Indexed In
- Academic Journals Database
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Case Report - (2019) Volume 11, Issue 3
Microbial Metabolism of Artemisinin by Penicillium janthinellum
Yanqin Liu, Jinyan Li, Fuli Luo and Yulian Zhan*Received: 02-Apr-2019 Published: 24-May-2019
Abstract
The microbial transformation of artemisinin by Penicillium janthinellum was investigated. During 6 days at 28°C and 180 rpm, artemisinin was transformed to a product by Penicillium janthinellum. The product was identified as 4α-hydroxy-1-deoxyartemisinin. This is the first report of biotransformation of artemisinin by Penicillium janthinellum.
Materials and Methods
General
1H NMR (nuclear magnetic resonance) and 13C NMR spectra were recorded in CDCl3 (chloroform-d) on a Bruker Avance III HD 600 MHz spectrometer. Chemical shifts were reported in ppm (δ), and J-values were reported in Hz.
Microorganism
The strain Penicillium janthinellum CGMCC 3.5951 was obtained from China General Microbiological Culture Collection Centre.
Medium
All culture and biotransformation experiments were carried out in the following medium: Potato infusion is made by boiling 200 grams of sliced potatoes in 1 litre of deionized water for 30 minutes and then filtering the broth through cheesecloth. Deionized water is added such that the total volume of the suspension is 1 litre. 20 grams dextrose is then added and the medium is sterilized by autoclaving at 121°C for 30 minutes.
Biotransformation of artemisinin (1) by Penicillium janthinellum
The mycelia were transferred into 250 mL Erlenmeyer flasks containing 60 mL of medium from the surface of agar slants. Cultures were cultivated for 48 h on a rotary shaker at 28°C and 180 rpm, and used to inoculate 37, 250 mL shake flasks that contained 60 mL of medium. The cultures were then incubated for 48 h using the same conditions as before. Artemisinin (Mediplantex, Vietnam) was dissolved in acetone (25 mg/mL), and 0.4 mL of this solution was added to each flask. A total of 370 mg of artemisinin was transformed. The cultures were incubated for additional 6 days at 28°C and 180 rpm. The mycelia were separated by filtration and discarded. The filtrate was extracted three times with an equal volume of ethyl acetate (EtOAc). The extract was evaporated to dryness under vacuum to afford a residue.
Chromatographic conditions
A total of 1.70 g of residue was obtained from the broth. The residue was purified by silica gel column chromatography, using a petroleum ether (60-90°C)-acetone mobile phase in a gradient mode, eluting with 10 to 30% acetone.
Introduction
Artemisinin (qinghaosu) is a sesquiterpene lactone endoperoxide, isolated from the Chinese herb Artemisia annua L., and the endoperoxide bridge is responsible for its activities. Today, artemisinin and its derivatives, dihydroartemisinin and artesunate, are used in the first-line treatment for multidrug-resistant malaria [1,2]. Besides the antimalarial activity, artemisinin and its derivatives also exhibited antifungal [3], antiangiogenic [4,5], antiinflammatory [6], and antitumor activities [7-10]. There has been much interest in the structural modification of artemisinin and its derivatives in the search for analogues which might serve as clinical alternatives. In this study, we report the biotransformation of 1 by Penicillium janthinellum, and one product was obtained.
Discussion and Conclusion
The biotransformation of artemisinin by Penicillium janthinellum gave 30 mg of 2 (yield 8.1%).
The structure of the product was identified on the basis of its spectroscopic data. Data of 1H and 13C NMR spectra of the product was in agreement with the reported literatures’ data [11,12]. So the product was identified as 4α-hydroxy-1- deoxyartemisinin (Figure 1).
