Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- CiteFactor
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review - (2020) Volume 12, Issue 5
Medicine Interchangeabilty in Brazil, is it Safe? A Systematic Review for the Last 15 Years about Oral Drugs
Gabriel Silva Lima*, Gustavo Reis Sampaio and Denis de Melo SoaresReceived: 13-May-2020 Published: 30-Oct-2020, DOI: 10.35248/0975-0851.20.12.402
Abstract
Objectives: The goal of this study was to review studies about bioequivalence test (BE) for oral drugs interchangeability in Brazil.
Methods: We searched two databases with a strict inclusion process: Conducted in Brazil; humans volunteers; cover the period from 2004 to 2019; be a comparative study between oral formulations; at least one Brazilian formulation under test and published in periodic; Two reviewers independently extracted the data.
Results: 4628 articles screened; 68 articles were included. 67 applying clinical assays and 1 article Chow and Liu methodology. Across studies that evaluated BE by clinical assays 66 demonstrated BE comparing generic or similar to their reference medicine.
Conclusion: These data can be used to inform interventions to change the public’s beliefs about a safe use of generic or similar drugs; and avoiding substitution between copies.
Keywords
Bioequivalence; Interchangeability; Oral Drugs
Introduction
In Brazil, 3 medicines types are known: i) reference medicine, responsible for introduction of a new drug on market and has patent protection, ii) generic medicines and iii) similar medicine both developed after innovative medicine patent protection expires [1,2]. According to ANVISA (Brazilian Health Surveillance Agency) guidance, similar medicines may differ in characteristics relative to size, shape, shelf-life, packaging, labeling and excipients to their reference [2]. Generic medicines it may differ only in excipients and a simplified version of the chemical name is displayed on the packaging including also a yellow stripe with the letter “G” to indicate it [2].
Concerning oral administration medicines, a resolution n°. 391 of 1999 introduces generics into Brazilian Pharmaceutical market and since the beginning it was essential to prove pharmaceutical equivalence (PE) as well as bioequivalence (BE) to their reference [1]. About similar medicines, proceeding a resolution n°. 134 of 2003, they all must to follow PE and BE requirements. Before that legislation, similar medicine met PE tests only [2].
For instance, it is assumed that two medicines (X and Y) are under these tests. PE is achieved when just in vitro tests are made to evaluate whether both contain the same concentration drug as also attending all quality requirements [3]. Otherwise BE is a comparative study of bioavailability profile of these medicines in vivo. X and Y fulfil BE whether bioavailability parameters as Peak concentration (Cmax), time to peak concentration and extent of absorption (area under the curve) not present a significant statistical difference after administration on the same dose under same conditions in patients [4]. Therefore, PE not assures a safety substitution between medicines [5].
In addition, bioequivalence tests are performed to evaluate plasma concentration of the active moiety or metabolite in function of time. From these data, concentration time curves are used to assess the pharmacokinetic parameters mentioned [6]. According to Brazilian legislation BE between two formulations is established obeying 90% confidence interval, within range of 80% and 125% for Cmax and AUC [7]. Although that, due of an initial laws gap about similar medicines, attached by several quality deviations leading to some generic recall, a lack of confidence among population and prescribers remains. In addition, BE between medicine copies (Generic and Similar) are not guarantee of treatment success. But it is common for patients to replace one by other [8].
Is it safe change a reference oral drug by its copies? If copies are interchangeable to its reference why is it not advisable a substitution between them? The review purpose is to bring studies to clarify Brazilian interchangeability scenario.
Literature Search
Search and information sources
This review was conducted up to December 23, 2019, using search terms as "bioequivalence and Brazil", "Oral drugs bioequivalence and Brazil" in attempt to cover all inclusion criteria and article objectives. Electronic databases Pubmed and Academic Scholar were used.
Study selection
Two reviewers (L.G.S and S.G.R) screened title and abstracts followed by full texts lectures of relevant articles. A third reviewer (S.D.M) analyzed and resolved any disagreements.
Inclusion and exclusion criteria
Articles must to attend these following inclusion criteria: conducted in Brazil, in human volunteers, cover the period from 2004 to 2019, be a comparative study between oral formulations; at least one Brazilian formulation under test and published in periodic. In addition, biowaiver monographs, comparison between different dosage forms and articles with incomplete or inaccessible data were excluded.
Synthesis of results
Figure 1 summarizes the methods used for data synthesis. Data extraction division was executed in order to separate methodologies applied. For instance, the idea of Chow and Liu methodology is to assure statistically BE between copies based on independent data from BE studies executed when compared to their reference [9].
