Indexed In
- Academic Journals Database
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2024) Volume 16, Issue 3
Microbial Secondary Metabolites as Immunomodulators
Fariba Mahmoudi* and Behzad BaradaranReceived: 21-Sep-2020, Manuscript No. JMBT-24-6553; Editor assigned: 24-Sep-2020, Pre QC No. JMBT-24-6553 (PQ); Reviewed: 08-Oct-2020, QC No. JMBT-24-6553; Revised: 01-Aug-2024, Manuscript No. JMBT-24-6553 (R); Published: 29-Aug-2024, DOI: 10.35248/1948-5948.24.16.615
Abstract
One auspicious strategy for immune system improvement and enhancement of immune function is immunomodulatory therapies which can help to restitute the balance of immunity.
Today due to the growing trend of immune disorders and new viral diseases as well as the increasing incidence of cancer, there is a greater demand to produce immunomodulating compounds with more efficacy and fewer side effects. Bacterial derivatives are a very fertile ground for discovering a lot of new compounds with various medical properties. Many natural products from bacterial sources like secondary metabolites are endowed with promising immunomodulating activities, which representing importance and value of this topic in the drug discovery and there is a clear need for a coherent source for study in this area. The aim of this review is to highlight the work on immunomodulatory effects of bacterial secondary metabolites and natural immunomodulators from microbial origin.
Keywords
Immunomodulators; Immunomodulation; Bacterial secondary metabolites; Microbial secondary metabolites
Introduction
The immune system has a crucial performance in modifying disease progress, hence modulating of immune function could be a good strategy for treatment of diseases. One of the most promptly promoting area of medical biotechnology research is immunology which has the great promises with regard to the prevention and treatment of a wide range of disorders such as the inflammatory diseases. In addition infectious diseases which now are considered immunological disorders primarily, may involve in an immunosuppressive state and also neoplastic diseases, viral infections, organ transplantation and different autoimmune diseases are dependent on the function of the immune system and in each case depending on the type of disorder, may need to suppress or stimulate the immune system. So, discovering new effective compounds which could improve immune system function is essential. As it mentioned one efficient approach for enhance immune function is immunomodulatory therapies, on the other hand bacterial secondary metabolites have a potent background for new discovering and based on multiple scientific studies many natural products from microbial sources are endowed with immunomodulating effects and by using genetic engineering it became possible to fully exnatural press biologically active copies of such powerful molecules from bacteria.
Overview of the immune system
Humans may encounter to numerous of pathogenic organisms via ingestion, inhalation and the other ways, while the immune system has expanded and evolved to protect the body against this wide range of pathogenic agents that are themselves continually evolving and also helps the host to eradicate allergenic substances and toxins which could penetrate via mucosal surfaces. The main part of immune system’s ability to organize a response to an invasive pathogen, allergen or a toxin, is its ability to differentiate self from non-self. Here we refer to key mechanisms used by the immune system to reacts against microorganisms and other exogenous agents and the settings which in the disordered immune system intensifies tissue damage. The distinguish self from non-self, is the foundation of immune function, this nonself could be an invasive organism, a transplanted organ or an endogenous cell that can be misdiagnosed as a foreign [1]. The immune response has two branches: Innate and adaptive immunity that in most cases host harmonized both mechanisms to identify and eliminate pathogens Both these processes have two parts; humoral and cellular.
Innate and acquired immune responses perform in harmonized form to eradicate problem. In some cases, innate responses are enough to overcome the disruptive agent and in many other cases, certain proteins and cells of innate immune system, for example Antigen Presenting Cells (APC), process the agent fragments which then present to adaptive immune system to neutralize these pathogens. Dendritic Cells (DC) are known to be the key antigen-presenting cells which they have an important role in linking innate to adaptive immunity. Dendritic cells capture, processed and present the antigen to activate the naive T cells and for the start of the primary immune reaction [2]. It has been shown that DCs are participate in inappropriate and uncontrolled immune responses including allergy, autoimmunity, cancer and transplant rejection.
Materials and Methods
Progress of adaptive immune responses against a new pathogen in the body is comparatively takes more time than innate immune responses adaptive immune responses mediated by B - and T-lymphocytes which exerts more effective immune responses and NK cells run innate immune response in collaboration with mature cells originating from tri lineage myeloid stem cells. During confront to specific antigens, Blymphocytes differentiate into antibody producing plasma cells in the bone marrow.