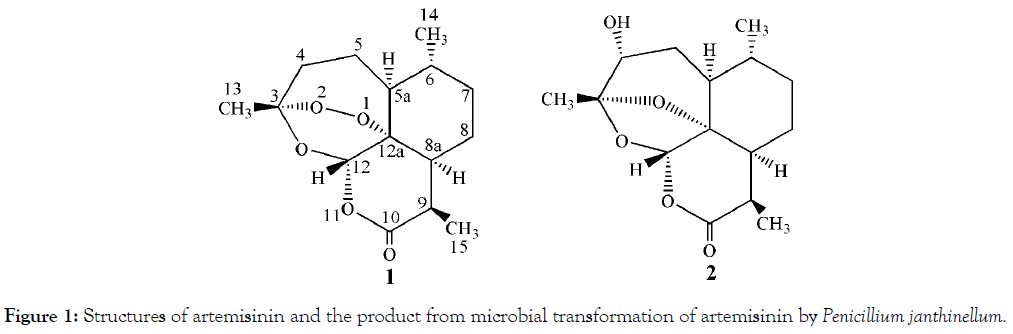
Figure 1. Structures of artemisinin and the product from microbial transformation of artemisinin by Penicillium janthinellum.
4α-hydroxy-1-deoxyartemisinin (2): Colourless needles (from acetone); 1H-NMR (CDCl3, 600 MHz) δ 5.63 (1H, s, H-12), 3.62 (1H, brs, H-4β), 3.19 (1H, m, H-9), 2.06 (1H, m, H-8a), 1.98 (1H, m, H-5α), 1.92 (1H, m, H-8α), 1.82 (1H, m, H-7α), 1.57 (3H, s, Me- 13), 1.55-1.47 (2H, m, H-5a, H-5β), 1.28 (1H, m, H-6), 1.20 (3H, d, J=7.1 Hz, Me-15), 1.11 (1H, m, H-7β), 1.00 (1H, m, H-8β), 0.93 (3H, d, J=6.3 Hz, Me-14); 13C NMR (CDCl3, 150 MHz) δ 171.6 (s, C-10), 109.1 (s, C-3), 99.2 (d, C-12), 83.1 (s, C-12a), 69.3 (d, C-4), 42.3 (d, C-8a), 40.8 (d, C-5a), 35.3 (d, C-6), 33.6 (t, C-7), 32.9 (d, C-9), 30.5 (t, C-5), 23.7 (t, C-8), 20.7 (q, C-13), 18.6 (q, C-14), 12.8 (q, C-15).
The effectiveness of artemisinin is impaired by its toxicities [13] and low solubility [14]. Chemical and biological modifications of artemisinin have been studied [15-19]. Microbial transformation is an effective route to obtain artemisinin derivatives. The microorganisms, such as Aspergillus niger, Aspergillus terreus, Rhizopus stolonifer, Cunninghamella elegans, Eurotium amstelodami, Mucor polymorphosporus, Penicillium simplicissimum, Streptomyces griseus [20-22] were used in the biotransformation of artemisinin. Here we first report the biotransformation of artemisinin by Penicillium janthinellum.
In conclusion, we investigated the biotransformation of artemisinin by Penicillium janthinellum, and obtained a product, 4α-hydroxy-1- deoxyartemisinin. This is the first report of biotransformation of artemisinin by Penicillium janthinellum.
Acknowledgment
This work was supported by Guangxi Undergraduate Program for Innovation and Entrepreneurship (No. 201610595197).
REFERENCES
- Poespoprodjo JR, Fobia W, Kenangalem E, Lampah DA, Sugiarto P, Tjitra E, et al. Dihydroartemisinin-piperaquine treatment of multidrug resistant falciparum and vivax malaria in pregnancy. Plos One. 2014;9(1):e84976.
- Luo XD, Shen CC. The chemistry, pharmacology, and clinical applications of qinghaosu (Artemisinin) and its derivatives. Med Res Rev. 1987;7(1):29-52.
- Galal AM, Ross SA, Jacob M, ElSohly MA. Antifungal activity of artemisinin derivatives. J Nat Prod. 2005;68(8):1274-1276.
- Lee S. Artemisinin, promising lead natural product for various drug developments. Mini Rev Med Chem. 2007;7(4):411-422.
- Soomro S, Langenberg T, Mahringer A, Konkimalla VB, Horwedel C, Holenya P, et al. Design of novel artemisinin-like derivatives with cytotoxic and anti-angiogenic properties. J Cell Mol Med. 2011;15(5):1122-1135.