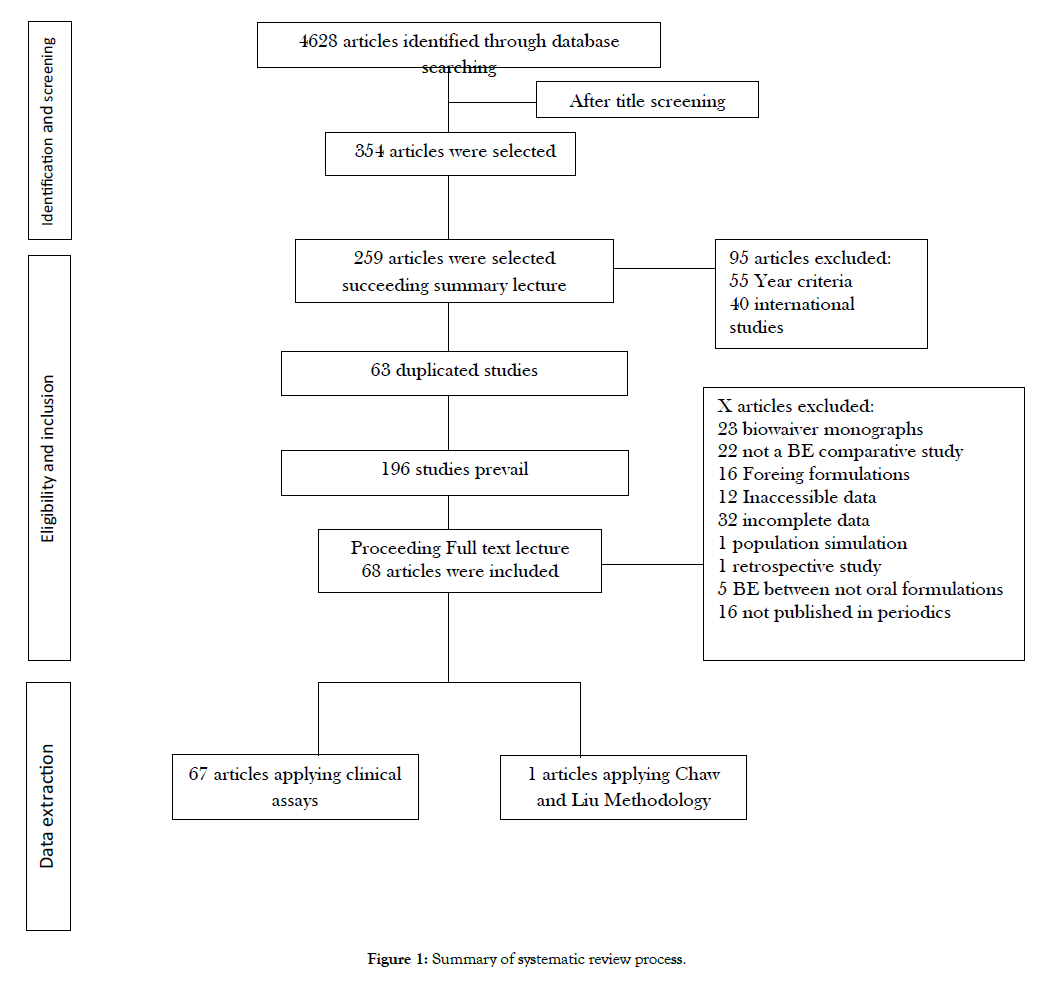
Figure 1: Summary of systematic review process.
Results
It was identified 67 studies which involved a total of 2185 subjects.
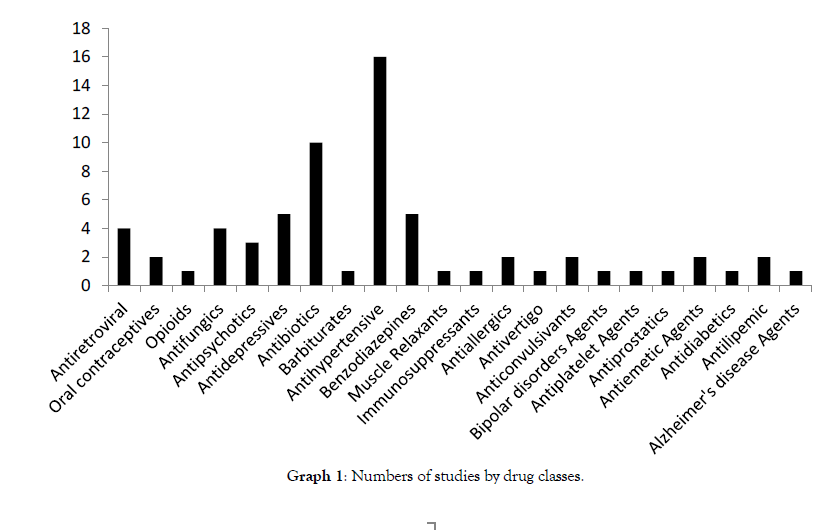
Graph 1 shows BE studies divided by classes and indications.