Concurrently, APCs activated T-cells, transfer to the thymus and get differentiated to the cytotoxic T-cells (CD8+) and helper Tcells (CD4+) under efficient stimulation by Antigen Presenting Cells (APC) acquire T-Cell Receptor (TCR). The CD4+ helper Tcell subtypes of T-cells differentiate into several subtypes at the outside of the thymus: TH1, TH2 and TH3 which each of these cells synthesize the different cytokines (IL-2 and IFN-γ). TH1 Tcells synthesize cytokines that stimulate proliferation and differentiation of T-lymphocytes and NK cells. These cytokines do an essential function in Cell Mediated Immunity (CMI). TH2 T-cells produce cytokine (IL-4, IL-5, IL-10 and IL-13) that stimulate B-lymphocytes proliferation for humoral immunity.
TH3 T-cells are essential in the production of antiinflammatory (IgA) antibodies that are necessary in secretory immunity. TH3 T-cells also have a key role in resting phases of immune response, thus the cytokines play an important role in different parts of immunity. It has been well known that B cells identify different specific antigens via BCRs, while CD8+ T-cells identify antigens as peptide/MHC class I complexes and CD4+ cells recognize antigens as peptide/MHC class II complex (Figure 1).
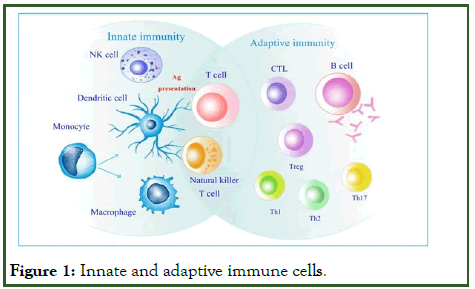
Figure 1: Innate and adaptive immune cells.
Immune system modulation
Immune system modulation or immunomodulation, is the method which use the therapy to manage and regulate the immune response, usually to avoid tissue injury as a result of an over activated response and refers to the all therapeutic strategies targeted for manipulating and modulating the immune system, according to the vital role of immune system and the quality of its performance, dysregulation of immune interactions can result in infections, inflammatory disorders and thus life-threatening. Immunomodulation is a type of novel treatments like phage therapy and cytokine therapy, with an aim to increase the immune potential and also maintain or restore balance to immune function as an alternative to control the disease during the course of time [3]. This approach raises hope for the prevention and treatment of a wide range of disorders such as cancer, the inflammatory diseases of central organs, gut, respiratory tract, skin and joints, also, viral infections and other kind of infectious diseases, organ transplantation and several autoimmune diseases so many immunomodulators are introduced and it was found that microbial types are significantly effective.
This approach can be useful in two form including immunostimulation and immunosuppression and also can be either specific or non-specific, related to a non-specific stimulation of the immune response immuno stimulation suggests a non-antigen dependent stimulation of the function of macrophages, natural killer cells, granulocytes, complement, lymphocytes and also the production of various effector molecules by activated cells. Being non-specific, expected to improve defense against diverse pathogens including viruses, bacteria, fungi, etc.
To prevent infection, the immunostimulation which also known as immune potentiation, can be utilized by vaccination through stimulation of humoral immunity, to confronting against an already settled infection by shifting the response to cell mediated immunity and to act against cancer by the use of tumor infiltrating lymphocytes, cytokines and tumor specific antibodies.
The suppression of immune response, well-known as immune suppression, is usually tried in overactive immune reaction, allergy, inflammation, autoimmunity and organ transplantation. As we mentioned immunomodulation can be either specific or non-specific, specific immunomodulation is restricted to a single determined antigen such as process of vaccination [4]. Nonspecific immunomodulation suggests for a more widespread alteration in immune responsiveness in both of innate and adaptive immunity, moves toward altered host reactivity to several various antigens.