- Yang Z, Ding J, Yang C, Gao Y, Li X, Chen X, et al. Immunomodulatory and anti-inflammatory properties of artesunate in experimental colitis. Curr Med Chem. 2012;19(26):4541-4551.
- Singh NP, Lai H. Selective toxicity of dihydroartemisinin and holotransferrin toward human breast cancer cells. Life Sci. 2001;70(1):49-56.
- Wu GD, Zhou HJ, Wu XH. Apoptosis of human umbilical vein endothelial cells induced by artesunate. Vascul Pharmacol. 2004;41:205-212.
- Efferth T, Benakis A, Romero MR, Tomicic M, Rauh R, Steinbach D, et al. Enhancement of cytotoxicity of artemisinins toward cancer cells by ferrous iron. Free Radic Biol Med. 2004;37(7):998-1009.
- Efferth T, Dunstan H, Sauerbrey A, Miyachi H, Chitambar CR. The anti-malarial artesunate is also active against cancer. Int J Oncol. 2001;18(4):767-773.
- Parshikov IA, Muraleedharan KM, Avery MA, Williamson JS. Transformation of artemisinin by Cunninghamella elegans. Appl Microbiol Biotechnol. 2004;64(6):782-786.
- Zhan Y, Liu H, Wu Y, Wei P, Chen Z. Williamson JS. Biotransformation of artemisinin by Aspergillus niger. Appl Microbiol Biotechnol. 2015;99(8):3443-3446.
- Kamchonwongpaisan S, McKeever P, Hossler P, Ziffer H, Meshnick SR. Artemisinin neurotoxicity: Neuropathology in rats and mechanistic studies in vitro. Am J Trop Med Hyg. 1997;56(1):7-12.
- Lin AJ, Lee M, Klayman DL. Antimalarial Activity of New Water-Soluble Dihydroartemisinin Derivatives. 2. Stereospecificity of the Ether Side-Chain. J Med Chem. 1989;32(6):1249-1252.
- Gaur R, Darokar MP, Ajayakumar PV, Shukla RS, Bhakuni RS. In vitro antimalarial studies of novel artemisinin bio transformed products and its derivatives. Phytochemistry. 2014;107:135-140.
- Zhan J, Zhang Y, Guo H, Han J, Ning L, Guo D. Microbial metabolism of artemisinin by Mucor polymorphosporus and Aspergillus niger. J Nat Prod. 2002;65:1693-1695.
- Goswami A, Saikia PP, Barua NC, Bordoloi M, Yadav A, Bora TC, et al. Bio-transformation of artemisinin using soil microbe: Direct C-acetoxylation of artemisinin at C-9 by Penicillium simplicissimum. Bioorg Med Chem Lett. 2010;20:359-361.
- Acton N. Semisynthesis of 3-β-Hydroxyartemisinin. J Nat Prod. 1999;62:790-793.
- Sy LK, Hui SM, Cheung KK, Brown GD. A rearranged hydroperoxide from the reduction of artemisinin. Tetrahedron. 1997;53(22):7493-7500.
- Yu H, Zhu B, Zhan Y. Microbial transformation of artemisinin by Aspergillus terreus. Biores Bioprocess. 2017;4(1):33.
- Liu JH, Chen YG, Yu BY, Chen YJ. A novel ketone derivative of artemisinin biotransformed by Streptomyces griseus ATCC 13273. Bioorg Med Chem Lett. 2006;16:1909-1912.
- Parshikov IA, Miriyala B, Muraleedharan KM, Avery MA, Williamson JS. Microbial transformation of artemisinin to 5-hydroxyartemisinin by Eurotium amstelodami and Aspergillus niger. J Ind Microbiol Biotechnol. 2006;33:349-352.
Citation: Liu Y, Li J, Luo F, Zhan Y (2019) Microbial Metabolism of Artemisinin by . J Microb Biochem Technol 11:3. doi:10.4172/1948-5948.1000414
Copyright: © 2019 Liu Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : This work was supported by Guangxi Undergraduate Program for Innovation and Entrepreneurship (No. 201610595197).

