Indexed In
- Online Access to Research in the Environment (OARE)
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Scimago
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2024) Volume 15, Issue 4
Maturation and Larval Production of Japanese Female Eel (Anguilla japonica) Produced by Induction of Early Metamorphosis for Long-Term Breeding
Yutaka Kawakami*Received: 26-Apr-2024, Manuscript No. JARD-24-25550; Editor assigned: 29-Apr-2024, Pre QC No. JARD-24-25550 (PQ); Reviewed: 13-May-2024, QC No. JARD-24-25550; Revised: 20-May-2024, Manuscript No. JARD-24-25550 (R); Published: 27-May-2024, DOI: 10.35248/2155-9546.24.15.858
Abstract
Our previous study reported artificial production of glass eels of Japanese eel (Anguilla japonica) by inducing early metamorphosis with Thyroid Hormone (TH) treatment. The purpose of this study was to clarify whether the artificial glass eels produced by TH treatment can grow and acquire fertility. TH-treated glass eels were feminized by feeding with an estradiol-supplemented diet. After being raised for 8 years, three females were brought to sexual maturity with artificial gonadotropic hormones. Fertilized eggs and hatched larvae were obtained from two of the three fish. Collectively, our findings show that the TH-treated glass eel grows smoothly, acquires fertility, and can reproduce successfully.
Keywords
Japanese eel; Thyroid hormone; Artificially produced glass eel; Artificial maturation
Introduction
The Japanese eel (Anguilla japonica) is an important commercial species in Japan owing to its high market value as a food source. However, production of artificial seeding for industry has not been established. To culture Japanese eel, 100% natural glass eels, which migrate to the Japanese coast and are collected in rivers, are used for seeding.
Since the early 1990s, the production of artificial glass eels has been studied [1,2]. In 2010, successful closed-cycle breeding of the Japanese eel was reported, facilitating studies of leptocephali, which have a leaf-shape body that is transparent and narrow with a high back [3,4]. Nevertheless, the production of artificial seeding for industry has not been established. One of the reasons is that the leptocephali do not feed, or grow, on biological baits such as rotifer (Brachionus plicatilis), which are used to produce seedlings of other fish species such as red seabream (Pagrus major) and/or Japanese flounder (Paralichthys olivaceus) [1,5]. Furthermore, the duration needed to reach the glass eel stage is long, for example, the average duration from hatched larva to glass eel was 299 days (range 153-754 days) at Shibushi laboratory national research institute of aquaculture, Fisheries research agency of Japan. Thus, it is inevitable that production costs will be higher and production efficiency will be lower for Japanese eel than for other fish species [6].
Thyroid Hormones (THs), including L-thyroxine (T4) and 3,5,3’- triiodo-L-thyronine (T3), are known to control metamorphosis in amphibians [7,8]. They are also important in early teleost development including metamorphosis [9-11]. To aid in the mass production of glass eels, Kawakami Y recently proposed a method of inducing early metamorphosis in leptocephali. Whereas metamorphosis to a glass eel naturally occurs in fully grown leptocephali of 50 mm TL or longer, metamorphosis can be artificially induced in 40 mm TL leptocephali using T4 [12]. However, while the artificial glass eels have been shown to feed and grow, it is not clear whether the induction of early metamorphosis has subsequent effects on development of the artificial eels. For example, do T4-induced glass eels grow to adult fish and have reproductive function? The aim of this study was to assess the viability of the artificial glass eels produced by T4 treatment. In particular, the purpose was to evaluate the presence or absence of fertility by inducing sexual maturity in females.
Materials and Methods
Glass eels and estradiol-17 β treatment to produce females
Leptocephali hatched from fertilized eggs of Japanese eel were fed on powdered shark eggs as in previous studies [12-14]. At around 40 mm TL, leptocephali were subjected to induction of metamorphosis by T4 treatment, as described in Experiments 3 and 4 [12].
