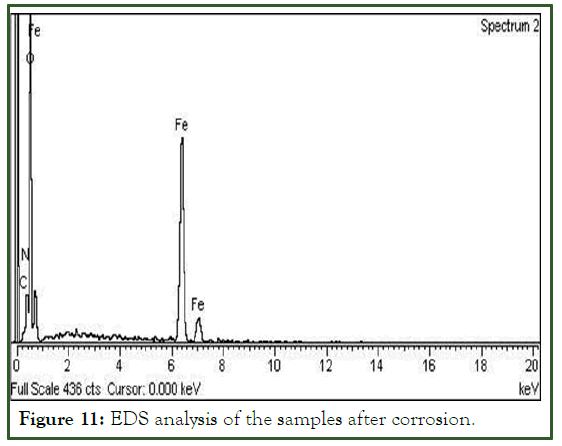
Figure 11: EDS analysis of the samples after corrosion.
Conclusion
This study has shown that the corrosion was occurred in a chemically harsh and aqueous medium in the crude oil. First chemical corrosion takes place and then initiated by the high velocity of crude oil leads to erosion-corrosion. Furthermore, mild steel ring gasket investigated in this paper revealed corrosion phenomena variations due to the variation of environment in which the material were used. It has been shown that variations in the corrosion/penetration rate occur partly due to differences in the microstructure. It was found that steels with a banded ferrite/pearlite structure perform poorly in terms of localized corrosion and this was attributed to a segregated distribution of the iron carbide phase cementite (Fe3C). By contrast, all other microstructural types were observed to have a uniform distribution of cementite. Insignificant differences were observed in the corrosion performance of steels having finegrained ferrite, ferrite/coarser and somewhat acicular pearlite/ pearlite or tempered marten site microstructures. In any event, the ferrite/coarser and somewhat acicular pearlite/pearlite material performed better in terms of both the average corrosion and penetration rates. It is suggested that a ferrite/coarser and somewhat acicular pearlite/pearlite structure may be more suitable under the conditions investigated in this study compared to a coarse banded ferrite/pearlite structure. This paper has demonstrated that steel microstructure is an important consideration when selecting a ring gasket material for a particular application.
References
- Niaz F, Khan MR, Ihsan-ul-Haque. Microstructural characterization of low carbon steel used in aircraft industry. JPMS Conference Issue, Materials. 2010;19-26. [Google Scholar]
- Al-Sultani KF, Al-Roubaiy AO, Duaa AA. Characterization of Corrosion Behavior of Low Carbon Steel Oil Pipelines by Crude Oil. Res J Recent Sci. 2016;5(4):1-5. [Google Scholar]
- Kartsonakis IA, Charitidis CA. Corrosion protection evaluation of mild steel: The role of hybrid materials loaded with inhibitors. Appl Sci. 2020;10(18):6594.
- Le L, Sofi M, Lumantarna E. The combined effect of stress and corrosion on mild steel. J Constr Steel Res. 2021;185:106805.
- Anadebe VC, Onukwuli OD, Omotioma M, Okafor NA. Optimization and electrochemical study on the control of mild steel corrosion in hydrochloric acid solution with bitter kola leaf extract as inhibitor. S Afr J Chem. 2018;71:51-61.
- Loto RT, Mbah E, Ugada J. Electrochemical data and analysis of the protection effect of Citrus sinensis on mild steel in weak acid electrolytes. Chem Data Collect. 2021;34:100736.
- Suganya S, Jeyalakshmi R. Long term study on the corrosion behaviour of buried mild steel under different native soil environments. Materials Today: Proceedings, Elsevier, 2021;47:957-963.
- Karthick M, Yoganandam K, Victor DPM. Investigation of mechanical properties and corrosion performance of CNT coated on EN8 mild steel. Materials Today: Proceedings, Elsevier, 2021;46:4224-4227.
- Lgaz H, Chung IM, Albayati MR, Chaouiki A, Salghi R, Mohamed SK. Improved corrosion resistance of mild steel in acidic solution by hydrazone derivatives: An experimental and computational study. Arab J Chem. 2020;13(1):2934-2954.
- Suraj R. Hardfacing and its effect on wear and corrossion performance of various ferrous welded mild steels. Materials Today: Proceedings, Elsevier, 2021;42:842-850.
- Tavangar R, Naderi R, Mahdavian M. Acidic surface treatment of mild steel with enhanced corrosion protection for silane coatings application: The effect of zinc cations. Prog Org Coat. 2021;158:106384.
- Rbaa M, Benhiba F, Abousalem AS, Galai M, Rouifi Z, Oudda H, et al. Sample synthesis, characterization, experimental and theoretical study of the inhibitory power of new 8-hydroxyquinoline derivatives for mild steel in 1.0 M HCl. J Mol Struct. 2020;1213:128155.
- De la Fuente D, DIaz I, Simancas J, Chico B, Morcillo M. Long-term atmospheric corrosion of mild steel. Corros Sci. 2011;53(2):604-617.
- Nathan SR, Balasubramanian V, Malarvizhi S, Rao AG. Effect of welding processes on mechanical and microstructural characteristics of high strength low alloy naval grade steel joints. Def Technol. 2015;11(3):308-317.
Citation: Khan ES, Iqbal A, Khan MA, Ahmad S, Sajjad T (2023) Microstructural Characterization of Mild Steel Used in Oil and Gas Pipeline. J Appl Mech Eng. 12:475.
Copyright: © 2023 Khan ES, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.