Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- ResearchBible
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- Scientific Indexing Services (SIS)
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2024) Volume 15, Issue 4
Metal Oxide Nanoparticles as Antimicrobial Agents: Mechanisms, Applications, and Future Perspectives
Abhishek Prakash TiwariReceived: 01-Jul-2024, Manuscript No. jnmnt-24-25983; Editor assigned: 04-Jul-2024, Pre QC No. jnmnt-24-25983(PQ); Reviewed: 18-Jul-2024, QC No. jnmnt-24-25983(QC); Revised: 25-Jul-2024, Manuscript No. jnmnt-24-25983(R); Published: 31-Jul-2024, DOI: 10.35248/2157-7439.24.15.736.
Abstract
Human beings are primarily affected by infectious diseases, which are caused by microbes. Excessive consumption of antibiotics gave rise to antimicrobial-resistant pathogens, which need clinical assistance. Therefore, there is a need for an alternate antimicrobial agent that should be safe and exhibit greater efficiency. For the same purpose, researchers have started working on metal oxide nanoparticles (MeO-NPs), which have proven an effective alternative to combat pathogens. Apart from their antimicrobial characteristics, MeO-NPs can act as drug carriers. Thus, it exhibits a wide spectrum of applications in the healthcare sector. The review highlights the action mechanism of metal oxide nanoparticles against microbes, their physical properties, safety issues, and future perspectives.
Keywords
Metal Oxide, Nanoparticles, Antimicrobial Agent
INTRODUCTION
Over the last couple of decades, the study of nanotechnology has developed ever since it began and has created a major influence on scientific evolutions [1,2]. As a consequence of science and technology by researchers all around the world, nanomaterials techniques and functions have broadened [3-5]. Metal oxide (MeO) nanoparticles (NPs) are a type of nanomaterials that ranges in size from 1 to 100 nm and appear in a range of form factors. MeO-NPs have unusual physico-chemical properties due to their small size, rendering versatility. Oxidation, diagnostics, drug administration, electronics, sensing [6], and electrochemical capacitors all use MeO-NPs. MeO-NPs are often used as antibacterial agents, which is why they have gained a great deal of interest presently [7]. The antibacterial property of MeO-NPs is mostly influenced by their various characteristics. Antimicrobial action is primarily conferred by the alkalinity of the CaOand MgO NP surfaces. Because of their input to alkaline nature in the media, these MeO-NPs are more soluble than unbiased ZnO NPs. The antimicrobial activity of ionized CeO2 NPs is governed by their electrostatic characteristic. TiO2 NPs are photocatalysts that can stop even UV resistance and inhibit bacteria from growing [8].
Hygiene plays an essential role in keeping oneself free from diseases. Hygiene and cleanliness can be maintained by keeping ourselves and our surroundings free from microorganisms. Several infection-causing microbial agents account for more than 10 million global deaths per year. Different potential antimicrobial metal-oxide examples can be seen from ancient societies, civilizations such as Mesopotamia, Indus Valley made use of Fe3O4, CuO, ZnO and various other metal oxides to clean the contaminated water and to enhance the healing process of wounds [9]. The antimicrobial characteristic feature of metal oxides was applied all over the historical period. However, metal-oxides to be used medically reduced in the early 20th century after the development of antibiotics. Microbial chronic diseases are amongst the highest death rates in the world, thus investigations for antimicrobial medicines that are resistive and suitable are required. MeO-NPs have the potential to be used as antibacterial agents against a wide variety of pathogens, along with drug-resistant variants. It mandates the synthesis of novel antimicrobial compounds for use in antibiotic prophylaxis, the food and beverage industry, potable water, and the manufacturing sector. There are so many distinct MeO-NPs with various physio-chemical characteristics; they can be used as antimicrobial agents instead of traditional medications [10]. They possess ahigh aspect ratio, therefore MeO-NPs are superior antibacterial agents than conventional anti-pathogenic agents; by modifying particular factors, MeO-NPs with unique molecular, electronic, magnetic, physical, and optical characteristics can be constructed. The dimension of the nanoparticle, the aqueous system applied, and the luminous source all influence antibacterial effectiveness. Metal NPs, like antibiotics, may distinguish prokaryotic from eukaryotic using their metal transport system. Metal NPs, on the other hand, trigger antimicrobial efficacy through several pathways. Despite this difference, every kind of resistance requires several gene alterations within the same bacterium given in Figure1. The action can be understood as the interaction of metal oxide ion with phospholipid bilayer, cytosolic protein binding and generation of reactive oxygen species (ROS). As compared to metal NPs metal-oxide NPs also possess similar pathways as multi-target drug resistant. Based on different synthesis processes there are different kinds of nanoparticles being generated which exhibit different antimicrobial mechanisms against wide microbial classes, this study of nanoparticles and their respective activity is depicted [Figure-1].