Graph 2 shows BE studies divided by types of medicines submitted to comparison. Across studies that evaluated BE by clinical assays 66 demonstrated BE comparing copies to their reference [10-75].
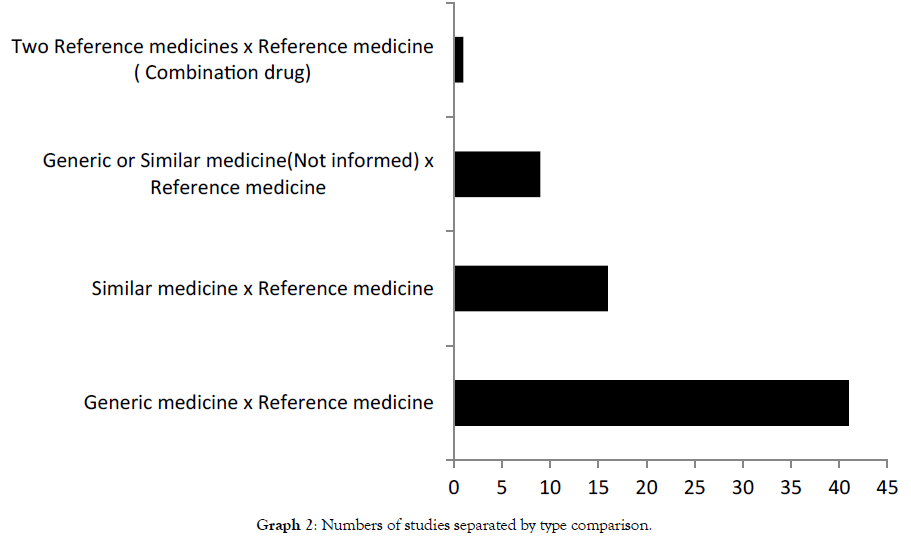
Only 1 study indicated not BE analyzing generic or similar with their reference medicine. A total of 22 subjects received a single 2-mg dose of similar and reference risperidone. Pharmacokinetic and statistical analyses were executed; both Cmáx and AUC parameters left the range established by legislation [76].
The study which applied Chow and Liu methodology has reported BE data comparing generic, similar and references drugs each other. During its meta-analysis between three formulations containing hydrochlorothiazide it was observed that all copies was BE when compared to their reference. In a comparison between copies, 1 of 3 combinations has concluded to non-bioequivalence. In this case, Cmax parameter left the range established by ANVISA, avoiding BE and consequently interchangeability for these combinations [77].
In addition, 8 formulations containing enalapril was compared to its reference drug, all of them fitted established limits. But it was observed that 14 of 28 combinations concluded by nonbioequivalence for Cmax parameter. Regarding AUC 6 of 28 combinations concluded non-bioequivalence [77].
Discussion
It is suggested that interchangeability is safe between copies with their reference. In addition, bioequivalence tests are performed to launch a copy on the market comparing pharmacokinetic parameters with their originator medicine [3,7]. Figure 2 summarizes the current legislation.
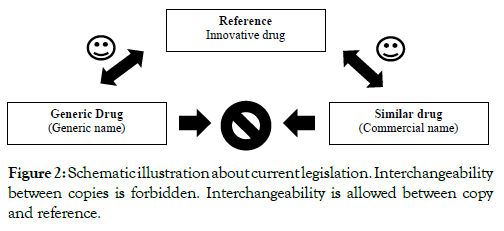
Figure 2: Schematic illustration about current legislation. Interchangeability between copies is forbidden. Interchangeability is allowed between copy and reference.
A typical case involves some professionals prescribe only reference medicines [78], but given this studies scenario the prescription of generic and similar might be more indicated.
Besides, prejudgment with medicines copies still remains among population, because of low price. They believe that price is78 synonymous with low quality raw material and added to the massive campaign by pharmaceutical industry to tarnish the image of generic, patients end up choosing the most expensive medicine [79].
Regarding the interchangeability between copies, even the legislation not allow [6], it was evidenced that it is not safe [77]. Bioequivalence studies allow a range of 80-125% in pharmacokinetic parameters, when comparing test drug with their reference as mentioned. Therefore, if one formulation has a deviation to upper side of the range when compared to another with deviation to opposite direction, when compared to each other they may possibly go beyond limits settled by legislation, not ensuring interchangeability between copies (generic and similar combinations).