At present the most current and commonly accepted approach to treat the microbial infections is chemotherapy. In the near futures available antibiotics may not act against pathogens which they are targeted, because of the rapid occurrence of drug resistant resulting evolution of microbial strains, all the microorganisms try to escape antibiotics which are proposed to destroy them and this endangered the health of human and animal community. Pathogens express specific virulence factors and aggressively suppress the normal reactions of the immune system and this leads more pathogenicity and disease progression. On the other hand, it is recognized that the transformed or inappropriate activity of the immune system also contributes to the pathology of such state. For instance, the over activation of immune responses leads to death in individuals with sepsis and those infected with an influenza virus or about COVID-19 immune system produce a generalized uncontrolled inflammatory reaction and occurs “cytokine storm” which is the most hazardous and potentially life-threatening event related to COVID-19 and also the chemokine system is involved in the pathogenesis of severe clinical cases of COVID-19. A number of challenges like these, leads growing interest in identifying and characterizing natural compounds with immunomodulatory effects. The availability of immune responses modulation, by either stimulation or suppression according to the demand, as a beneficial therapeutic strategy has been proved and it being used in many situation, including the prevention and treatment of infections, the suppression of inflammatory and autoimmune responses and the enhancement of antitumor immunity in patients with cancer. There is increasing attention in identifying and characterizing natural compounds with immunomodulatory activity ever since their use in modern medicine has been submitted [5].
Immunomodulators
An immunomodulator may be described as any biological or synthetic substance that can alter immune response either innate or adaptive or both arms of the immune system. Entirely these drugs generally categorized in two group:
• Immunosuppressants
• Immunostimulants
Each group operate with different mechanisms, depending on which component of immune system they affect, some of these can act with both the properties. There are also a next generation of immunosuppressants is upcoming which called tolerogens. Based on the source of these compounds, they are divided in to two group including: Synthetic immunomodulators and natural immunomodulators [6].
Each type of immunomodulator affects immune function through different mechanisms, here are some examples:
• Inhibitors of lymphocyte gene expression to reduce
inflammatory response (e.g. Glucocorticoids).
• Inhibitors of lymphocyte signaling to prevent immune cell
activation and proliferation (e.g. Cyclosporine).
• Cytotoxic agents to reduce lymphocyte proliferation (e.g.
Azathioprine).
• Antibodies against specific immune cell molecules (e.g.
Antithymocyte globulin).
• Cytokine inhibitors (e.g. TNF-α Inhibitors).
A strong immunomodulator have got many benefits compared to antimicrobials, immunomodulators do not affect directly microbes, by targeting the host rather than the pathogen they avoid selective pressure for the evolution of microbial resistance. In immunocompromised patients, traditional antimicrobials often act weakly and the importance of immunomodulators then can be realized. The antimicrobial compounds are specific whereas immunomodulators bring a wide spectrum capability against viral, bacterial and fungal infections and thereby provide non-specific emergency-therapeutic strategies in the occurrence of a strange pathogen or bio warfare agents In the today medicine, the potent applications of useful immunomodulators needs to be encouraged [7].
The ability of immunomodulator to stimulate natural and adaptive defense mechanisms, enables the body to help itself, such as cytokines stimulation. Using immunomodulatory treatments as anti-infective, often applied by use recombinant forms of the natural immunomodulators produced by the host immune system. They are mainly designed as adjunctive therapies for spread the efficacy of antibiotics and antivirals. For instance, type I Interferons (IFNs) have been prescribed clinically to enhance immune activity in patients with viral infections. Another form of immunomodulatory therapy and the most successful and cost-effective form is vaccination which is a medical interference for the prevention of infectious diseases. Also monoclonal antibodies are another type of immunomodulators and have been in clinical use for several decades which their application against infectious diseases is not routine and focuses mainly on inhibiting harmful inflammatory responses. The natural immunomodulators could strengthen weak immune systems and to balanced immune systems that are overactive. Recently, mesenchymal stem cells have been suggested to be auspicious therapeutics for autoimmune disorders including systemic sclerosis, systemic lupus erythematosus, Crohn’s disease and allergic diseases.
Bacterial immunomodulators are broadly prescribed drugs. They form a heterogeneous group of drugs usually produces of standardized lysates, extracts or metabolites of different bacterial strains used for alter immune response. The famous bacterial genus Streptomyces of actinomycetes, has long been known with its important role in natural product discovery and a wide capacity to produce various kind of medically important secondary metabolites, such as antibiotics, anti-tumor agents, immunomodulators and enzyme inhibitors.