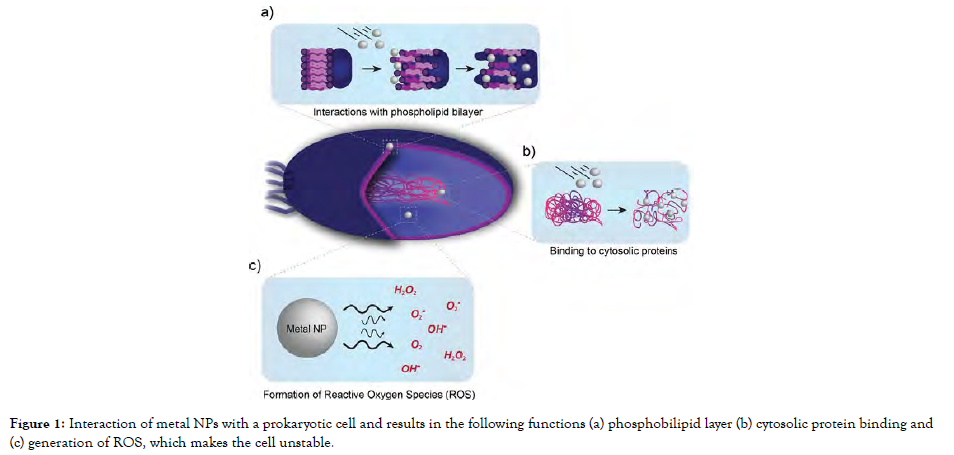
Figure 1: Interaction of metal NPs with a prokaryotic cell and results in the following functions (a) phosphobilipid layer (b) cytosolic protein binding and (c) generation of ROS, which makes the cell unstable.
Fabrication of metal-oxide nanoparticles
For a specific purpose, metal-oxide nanoparticles can be readily fabricated or chemically transformed. The characteristics and uses of MeO-NPs are determined by the synthesis process. Most scientists favoured chemical approaches rather than commonly available MeO-NPs for determining antimicrobial effect, and we have briefly outlined the most widely adopted techniques below:
Chemical method
During the synthesis method, the resulting mixture is gradually heated up, and this is the most popular chemical technique. The key phases in this technique are monomer synthesis and aggregation, polymerization, and maturation, which culminate in strongly monodisperse MeO-NPs. The growth and boiling temperature of the solvent determine the particle size and diameter. Chemically fabricated CdO, CeO2, Fe2O3, MgO, ZnO, V2O5, TiO2, Fe3O4, and graphene oxide nanoparticles were investigated for possible antimicrobial effects [Figure-2].
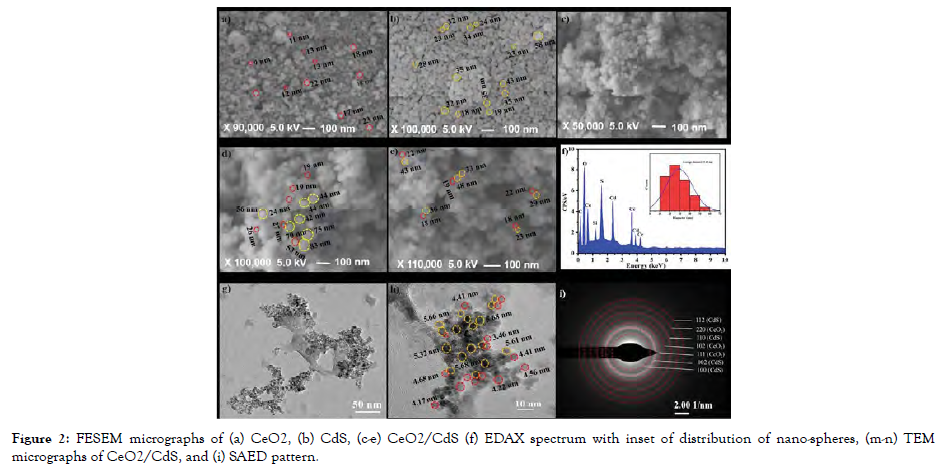
Figure 2: FESEM micrographs of (a) CeO2, (b) CdS, (c-e) CeO2/CdS (f) EDAX spectrum with inset of distribution of nano-spheres, (m-n) TEM micrographs of CeO2/CdS, and (i) SAED pattern.