This may result in a modified therapeutic response because these formulations are, mostly, non-bioequivalent when compared to each other [77-79]. It is clear that when therapeutic response is lower than expected, drug does not achieve appropriate amounts to produce an expected effect in the body [80]. Same direction, an excess in absorption may lead to drug toxic levels in the organism [81].
For example, in studies involving contraceptive formulations copies were bioequivalent only in comparison to their reference. But security between copies cannot be guaranteed. Therefore, recurrent practices in women switching generic formulations should be avoided. In addition, the alert is also valid for medicines used to control the chronic diseases most common here in Brazil, as well as for drugs with narrow therapeutic window.
Conclusion
Across analysis it was evidenced a majority of results indicating a safe substitution between similar or generic to their reference. Generic and similar drugs frequently are not bioequivalent when compared each other. Orientation guidance for health professionals is needed. Pharmacists dealing directly with patient has to be aware about the insecurity of interchangeability between generic and similar, especially those with narrow therapeutic window and regular use. These data can be used to inform interventions to change the public’s beliefs about a safe use of generic or similar drugs; and avoiding substitution between copies.
Conflict Of Interests
The authors whose names are listed immediately above certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
REFERENCES
- BRASIL. Ministério da Saúde. Agência Nacional de Vigilância Sanitária. Resolução n°391, de 9 de agosto de 1999. A Agência Nacional de Vigilância Sanitária aprova o regulamento técnico para medicamentos genéricos. Diário Oficial da União. Brasília, 1999.
- BRASIL. Ministério da Saúde. Agência Nacional de Vigilância Sanitária. Resolução nº 31, de 11 de agosto de 2010. Dispõe Sobre a Realização dos Estudos de Equivalência Farmacêutica e de Perfil de Dissolução Comparativo. Diário Oficial da União. Brasília, 2010.
- https://trove.nla.gov.au/work/9339762.
- Storpirtis S, Oliveira PGD, Rodrigues D, Maranho D. Considerações biofarmacotécnicas relevantes na fabricação de medicamentos genéricos: fatores que afetam a dissolução e a absorção de fármacos. Rev Bras Cienc Farm. 1999;35(1):1-16.
- Storpirtis S, Marcolongo R, Gasparotto FS, Vilanova CM. A equivalência farmacêutica no contexto da intercambialidade entre medicamentos genéricos e de referência: bases técnicas e científicas. Infarma. 2004;16(9-10):51-56.
- Paumgartten FJR, Oliveira ACAXD. Non-bioequivalent prescription drug interchangeability, concerns on patient safety and drug market dynamics in Brazil. Ciencia & saude coletiva. 2017;22:2549-2558.
- BRASIL. Ministério da Saúde. Agência Nacional de Vigilância Sanitária. Resolução n°1170, de 19 de abril de 2006. A Agência Nacional de Vigilância Sanitária Guia para provas de biodisponibilidade relativa/bioequivalência de medicamentos. Diário Oficial da União. Brasília, 2006.
- Schramm SG. Emprego de meta-análise para avaliação da intercambialidade entre medicamentos (Doctoral dissertation, Universidade de São Paulo), 2008.
- Chow SC, Liu JP. Design and analysis of bioavailability and bioequivalence studies. CRC press. 1999.
- Serra CHR, Koono EEM, Kano EK, Schramm SG, Armando YP, Porta V. Bioequivalence and pharmacokinetics of two zidovudine formulations in healthy Brazilian volunteers: An open-label randomized, single-dose, two-way crossover study. Clin Therapeut. 2008;30(5):902-908.
- Junior EA, Duarte LF, Pirasol Vanunci ML, Teixeira ML. Bioequivalence of two oral contraceptive drugs containing ethinyl-estradiol and gestodene in healthy female volunteers. J Bioequiv Availab. 2010;2(6):125-30.
- Silva MF, Schramm SG, Kano EK, Koono EEM, Porta V, Serra CHR. Bioequivalence evaluation of single doses of two tramadol formulations: A randomized, open-label, two-period: Crossover study in healthy brazilian volunteers. Clinical Therapeut. 2010;32(4):758-765.
- Pereira R, Fidelis S, Vanunci ML, Oliveira CH, Mendes GD, Abib E, et al. Bioequivalence study of two fluconazole capsule formulations in healthy volunteers. Int J Clin Pharmacol Therapeut. 2004;42(1):39-42.
- Filho JHS, Bonifácio FN, Bedor DCG, Ramos VL, de Sousa CEM, Sardón LLF, et al. Relative bioavailability of two formulations of venlafaxine extended-release 75-mg capsules in healthy Brazilian male volunteers: A single-dose, randomized-sequence, open-label, two-period crossover study in the fasting and fed states. Clin Therapeut. 2010;32(12):2088-2096.