The resulting T4-induced glass eels were raised as follows. The feed initially comprised larvae of Chironomus sp. and/or sludge worms (Tubifex tubifex) and was gradually switched to a compound feed specific for eels (Hayashikane, Shimonoseki, Japan). The fish that started to feed were feminized by adding estradiol-17 β (E2) (Sigma-Aldrich, Steinheim, Germany) to the compound feed. During this time, the fish were kept in fresh water at a temperature range of 15 °C to 27 °C, and no temperature control was performed. The fish continued to be fed with E2 for approximately 6 months, after which they were raised on regular compound feed. Individual identification of the eels in this study during these stages was not performed.
Breeding and fertilization
To obtain fertilized eggs and/or larvae, artificial maturation was started on January 2022 and performed by hormonal treatment. In brief, three immature male eels (250 g-300 g Body Weight (BW)) were purchased from a commercial supplier, and induced to maturation by injection of recombinant Japanese eel luteinizing hormone (rLH, ARK Resource, Kumamoto, Japan), as described in Ohta H et al, [15]. After confirming sperm motility activity, semen samples from three individuals were each diluted with K30 artificial seminal plasma, stored at 4 °C, and used within a few weeks [16].
Female eels raised from artificially induced glass eels were injected with recombinant Japanese eel follicle-stimulating hormone (500 µg/kg BW) (rFSH, ARK Resource, Kumamoto, Japan) once a week as described by Kazeto Y et al, (Figure 1) [17]. Oocyte maturation was performed under artificial seawater at 15°C, except for the final stage (20 °C) when rLH (500 µg/kg BW) and 17 α-hydroxyprogesterone (2 mg/kg BW) (17 α-OHP, Sigma) were injected. Oocytes were obtained by gently stripping the ovulating female and subsequently fertilized with the refrigerated sperm. Fertilized eggs were maintained in a 100-L cylindrical polycarbonate tank (20,000-50,000 eggs per tank) for 1.5 days at 25°C and hatching larvae were transferred to a 10-L acrylic tank (50-100 individuals per liter) at 25 °C with artificial seawater. All fish handling, husbandry, and sampling methods were approved by the Institutional animal care and use committee.

Figure 1: Artificially produced female fish. A) Fish 1; B) Fish 2; C) Fish 3. Note: Top images show fish at the start of artificial maturation; bottom images show fish prior to egg collection.
Results and Discussion
In this study, eight glass eels produced between October 2013 and January 2014 by T4 treatment were raised in running fresh water for about 8 years. The age of the glass eels was 180 days or less (about 6 months); therefore, the fish were about 8-9 years old at the start of the maturation process, and approximately 9 years old at final oocyte maturation [12].
About 6 months after initiating feeding in the first year after metamorphosis, the glass eels were feminized by using a diet supplemented with E2. Four of the eight fish died for the various reasons, such as escape. Of the remaining four, three were selected as female fish, while the remaining fish was determined to be male and the maturation process was stopped (Figures 1A-1C).
Of the three female fish, fertilized eggs and hatched larvae were obtained with Fish 1 and Fish 2 (Table 1). Figure 2A, indicates the rate of BW gain of Fish 1 after starting weekly rFSH injections. The increase in BW followed a smooth curve until week 12, when the final oocyte maturation process was started (Figure 2A). Two days after the week-12 rFSH injection, BW had increased to more than 120% of the initial value (Figure 2A). In this study, a BW of 120% or more was set as one of the criteria for the possibility of transition to the final maturation process. At the same time, observation of the ovarian eggs by cannulation showed that the eggs had reached the target size and clarity; therefore, rLH was injected in the afternoon (Figure 2A and 3A). The next day, the oil droplets in the oocytes were enlarged and 17α-OHP was injected in the afternoon (Figure 2A and 3B). The following day, egg collection and artificial insemination were performed (Figure 2A). For Fish 1, the fertilization rate was 61.9% and the hatching rate was 32.2% (Table 1). Figure 3C, shows fertilized eggs derived from Fish 1, with the blastocoel clearly observed, while Figure 3D, shows embryos 30 hours after fertilization. Larvae were observed 1-day post hatching (dph) and normal growth was observed up to 7 dph (Figure 3E and 3F).
| No. | TL (mm) | BW (g) | Age (y) | Fertilization rate (%) | Hatching rate (%) |
|---|---|---|---|---|---|
| 1 | 514 | 432.5 | 8 | 61.9 ± 3.82 | 32.2 ± 9.29 |
| 2 | 516 | 504.9 | 8 | 21.2 ± 1.81 | 1.54 ± 0.42 |
| 3 | 467 | 331.6 | 8 | 0 | 0 |
Note: E2: Estradiol-17ß, T4: L-thyroxine, TL: Total Length, BW: Body Weight, Mean ± SE.