Biological method
To accelerate the synthesis of MeO-NPs, botanical extracts or microbiological secretions containing precursor chemicals are swirled consistently using a magnetic stirrer at 100-120 °C for 24 hrs. After centrifuging the mixture, the liquid is removed. In a watch glass, the pelleted MeO-NPs are gradually dehydrated. CuO, Fe3O4, and ZnO nanoparticles were biosynthesized and respective antimicrobial properties were investigated [Figure-3].
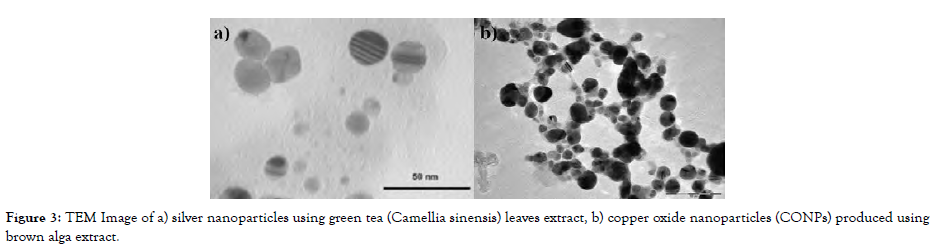
Figure 3: TEM Image of a) silver nanoparticles using green tea (Camellia sinensis) leaves extract, b) copper oxide nanoparticles (CONPs) produced using brown alga extract.
Sol-gel method
The precipitation and hydrolysis of precursor are used in this wet path technique. The gel characteristics have an impact on the synthesized crystallized nanoparticle. Furthermore, the hydroxylation and precipitation processes are governed by spinning, precursor content pH, and temperature. It is possible to achieve a specified nanoparticle with monodisperse or amorphous phases. The reaction products' homogeneity can be regulated. The antimicrobial effects of nanoparticles such as TiO2 and ZnO have been developed and verified [Figure-4].
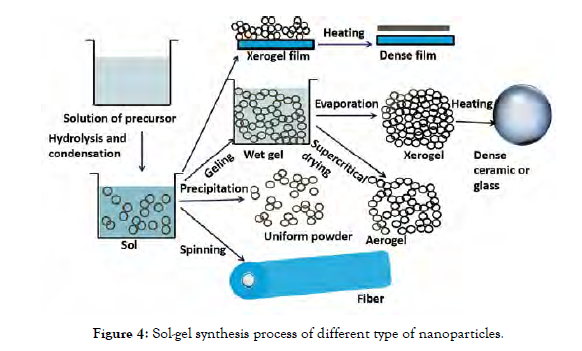
Figure 4: Sol-gel synthesis process of different type of nanoparticles.
Co-precipitation method
On adding ammonium hydroxide/sodium hydroxide, a salt precursor transforms itself into its corresponding metal hydroxide in the aqueous phase. To get the appropriate MeOs, the chloride salts are rinsed and on the application of heat, hydroxides are removed. Transient nucleation is continued by nuclei expansion onto the substrate through solute dispersion in co-precipitation. The shape and grain size cannot be regulated due to kinematic constraints; however, the type of salts involved, ionic concentration, acidity, and temperature may be regulated. The antimicrobialeffects of Fe3O4 and ZnO nanoparticles have been investigated [Figure-5].
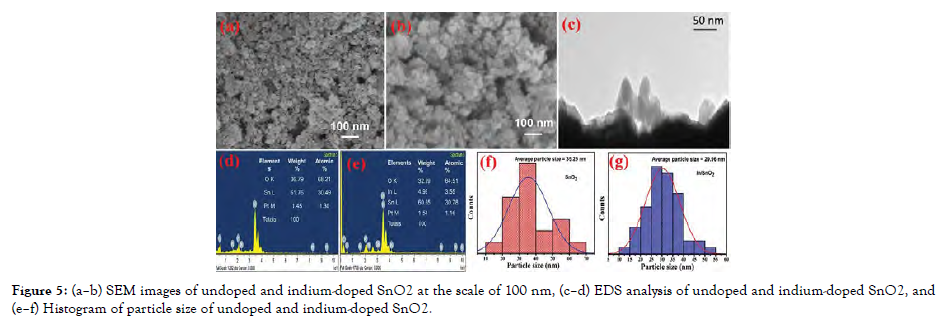
Figure 5: (a–b) SEM images of undoped and indium-doped SnO2 at the scale of 100 nm, (c–d) EDS analysis of undoped and indium-doped SnO2, and (e–f) Histogram of particle size of undoped and indium-doped SnO2.