- Kano KE, Porta V, Koono EEM, Schramm SG, Serra CHR. Bioequivalence study of two oral formulations of cefadroxil in healthy volunteers. Arzneimittelforschung. 2008;58(1):42-47.
- Silva MF, Schramm SG, Kano EK, Koono EEM, Manfio JL, Porta V, et al. Metronidazole immediate release formulations: A fasting randomized open-label crossover bioequivalence study in healthy volunteers. Arzneimittelforschung. 2012;62(10):490-495.
- Alves AJ, Aquino TM, Neto JL, Filho SDS, Junor HJ, Gaspar FL, et al. Bioequivalence between two metronidazole formulations. Lat Am J Pharm. 2007;26(2):266.
- Porta V, Chang KH, Storpirtis S. Evaluation of the bioequivalence of capsules containing 150 mg of fluconazole. Int J Pharmaceut. 2005;288(1):81-86.
- Catia P, Eduardo B, Carmen MD, Danilo CG, Mota TG, Santana DP. Bioequivalence study analyzed in face of the brazilian generic drugs policy. Lat Am J Pharm. 2007;26(6):859-65.
- Dalmora SL, Sangoi MDS, Nogueira DR, D'Avila FB, Moreno RA, Sverdloff CE, et al. Determination of phenobarbital in human plasma by a specific liquid chromatography method: Application to a bioequivalence study. Química Nova. 2010;33(1):124-129.
- Rezende KR, Mundim IM, Teixeira LS, Souza WC, Ramos DR, Cardoso CR, et al. Determination of captopril in human plasma, using solid phase extraction and high-performance liquid chromatography, coupled to mass spectrometry: Application to bioequivalence study. J Chromatography B. 2007;850(1-2):59-67.
- Borges NC, Guermani A, Mazucheli JA, Mendes GD, Moreno RA. Digoxin bioequivalence study: determination in human plasma by microparticle enzyme immunoassay. Int J Clin Pharmacol Therapeut. 2007;45(6):366-372.
- Borges NCDC, Rigato HM, Oliveira PR, Nogueira DR, Moreno RA, Dalmora SL. Liquid chromatography-tandem mass spectrometry method for the determination of propranolol in human plasma and its application to a bioequivalence study. J Liquid Chromatogra Related Technol. 2008;31(19):2927-2941.
- Cavedal LE, Mendes FD, Domingues CC, Patni AK, Monif T, Reyar S, et al. Clonazepam quantification in human plasma by high-performance liquid chromatography coupled with electrospray tandem mass spectrometry in a bioequivalence study. J Mass Spectrometry. 2007;42(1):81-88.
- Andrade SS, Kano EK, Brioschi TMDLS, Koono EEM, Dos Serra CH, Porta V. Bioavailability study of two oral formulations of didanosine in healthy volunteers. Arzneimittelforschung. 2006;56(5):359-364.
- Silva LF, Moraes MO, Santana GS, Frota FB, De GN, Moraes ME. Phentolamine bioequivalence study. International journal of clinical pharmacology and therapeutics. 2004;42(1):43-49.
- Gonçalves JCS, Monteiro TM, Neves CSM, Gram KRS, Volpato NM, Silva VA, et al. On-line solid-phase extraction coupled with high-performance liquid chromatography and tandem mass spectrometry (SPE-HPLC-MS-MS) for quantification of bromazepam in human plasma: an automated method for bioequivalence studies. Therapeut Drug Monitoring. 2005;27(5):601-607.
- Ruenis APDB, Moreno RA, Abib-Junior E, Simoes RP, Franco LM, Groppo FC, et al. Comparative bioavailability of clarithromycin formulations in healthy brazilian volunteers. Int J Clin Pharmacol Therapeut. 2005;43(8).
- Brioschi TMDLS, Schramm SG, Kano EK, Koono EEM, Ching TH, Serra CHDR, et al. Pharmacokinetics and bioequivalence evaluation of cyclobenzaprine tablets. BioMed Res Int. 2013;2013:281392.
- Daher A, Pitta L, Santos T, Barreira D, Pinto D. Using a single tablet daily to treat latent tuberculosis infection in Brazil: bioequivalence of two different isoniazid formulations (300 mg and 100 mg) demonstrated by a sensitive and rapid high-performance liquid chromatography-tandem mass spectrometry method in a randomized, crossover study. Memórias do Instituto Oswaldo Cruz. 2015;110(4):543-550.
- Franco GC, Baglie S, Ruenis AP, Franco LM, Cogo K, Franco YO, et al. Bioequivalence study of two commercial amoxicillin suspension formulations in healthy. Int J Clin Pharmacol Therapeut. 2014;52(5):425-430.