Streptomyces are gram-positive soil bacteria and Biosafety level 1 (BS1-1) organisms which not known to consistently cause disease in healthy adult humans. About 75% of used antibiotics and more than half of the naturally occurring antibiotics have been derived from Streptomyces.
Results
Bacterial metabolites
Bacterial metabolites are the intermediates and products of metabolism, which they categorized into both primary and secondary metabolites, primary metabolites are acknowledged essential for suitable growth of microorganism. Secondary metabolites do not play a role in growth, development and reproduction and produced during the stationary phase of growth, metabolites are the bioactive compounds with small molecular weight, produced by microbes, to suppress organisms that are harmful and regulate other organisms beneficial to them and also develop their own growth and development (Table 1) [8].
| Primary metabolites | Secondary metabolites |
|---|---|
| Synthesized during the log phase of bacterial growth | Synthesized at the beginning of stationary phase of bacterial growth |
| Used in food and feed industry | Used in medicine, cosmetics and agriculture as preservatives |
| Have a simple chemical structure | Have an unusual chemical structures |
| Terminal products involved in the synthesis of macromolecules, coenzyme | Not vitally important for cell growth |
| Main source of energy for cellular metabolism and life support | Participate in intercellular communication, cell protection and competition for food and space |
| Provide energy reserve for communication of cells | Protect bacteria during the period of adverse con |
Table 1: Key biological properties of primary and secondary metabolites of bacteria. Primary metabolites Secondary metabolites
The most significant, features of the bioactive microbial metabolites are; microbial origin, special chemical structures and their interaction with the environment. Natural products including the microbial metabolites may be basically applied in three methods including; using the natural/fermentation product directly in the medicine, industry or in other fields or applying metabolites as starting material for subsequent microbiological modification or chemical changes (derivatization) [9]. They could be applied as templates in the Rational Drug Design (RDD) and also used as lead compounds for chemical synthesis of new analogs (Figure 2).
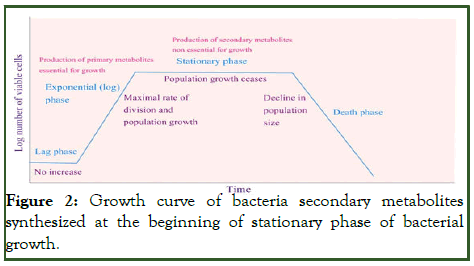
Figure 2: Growth curve of bacteria secondary metabolites synthesized at the beginning of stationary phase of bacterial growth.
Secondary metabolites as immunomodulators
Louis Pasteur first implied in 1885, that the human immune system can be affected by microorganisms. Microbial secondary metabolites are now being used for different applications that were mentioned and the immunomodulation is one of these divers applications. Some microbial compounds with potency of suppressing the immune response have been discovered. They are used in different organ transplants. Cyclosporin A was firstly introduced as a narrow-spectrum antifungal peptide produced by the mold, Tolypocladium nivenum, by aerobic fermentation.
One of the well-known immunomodulator drugs is tacrolimus which was discovered in 1987 in Japan it is 822-Da polyketide macrolide antibiotic produced by Streptomyces tsukubaensis and extracted from the fermentation broth of this genus, Macrocyclic polyketides are predominantly produced by Streptomyces and filamentous bacteria. Tacrolimus is widely used as anti-fibrotic agent and potent immunosuppressant and inhibits T-cell activation and reduces the IL-2 production by inhibiting calcineurin. It was approved by the FDA for use as an immunosuppressant in liver transplantation and it is recommended for control rejection of organ transplants like kidney, heart, intestines, lung and for the prevention of graft-vs-host disease. Furthermore, its use has been prolonged to include trachea, bone marrow, pancreas, cornea and limb transplants by reducing activity of the patient's immune system. Topically, it is also used against widespread inflammatory skin diseases like atopic dermatitis. Recently, it has been reported that tacrolimus reduces collagen synthesis in human lung fibroblastic cells by inhibiting tumor growth factor-b-induced signaling and this factor plays a critical role in tissue fibrosis.