Electrochemical
The core metal will be retained on the anode and converted into metal complexes using an electrolytic mixture and a two-electrode system. Tetra-alkyl-ammonium compounds are applied as a buffering electrolyte to keep the metal complexes stable. To eliminate dissolved oxygen content, electrolysis is performed in a nitrogen flow, and metallic ions move to the negative electrode while the core metal oxidizes at the positive electrode. The metal is oxidized to corresponding MeOs by the leftover oxygen present in the electrolytic system. Ammonium actuators protect powder particles from clumping together. The cluster size is determined by the current density. The harmful effects of Cr2O3, CuO, and CuOmultiple-armednanoparticles on microbes are already proven [Figure-6].
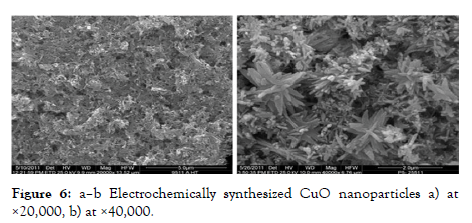
Figure 6: a–b Electrochemically synthesized CuO nanoparticles a) at ×20,000, b) at ×40,000.
Wet chemical method
The wet chemical approach is a very simple and cost-effective process which can be used on any material, regardless of form or curvature, and has a wide range of applications. The components are blended in deionization water for 30 minutes, then amalgamated and heated for 45 minutes before being centrifuged. CuO nanotubes, Fe3O4 nanoparticles, and ZnO nanoparticles have all been fabricated and analyzed on microbes [Figure-7].
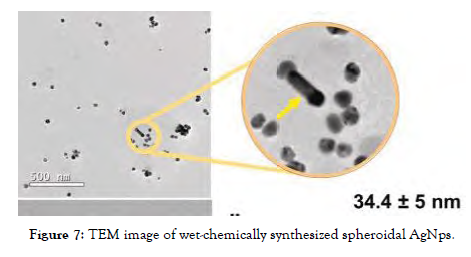
Figure 7: TEM image of wet-chemically synthesized spheroidal AgNps.
Pyrolytic method
When heating the reactant components, the first step is to atomizing the mixture, blend it along with soluble polymeric materials and incorporate this into the polymeric layer. Melting, agglomeration, and clustering are mostly averted by such measures. The MeO-NPs, in combination with the substrate, can function as promoters. Consequently, the approach is ineffective for synthesizing hybrid MeO-NPs. CO3O4 nanostructures are already synthesized and evaluated for antimicrobial properties [Figure-8].
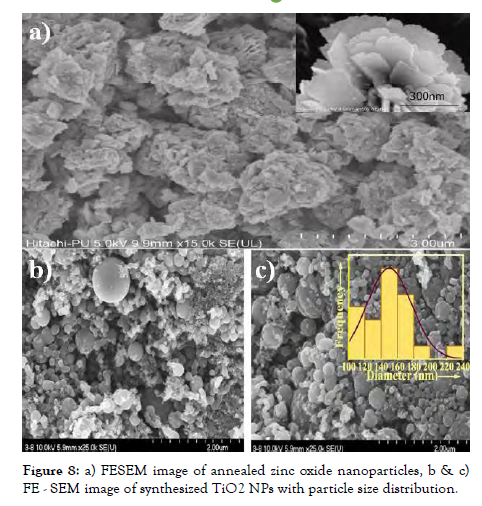
Figure 8: a) FESEM image of annealed zinc oxide nanoparticles, b & c) FE - SEM image of synthesized TiO2 NPs with particle size distribution.
Microwave method
The components are diluted in distilled water individually and mixed together in a 100 mL solution, which is then stirred repeatedly for 10 minutes at ambient temperature till it transformsinto a white gel. Microwave radiation can be used to sterilize the concoction, which is then allowed to cool gradually at ambient temperature. The residue is filtered using a vacuum, rinsed with distilled water and 100% ethanol, and then dehydrated for 1 hr in a vacuum at 80°C. CaO and MgO nanoparticles were prepared and studied for bioactivity.