- Mendes GD, Oliveira CH, Sucupira M, Donato JL. Cyclosporine bioequivalence study; quantification using fluorescence polarization immunoassay (FPIA). Int J Clin Pharmacol Therapeut. 2004;42(2):125-132.
- Suenaga EM, Ifa DR, Cruz AC, Pereira R, Abib E, Tominga M, et al. Automated determination of venlafaxine in human plasma by on-line SPE-LC-MS/MS. Application to a bioequivalence study. J Separation Sci. 2009;32(4):637-643.
- Montovani PAB, Pinto AMP, Santos MBD, Vieira DL, Prado AWD, Manfio JL. Bioavailability of two oral formulas of secnidazole in healthy volunteers. Brazilian J Pharm Sci. 2009;45(4):687-692.
- Mendes GD, Arruda A, Chen LS, Magalhães JCA, Alkharfy KM, De Nucci G. Quantification of cyproheptadine in human plasma by high-performance liquid chromatography coupled to electrospray tandem mass spectrometry in a bioequivalence study. Biomed Chromatogra. 2012;26(1):129-136.
- Salvadori MC, Moreira RF, Borges BC, Andraus MH, Azevedo CP, Moreno RA, et al. Simultaneous determination of losartan and hydrochlorothiazide in human plasma by LC/MS/MS with electrospray ionization and its application to pharmacokinetics. Clin Experiment Hypertension. 2009;31(5):415-427.
- Val L, Chen LS, Mendes GD, Nucci GD. Comparative bioavailability of betahistine tablet formulations administered in healthy subjects. Arzneimittelforschung. 2010;60(07):440-444.
- Sérgio LD, Nogueira DR, Londero LF, Santana DP, Goncalves TM. Determination of Phenytoin in Human Plasma by a Validated Liquid Chromatography Method and its Application to a Bioequivalence Study. Lat Am J Pharm. 2009;28(2):247-53.
- Guilherme MC, Pereira DG, Galuppo MP, Mendes GD, Donato JL, De Nucci G. Bioequivalence of two lithium formulations in healthy volunteers. Arzneimittelforschung. 2006;56(7):524-528.
- Silva DO, Oliveira CH, Mendes GD, Galvinas PAR, Astigarraga REB, Nucci GD. Quantification of chlordesmethyldiazepam by liquid chromatography–tandem mass spectrometry: application to a cloxazolam bioequivalence study. Biomed Chromatogra. 2009;23(12):1266-1275.
- Borges NCC, Mendes GD, Silva DO, Rezende VM, Astigarraga REB, Nucci GD. Quantification of carvedilol in human plasma by high-performance liquid chromatography coupled to electrospray tandem mass spectrometry: application to bioequivalence study. J Chromatogra B. 2005;822(1-2):253-262.
- Sena RC, Brasil COP. Bioequivalence test applied to a new lamivudine/zidovudine combined formulation tablet. Lat Am J Pharm. 2009;28(3):433-437.
- Koono EEM, Kano EK, Schramm SG, Serra CHR, Porta V. Bioequivalence evaluation of two different tablet formulations of tinidazole in healthy volunteers. Arzneimittelforschung. 2008;58(11):598-601.
- Manfio JL, Santos MB, Favreto WAJ, Weich A, Pugens AM, Donaduzzi CM. Bioequivalence study of two formulations of 100 mg capsule of itraconazole. Arzneimittelforschung. 2010;60(3):157-161.
- Borges NCDC, Mendes GD, Borges A, Oliveira SED, Astigarraga REB, Nucci GD. Ticlopidine quantification in human plasma by high-performance liquid chromatography coupled to electrospray tandem mass spectrometry. Application to bioequivalence study. J Mass Spectrometry. 2004;39(12):1562-1569.
- Laurito TL, Mendes GD, Santagada V, Caliendo G, Moraes MEA, Nucci G. Bromazepam determination in human plasma by high-performance liquid chromatography coupled to tandem mass spectrometry: A highly sensitive and specific tool for bioequivalence studies. J Mass Spectrometry. 2004;39(2):168-176.
- Baglie S, Rosalen PL, Franco LM, Ruenis APDB, Baglie RCC, Franco GCN, et al. Comparative bioavailability of 875 mg amoxicillin tablets in healthy human volunteers. Int J Clin Pharmacol Therapeut. 2005;43(7):350-354.
- Bragatto MS, Santos MB, Pinto AMP, Gomes E, Angonese NT. Comparison between Pharmacokinetic and Pharmacodynamic of Single-Doses of Furosemide 40 mg Tablets. J Bioequiv Availab. 2011;3:191-197.
- Kano EK, Koono EEM, Schramm SG, Serra CHDR, Junior EA, Pereira R, et al. Average bioequivalence of single 500 mg doses of two oral formulations of levofloxacin: A randomized, open-label, two-period crossover study in healthy adult Brazilian volunteers. Brazilian J Pharmaceut Sci. 2015;51(1):203-211.