Table 1: Results of artificial maturation using artificially produced female eels (Anguilla japonica), derived from glass eels produced by induction of metamorphosis using T4 and feminized by E2 treatment.
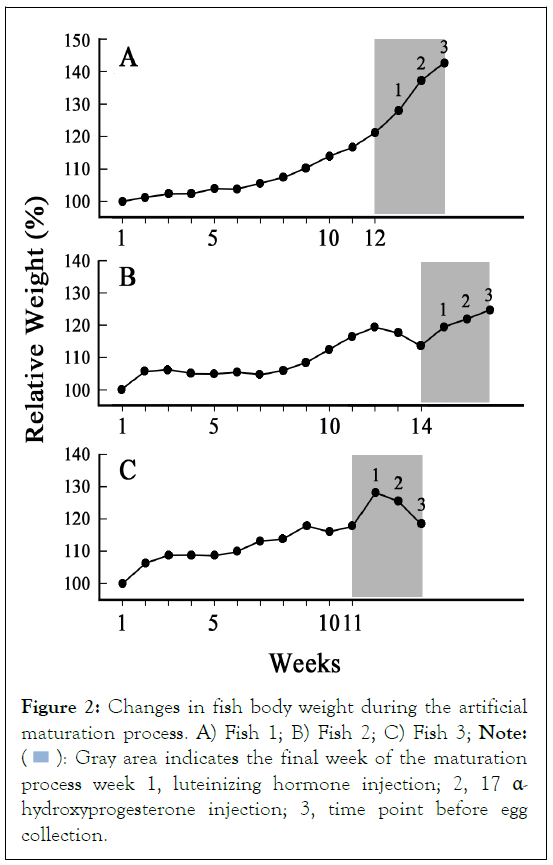
Figure 2: Changes in fish body weight during the artificial maturation process. A) Fish 1; B) Fish 2; C) Fish 3; Note:  Gray area indicates the final week of the maturation process week 1, luteinizing hormone injection; 2, 17 α-
hydroxyprogesterone injection; 3, time point before egg
collection.
Gray area indicates the final week of the maturation process week 1, luteinizing hormone injection; 2, 17 α-
hydroxyprogesterone injection; 3, time point before egg
collection.
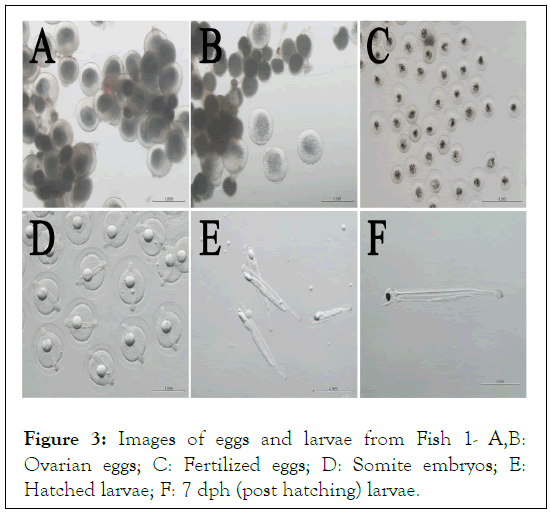
Figure 3: Images of eggs and larvae from Fish 1- A,B: Ovarian eggs; C: Fertilized eggs; D: Somite embryos; E: Hatched larvae; F: 7 dph (post hatching) larvae.