Hydrothermal method
Due to the extreme precision for particulate size and form, bidirectional MeO-NPs may be formed using hydrothermal techniques, which are conducted inside autoclaves/reactors at 2000 psi and 200°C in an aqueous phase. In ideal circumstances, fluid solvent-based are hard to break down so they are dispersed and again crystallized in a diverse methodology. Optimizing the hydrothermal conditions facilitates the production of several types of nanoparticles. Antimicrobial effects of MgO nanoparticles, ZnO nanowires, and ZnO nanofibers have been developed and characterized [Figure-9].
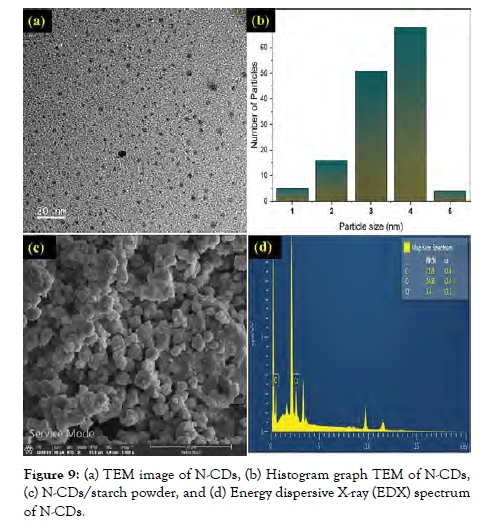
Figure 9: (a) TEM image of N-CDs, (b) Histogram graph TEM of N-CDs, (c) N-CDs/starch powder, and (d) Energy dispersive X-ray (EDX) spectrum of N-CDs.
Sono chemical method
Physical factors such as particle acceleration and collision affect size distribution, concentration, topologies, and ratios in conventional sonochemical fabrication, typically indicating higher acoustic sonication of metal ions and oxygen in suspension. This is among the finest techniques for depositing nanoparticles upon composite polymers because energetic radicals and atoms interact with metal cations to produce MeOs. CuO nanoparticles were prepared to employ increased ultrasonic, and their antimicrobial properties were investigated [Figure-10].
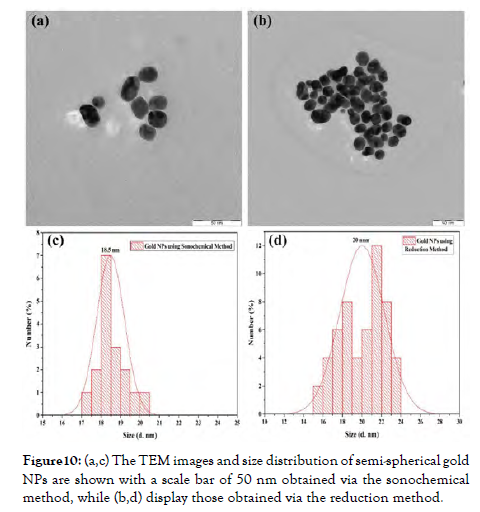
Figure 10: (a,c) The TEM images and size distribution of semi-spherical gold NPs are shown with a scale bar of 50 nm obtained via the sonochemical method, while (b,d) display those obtained via the reduction method.
Antimicrobial mechanism of metal-oxide nanoparticles
MeO-NPs seem to be of interest to researchers as antimicrobials due to their distinct morphological, pharmacological, electrochemical, magnetic, photonic, and biochemical processes. Evidence on the cellular mechanism of MeO-NPs' antimicrobial effects is still in its adolescence, and deciphering the underlying pathways of MeO-NPs is currently a work in progress as shown in Figure 2.
The production of ROS is the primary mechanism of antibacterial action. Other mechanisms of action include electrostatic interaction cellular damage, disruption of metal ion balance, protein and enzymatic instability, and cytotoxicity [Figure-11 and Figure 12].
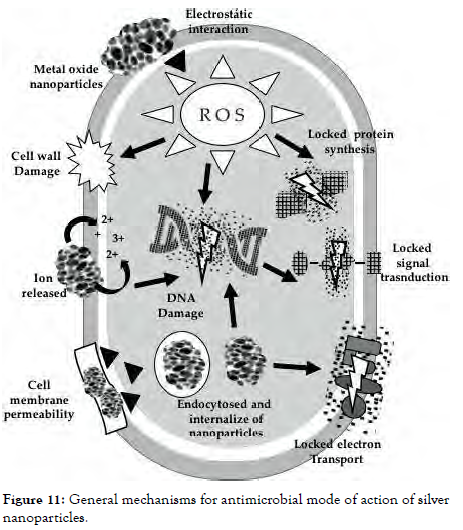
Figure 11: General mechanisms for antimicrobial mode of action of silver nanoparticles.