- Pinto GA, Pastre KIF, Bellorio KB, Teixeira LS, Souza WC, Abreu FC, et al. An improved LC-MS/MS method for quantitation of indapamide in whole blood: application for a bioequivalence study. Biomed Chromatogra. 2014;28(9):1212-1218.
- Carvalho V, Costa F, Riccio M, Bernasconi G, Noboli A. Bioequivalence between two fixed dose combinations of dutasteride and tamsulosin in male subjects under fasting and fed conditions. Int Annals Med. 2017;1(10).
- Vespasiano CFP, Accennato VAC, Costa F, Riccio MF, Bernasconi G. Bioequivalence between two Prolonged Release Tablets of Desvenlafaxine Succinate in Healthy Subjects under Fasting and Fed Conditions. J Bioeq Stud. 2018;4(1):101.
- Abib Jr E, Duarte LF, Pereira R, Pozzebon JM, Tosetti D, Custodio JMC. Gabapentin bioequivalence study: Quantification By liquid chromatography coupled to mass spectrometry. J Bioequiv Bioavailab. 2011;3(8):187-190.
- Oliveira PR, Rincon LG, Chellini PR, Baratta J, Siqueira AL, Soares TH, et al. Comparative study of relative bioavailability between two formulations containing Clozapine 100 mg in patients with schizophrenia. Rev Med Minas Gerais. 2015;25(1):64-69.
- Vespasiano CFP, Laurito TL, Iwamoto RD, Moreno RA, Mendes GD, Nucci GD. Bioequivalence study between a fixed-dose single-pill formulation of nebivolol plus hydrochlorothiazide and separate formulations in healthy subjects using high-performance liquid chromatography coupled to tandem mass spectrometry. Biomed Chromatogra. 2017;31(5):e3884.
- Suenaga EM, Val LDC, Tominaga M, Filho JHS, Soares G, Vioto M, et al. A fast and sensitive UHPLC–MS/MS method for the determination of N-butylscopolamine in human plasma: application in a bioequivalence study. Biomedical Chromatography, 2017;31(3):e3823.
- Neto EMR, Marques LARV, Cunha GH, Pontes AV, Lobo PLD, Nucci G, et al. Bioavailability of different formulations of metformin hydrochloride in healthy volunteers: A comparative study. Int Arc Med. 2016;9.
- Filho HOS, Ilha JO, Silva LC, Borges A, Mendes GD, Nucci GD. Comparative bioavailability study with two amiodarone tablet formulations administered with and without food in healthy subjects. Arzneimittelforschung. 2007;57(9):582-590.
- Abib Jr E, Duarte LF, Suenaga EM, Cruz AC, Nakaie CR. Comparative bioavailability of two quetiapine formulations in healthy volunteers after a single dose administration. J Bioequiv Bioavailab. 2011;3(8):178-181.
- Borges NCDC, Mendes GD, Astigarraga REB, Zappi E, Mendes FD, Nucci GD. Comparative bioavailability study with two gemfibrozil tablet formulations in healthy volunteers. Arzneimittelforschung. 2005;55(7):382-386.
- Soares AKA, Moraes MO, Bezerra FAF, Nucci G, Moraes MEA. Determinação de captopril por HPLC acoplado a espectômetro de massa: aplicação em estudo de bioequivalência. Revista Brasileira em Promoção da Saúde. 2012;25(1):13-19.
- Borges NCDC, Mendes GD, Astigarraga REB, Galvinas P, Oliveira CH, Nucci GD. Verapamil quantification in human plasma by liquid chromatography coupled to tandem mass spectrometry: An application for bioequivalence study. J Chromatogra. 2005;827(2):165-172.
- Mendes GD, Sanfelice ATD, Borges NCC, Cavedal LE, Sverdloff C, Galuppo MP, et al. Comparative bioavailability of two ramipril formulations after single-dose administration in healthy volunteers. Int J Clin Pharmacol Therapeut. 2006;44(2):93-98.
- Oliveira CH, Silva RM, Santagada V, Caliendo G, Perissutti E, Galupo MP, et al. Comparative bioavailability of two losartan formulations in healthy human volunteers after a single dose administration. Int J Clin Pharmacol Ther. 2006;44(3):142-148.
- Moreira RF, Salvadori MC, Azevedo CP, Silva DO, Borges DC, Moreno RA, et al. Development and validation of a rapid and sensitive LC-ESI-MS/MS method for ondansetron quantification in human plasma and its application in comparative bioavailability study. Biomed Chromatogra. 2010;24(11):1220-1227.