Therefore, tacrolimus may be effective for the treatment of pulmonary fibrosis, it is noteworthy that its use in the acute inflammatory phase may intensify lung injury. Tacrolimus is approximately 100 times more potent than cyclosporine A, both of them have similar mechanism of action of inhibiting cellmediated and humoral immune responses [10]. Some approaches have been employed for enhancement production of tacrolimus, such as improvement of novel strains by metabolic engineering and optimization of fermentation parameters. One of the significant advances reported in tacrolimus biosynthesis is the enhancement in production of tacrolimus by genetic manipulations, this studies have investigated the genetic machinery involved in biosynthesis of tacrolimus and also their regulation. Isolation of a novel tacrolimus-producing microorganism, S. clavuligerus CKD1119, from soil samples in Korea have been reported by Kim and Park, this strain produced 58 mg/l tacrolimus in a 7-L jar fermenter.
Other important immunosuppressor agents include sirolimus (Rapamycin) and everolimus, which are macrocyclic lactones produced by the soil bacteria Streptomyces hygroscopicus. These agents bind to a key enzyme in cell-cycle progression, the mammalian Target of Rapamycin (mTOR), Unlike tacrolimus, do not affect calcineurin activity, they inhibit IL-2 receptor signal transduction and inhibits T-lymphocyte proliferation and downstream activation of the IL-2 and other T-cell growth factor receptors. Rapamycin first discovered in 1975 which is especially useful in kidney transplants as it lacks the nephrotoxicity seen in case of using cyclosporin A and tacrolimus. In additionto the immunosuppressive effects it used as an anti-fungal, antiinflammatory, anti-tumor and agent. Rapamycin is also usefule in treating Tuberous Sclerosis Complex (TSC), this is a congenital disorder that predispose pationts to benign tumor growth in the brain and other organs [11]. The anti-proliferative effect of rapamycin has also been used in aggregation with coronary stents to prevent restenosis (Figure 3).
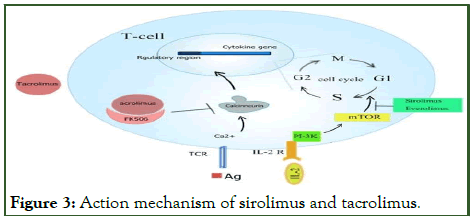
Figure 3: Action mechanism of sirolimus and tacrolimus.
Among the efforts have been made to increase the production yeald of rapamycin, we can mention mutagenesis of wild type Streptomyces hygroscopicus was operated using Ultra Violet (UV) radiation and a medium which optimized by using glycerol (one of the cheapest starting substrates by Plackett-Burman design and response surface methodology). On of the broad area which could be considered about immunomodulatory effects of bacterial metabolites is probiotic microorganisms. Gut microorganisms produce various nontoxic metabolites with clinical applications. Probiotic microorganisms in gut act symbiotically and modulate immunity. Several studies have reported that probiotics microorganisms produce antioxidants (glutathione) that help reducing oxidative stress. Some other benefits of probiotics include increasing defence against infectious diseases of the intestinal and urinary tracts, reducing symptoms of allergy, modulation and stimulation of the immune system, stimulating Bcells to enhanced IgA secretion, modulation of cytokine gene expression, reduction in cocarcinogen creation and regression of tumors.
Discussion
Gut microorganisms produce Short Chain Fatty Acids (SCFAs) from fermentation of fibers. SCFAs, such as propionate, acetate and butyrate, which are important metabolites in continuing intestinal health. Several studies have shown that SCFAs levels are typically reduced in mucosa of patients with Inflammatory Bowel Disease (IBD) as compared to healthy individuals. SCFAs, specially butyrate, also have significant immunomodulatory effects and promote cellular metabolism. SCFAs may signal through cell surface G-Protein Coupled Receptors (GPCRs), like GPR41, GPR43 and GPR109A, to stimulate signaling cascades that control immune functions. Transgenic mouse models strengthen the key role of these GPCRs in controlling intestinal inflammation.
According to a previous study that our group have done in 2016, secondary metabolites extracted from Streptomyces calvus could enhance cellular immunity and increase the proliferation of immune cells, also could stimulate the expression of IL-2, IFNand so show a potential perspective for treatment of immunocompromised patients by drug intervention, thus providing a promising basis for future biomedical studies.