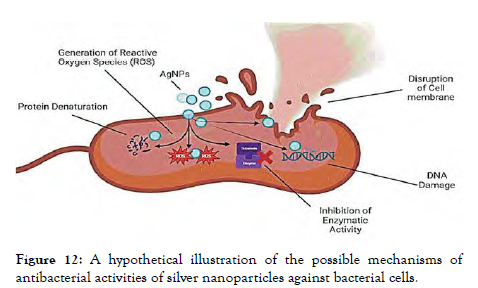
Figure 12: A hypothetical illustration of the possible mechanisms of antibacterial activities of silver nanoparticles against bacterial cells.
Electrostatic interaction damaging cell membrane
Metal cations are attracted to the electronegative functional groups of macromolecules on lipid bilayer. Due to the presence of carboxylic acid groups in the amino acids, the negative charge is retained upon the interface of bacterium and microbes at natural pH. Due to the charge disparity between the lipid bilayer and MeO, electrostatic interaction emerges, causing MeO to concentrate on the cellular membrane, allowing its entry. Microorganisms are damaged by the interaction of transmembrane with charged MeO. Gram-negative bacteria possess a higher repulsive force than Gram-positive bacteria.
Homeostatic imbalance
Metallic element homeostasis is fundamental for microorganism life as it helps coenzymes, metabolites, and enzymes control physiological processes. The biological activities of bacterium will be affected if they have an accumulation of metallic oxide ions. Metal oxide charged particles also could induce cell membrane breakdown by producing significant quantities of hydroxyl radicals as well as transport in bacterium owing to metal ion diffusion. According to other research, metal oxide nanoparticles progressively emit metal ions by chemisorptions, dissociation, and hydration; particles are corrosive and harsh to a bacterium, prompting cells to lyses.
Improper functions of enzymes and proteins
Another mechanism of antimicrobial activities demonstrated by metallic nanoparticles is protein disruption. Metal ions stimulate the reaction of polypeptide chains, culminating in the spontaneous generation of carboxylic acids linked to receptors. The carboxylation concentrations of macromolecules act as an indicator for protein oxidation damage. This decreases enzymatic oxidative metabolism which eventually leads to macromolecule breakdown.
Transduction signal inhibition
Nanoparticles of Metal oxide possess an electrical characteristic which interferes with nucleotides, causing cell division to be inhibited via modifying the Genomic sequence and plasmid replication operations in microorganisms. Metal oxides have been shown to influence signalling pathways in microorganisms. In bacteria, phosphotyrosine is indeed a critical feature of the signalling pathways. Nanostructures dephosphorylate phosphotyrosine fragments, blocking signalling pathways resulting in inhibiting bacterial growth.
Toxic activity of metal oxide nanoparticles
Scientists from all across the globe are collaborating to figure out how MeO nanoparticles interact with microorganisms. Despite this, the processes of toxic effects also are widely undefined. MeO nanoparticles had a differential expression of toxic effects, but it was less evident for its mass materials MeO nanoparticles and its mass materials have diverse perilous impacts. Morphology, shape, stability, the potential for traversing significant obstacles, agglomeration inside the matrix, producing oxidizing agents, the capability of penetrating inside the nucleus, incubation period, dosage, and other characteristics cause hazards for the application of MeO as antibacterial compounds in most species. The risking MeOnanoparticles depend greatly upon their quantity as well as length of therapy, and also cellular varieties. The fact that they are oxidants is the main topic of concern, accompanied by their potential to harm macromolecules and membranes. Despite the fact that there are some researches on the danger of MeO nanoparticles in the laboratory, additional research is needed. MeO nanoparticles immunomodulatory effects are influenced both by their physical properties and strains of microbes. As a result, they hold great potential as emerging antimicrobial compounds and possess a wide spectrum of uses in biomedicine.
Physical properties of metal oxide nanoparticles and their effect on antimicrobial activity
The characteristic features of metal oxide nanoparticles influence the antimicrobial activity. These physical properties are shape, size, smoothness, zeta potentials, coating and material doping. These properties and their respective influences have been described here.