- Ruenis APDB, Franco GCN, Baglie S, Tiberti LA, Franco LM, Groppo FC, et al. Bioequivalência de formulações orais de carbamazepina disponíveis no Brasil. Rev Bras Ciênc Saúde. 2004;113-124.
- Abib Jr E, Duarte LF, Pereira R, Lemes AB, Morais DC, Lima LG, et al. Study of relative bioavailability/bioequivalence of two formulations of valsartan in healthy volunteers for both sexes [estudo De Biodisponibilidade Relativa/bioequivalência De Duas Formulações De Valsartana Em Voluntários Sadios De Ambos Os Sexos]. Revista Brasileira de Medicina. 2012.
- Abib Jr E, Duarte LF, Pereira R, Lemes AB, Morais DC, Lima LG, et al. Study of relative bioavailability/bioequivalence of two formulations of donepezil hydrochloride in healthy volunteers for both sexes [estudo De Biodisponibilidade Relativa/bioequivalência De Duas Formulações De Cloridrato De Donepezila Em Voluntários Sadios De Ambos Os Sexos]. Revista Brasileira de Medicina. 2013.
- Mendes GD, Araujo MVFD, Paris EG, Astigarraga REB, Oliveira CHD. Biodisponibilidade comparativa de duas formulações de sertralina em voluntários humanos sadios após a administração de uma única dose. Rev Bras Med. 2004;61(1/2):80-84.
- Mendes FD, Chen LS, Borges A, Babadópulos T, Ilha JO, Alkharfy KM, et al. Ciprofibrate quantification in human plasma by high-performance liquid chromatography coupled with electrospray tandem mass spectrometry for pharmacokinetic studies. J Chro Matograb. 2011;879(24):2361-2368.
- Borges NC, Astigarraga RB, Sverdloff CE, Galvinas PR, Da Silva WM, Rezende VM, et al. A novel and sensitive method for ethinylestradiol quantification in human plasma by high-performance liquid chromatography coupled to atmospheric pressure photoionization (APPI) tandem mass spectrometry: Application to a comparative pharmacokinetics study. J Chromatogra B. 2009;877(29):3601-3609.
- Morita MR, Berton D, Boldin R, Barros FAP, Meurer EC, Amarante AR, et al. Determination of levocetirizine in human plasma by liquid chromatography–electrospray tandem mass spectrometry: Application to a bioequivalence study. J Chromatogra B. 2008;862(1-2):132-139.
- Kano EK, Serra CHR, Koono EEM, Andrade SS, Porta V. Determination of lamivudine in human plasma by HPLC and its use in bioequivalence studies. Int J Pharmaceut. 2005;297(1-2):73-79.
- Andraus MH, Wong A, Silva OA, Wada CY, Toffleto O, Azevedo CP, et al. Determination of bromazepam in human plasma by high-performance liquid chromatography with electrospray ionization tandem mass spectrometric detection: application to a bioequivalence study. J Mass Spectro. 2004;39(11):1348-1355.
- Padua AA, Astigarraga REB, Rezende VM, Mendes GD, Nucci GD. Lisinopril quantification in human plasma by liquid chromatography–electrospray tandem mass spectrometry. J Chromatogra B. 2004;809(2):211-216.
- Belotto KCR, Raposo NRB, Ferreira AS, Gattaz WF. Relative bioavailability of two oral formulations of risperidone 2 mg: A single-dose, randomized-sequence, open-label, two-period crossover comparison in healthy Brazilian volunteers. Clin Therapeut. 2010;32(12):2106-2115.
- Lopes RA, Neves FDAR. Meta-analysis for bioequivalence studies: interchangeability of generic drugs and similar containing Hydrochlorothiazide is possible but not with Enalapril Maleate. Brazilian J Nephrol. 2010;32(2):173-181.
- Massud M. Conflito de interesses entre os médicos e a indústria farmacêutica. Revista Bioética. 2010;18(1).
- Rumel D, Nishioka SDA, Santos AAMD. Intercambialidade de medicamentos: abordagem clínica e o ponto de vista do consumidor. Revista de Saúde Pública. 2006;40:921-927.
- Gidal BE, Tomson T. Debate: Substitution of generic drugs in epilepsy: Is there cause for concern?. Epilepsia. 2008;49:56-62.
- Pope ND. Generic substitution of narrow therapeutic index drugs. US Pharm. 2009;34(6):12-19.
Citation: Lima GS, Sampaio GR, Soares DM (2020) Medicine Interchangeability in Brazil, is it Safe? A Systematic Review for the Last 15 Years about Oral Drugs. J Bioequiv Availab 12:402. doi: 10.35248/0975-0851.20.12.402.
Copyright: © 2020 Lima GS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