According to Ali et al., Lawsonyl monocyclic terpene from the marine derived Streptomyces sp. could modulate LPS induced inflammatory response. It suggests that Lawsonone specifically inhibits the Con-A and LPS activated splenic T and B-cell proliferation and acts as an effective immunosuppressor and it acts as a potent inhibitor of the LPS-induced NO and production of pro-inflammatory cytokine (IL-1 β, IL-6 and TNF- α) [12]. In addition, it showed cytotoxicity activity against cancer cell lines. May be through down regulation of anti-cancer mediators such as NF-κB etc. Moreover, its inhibitory effect on the splenic CD4, CD8 and CD19 cell population submits its immunopharmacologic applications to alter the immune system during many diseases.
Conclusion
Over viewing our existing knowledge and the future perspectives promises that there is continuous increase of the new microbial metabolites, immunomodulatory treatment is a promising research area in the medical sciences, microbial secondary metabolites have a potential in the modulation of immune responses, this represents important practical outcomes in the human therapy and remarkable point is the qualitative improvement. Cloning and genetic engineering offer alternative approaches and the chance of incorporating the suitable biosynthetic pathways to achieve more optimized results. Natural products, in general, from microbes can be expected to play an important role in the ongoing transition from the experimental screening to the really rational drug design, further studies can be suggested to determine the precise action of secondary metabolites on immune function and inflammation because these findings will be key way in the medical field and for better human health.
References
- Abel AM, Yang C, Thakar MS, Malarkannan S. Natural killer cells: Development, maturation and clinical utilization. Front Immunol. 2018;9:1869.
[Crossref] [Google Scholar] [PubMed]
- Agarwal SS, Singh VK. Immunomodulators: A review of studies on Indian medicinal plants and synthetic peptides. Part-I: Medicinal plants. Proc Indian Natl Sci Acad Biol Sci. 1999;65(3-4):179-204.
- Ali A, Khajuria A, Sidiq T, Kumar A, Thakur NL, Naik D, et al. Modulation of LPS induced inflammatory response by Lawsonyl monocyclic terpene from the marine derived Streptomyces sp. Immunol Lett. 2013;150(1-2):79-86.
[Crossref] [Google Scholar] [PubMed]
- Alshatwi AA. Bioactivity-guided identification to delineate the immunomodulatory effects of methanolic extract of Nigella sativa seed on human peripheral blood mononuclear cells. Chin J Integr Med. 2014:1-6.
[Crossref] [Google Scholar] [PubMed]
- Azad MA, Sarker M, Wan D. Immunomodulatory effects of probiotics on cytokine profiles. Biomed Res Int. 2018;2018:1-0.
[Crossref] [Google Scholar] [PubMed]
- Barreiro C, Martínez-Castro M. Trends in the biosynthesis and production of the immunosuppressant tacrolimus (FK506). Appl Microbiol Biotechnol. 2014;98:497-507.
[Crossref] [Google Scholar] [PubMed]
- Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121-141.
[Crossref] [Google Scholar] [PubMed]
- Bell RG. IgE, allergies and helminth parasites: A new perspective on an old conundrum. Immunol Cell Biol. 1996;74(4):337-345.
[Crossref] [Google Scholar] [PubMed]
- Sánchez-Berna I, Santiago-Diaz C, Jimenez-Alonso J. Immunomodulatory properties of stem mesenchymal cells in autoimmune diseases. Med Clin. 2015;144(2):88-91.
[Crossref] [Google Scholar] [PubMed]
- Chaplin DD. Overview of the immune response. J Allergy Clin Immunol. 2010;125(2):S3-S23.
[Crossref] [Google Scholar][PubMed]
- Chen JR, Yang ZQ, Hu TJ, Yan ZT, Niu TX, Wang L, et al. Immunomodulatory activity in vitro and in vivo of polysaccharide from Potentilla anserina. Fitoterapia. 2010;81(8):1117-1124.
[Crossref] [Google Scholar] [PubMed]
- Choi SS, Hur YA, Sherman DH, Kim ES. Isolation of the biosynthetic gene cluster for tautomycetin, a linear polyketide T cell-specific immunomodulator from Streptomyces sp. CK4412. Microbiology. 2007;153(4):1095-102.
[Crossref] [Google Scholar] [PubMed]
Citation: Mahmoudi F, Baradaran B (2024) Microbial Secondary Metabolites as Immunomodulators. J Microb Biochem Technol. 16:615.
Copyright: © 2024 Mahmoudi F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