Shape and Size
Shape and size of nanoparticles play an essential role in affecting the antimicrobial properties. Different sizes influence bacterial layers in different ways. Size smaller than 30nm enables aggregation and entry in bacterial cells inflicting disruption, thereby killing the cell this usually occurs at ≈10 nm. On interaction with the bacterium, increasing the size by more than 10 nm enhances the permeability. The aspect ratio is influenced by the particular area of nanomaterials that influences overall reactivity. Whereas, based on the method adopted for synthesizing nanoparticles there can be varied types of structures such as nanowires, nanotubes, nanocubes, nanoneedles etc.Depending on the optic and luminous intensities, data suggests that metal oxide nano-needles possess better antimicrobial activity as compared to metal oxide nanocubes.
Zeta potentials
If negative-charged surfaces can reduce contact with microorganisms that are negatively charged, a negative-charged nanomaterial surface might possess a similar impact, impairing bacterial adherence. Electrostatic attraction originates when nanomaterials are charged positively, increasing their concentration in the negatively charged lipid bilayer, and afterwards, nanoparticles enter into the bacterium, activating additional processes. Also, the nanoparticles' surface possessesa great level of ruggedness, hence bacterium membranes are unable to cling to them, thereby reducing bacterium adherence.
Material doping
Chemical doping of nanomaterials is a process that involves modifying and functionalizing the coating of nanostructures in place to control and modulate their activities against microorganisms therefore strengthening overall antimicrobial properties. The concentration of oxygen molecules on the interface aids in the creation of the passivation of chemicals and the antibacterial ability increases as the aspect ratio rises.
CONCLUSION
Metal oxide nanoparticles exhibit antimicrobial properties which gives a positive instinct to be used as an antibiotic in future. With the advancing science and technology, nanoparticle applications are actively growing in different sectors. Metal-oxide nanoparticles possess multi-drug resistant features which adds some important features to biosynthetic materials. Different metal-oxide nanoparticles inhibit different classes of microorganisms such as bacteria, fungi, viruses and many more, which have been examined in the past few years. The antimicrobial property of metal oxide nanoparticles may extend its application in the treatment of contaminated water, packaging and storage, surface coating, textile industry and pharmaceutical industries.
Conflict of interests
The authors declare no conflict of interest.
REFERENCES
- Liu H, Brown MD. Scanning tunnelling microscopy characterization of gold film surfaces processed using an argon radio frequency (RF) plasma. Thin Solid Films. 1999; 349(1-2): 78-83.
- Suzuki Y, Enoki H, Akiba E. Observation of HOPG by STM and contact AFM in various gas atmospheres under pressures up to 1.1MPa. Ultramicroscopy.2005; 104(3-4): 226-232.
- Yıldız, D, Gürlü O. Apparent corrugation variations on moiré patterns on highly oriented pyrolytic graphite. Materials Today Communications. 2016; 8, 72-78.
- Stevens F, Kolodny L A, Beebe T P. Kinetics of Graphite Oxidation: Monolayer and multilayer etch pits in HOPG studied by STM. Journal of Physical Chemistry B.1998; 102(52): 10799-10804.
- Patrick DL, Cee V J, Purcell T J, Beebe T P. Defect pinning in monolayer films by highly controlled graphite defects: molecule corrals. Langmuir.1996; 12(7): 1830-1835.
- A Bryant. Imaging in real time with the tunneling microscope.1986.
- Tatar R C, Rabii S. Electronic Properties of Graphite: A Unified Theoretical study. Physical review. 1982;25(6): 4126-4141.
- Liang H, Cheng F, Sun W, Shao X, Wang. Two-dimensional molecular porous networks constructed by surface assembling. Coordination Chemistry Reviews.2009; 253(23-24): 2959-2979.
- Liu H, Brown MD. Studies using AFM and STM of the correlated effects of the deposition parameters on the topography of gold on mica. Thin Solid Films.1997; 300(1-2): 84-94.
- López J C, Passeggi M C, Ferrón J. Surface superstructures in highly oriented pyrolytic graphite surfaces after AR+ bombardment. Surface Science.2008; 602(3): 671-676.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Abhishek PT (2024) Metal Oxide Nanoparticles as Antimicrobial Agents: Mechanisms, Applications, and Future Perspectives. J Nanomed Nanotech. 15: 736.
Copyright: ©2024 Abhishek PT. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