For Fish 2, the fertilization rate was 21.2% and the hatching rate was 1.5% (Table 1). Fish 2 was found to be silvered in the autumn of its 7th year (autumn 2020), and it remained in a silvered state at the start of maturation, in contrast to Fish 1 and Fish 3 (Figures 1A-1C). This may be a reason why Fish 2 did not produce good quality fertilized eggs or hatched larvae. In addition, weight gain in Fish 2 slowed down at the end of the artificial maturation process (Figure 2B). Although the fish had a BW of 120% at week 12, no transparent oocytes were observed; therefore, we decided to extend the maturation process by 2 weeks. It can be seen that the BW decreased from week 12 to week 14 before the final oocyte maturation was started and this may also have been a reason for the low fertilization and hatching rates of Fish 2 (Figure 2B).
Lastly, Fish 3 produced no fertilized eggs (Table 1). This fish showed low initial reactivity to rFSH, and its weight gain was not remarkable even in the late maturation period (Figure 2C). Its BW was as low as 331.6 g, which might not be suitable for a female parent fish; however, the maturation of oocytes was confirmed as for Fish 1 and Fish 2 (Figure 2C). Nevertheless, no fertilized eggs were obtained from Fish 3 (Table 1).
In this study, we assessed whether three female parent fish raised from T4-induced glass eels could reach sexual maturation and reproduce. We confirmed that all three fish had ovaries, and fertilized eggs and hatched larvae were obtained from two. Thus, glass eels treated with T4 are considered to have no problem in terms of subsequent growth and maturity. The three fish used in this study were derived from the glass eels reported by Kawakami Y et al, but they were produced by several T4 methods; in particular, some of the early T4 methods led to artificial glass eels with morphological abnormalities, especially vertebrae formation [12]. Individual identification of the glass eels in this study was not performed; therefore, it was not known which method was used to produce each individual. However, the results of Fish 1 and 2 indicate that T4-treated glass eels have reproductive ability. Fish 3 had grown for 8 years and reached only 331.6 g BW (Table 1). The probable cause of this low BW was that more than eight fish were raised in a 500-L capacity tank, leading to differences in growth, rather than that metamorphosis was artificially induced using T4. In addition, the short body shape of Fish 3 seems to be the result of inheriting characteristics of a glass eel produced by early metamorphosis induction (Figure 1) [12].
In Japanese eels, E2 treatment is now commonly used to produce mature female parent fish [18]. As a rule of thumb, 2-year-old or 3-year-old fish have been used for artificial maturation owing to the deterioration of oocyte quality due to overripening of the ovaries in older fish (personal communications). Although there are no published data to confirm this as yet, it is also evident from our experience. It is generally thought that wild Japanese male eels of at least 4 years in age migrate and spawn, while wild females are several years older [19]. Based on empirical rules, therefore, it is currently difficult to explain the phenomenon that the egg quality of feminized eels deteriorates after 3 years and their performance as female fish for maturation deteriorates. It will be necessary to clarify whether this is due to the influence of aquarium breeding or to differences in growth under natural conditions [20].
Conclusion
In recent years, Japanese eels have been released into rivers and lakes in Japan in order to maintain eel resources and, although their origins vary, there have been reports of the release of fully grown adult eels originated from wild glass eels raised under aquaculture (personal communications). However, there is little information about the condition of the ovaries of the released eels subsequent to their release or the performance of the fish in reproduction. Furthermore, artificially produced eels may be used for release in the future. The fact that we obtained fertilized eggs and hatched larvae from the long-term breeding of artificial produced eels in this study may be a very significant finding from the perspective of the effectiveness and pros and cons of such release projects.
Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgements
Although some of our important fish died due to the poor breeding environment, we would like to express our appreciation to the staff of the Shin Nippon Biomedical Laboratories, Ltd. for long-term maintenance of the fish. We would also like to thank for English language editing.
References
- Tanaka H, Kagawa H, Ohta H. Production of leptocephali of Japanese eel (Anguilla japonica) in captivity. Aquaculture. 2001;201:51-60.
- Tanaka H, Kagawa H, Ohta H, Unuma T, Nomura K. The first production of glass eel in captivity: Fish reproductive physiology facilitates great progress in aquaculture. Fish Physiol Biochem. 2003;28:493-497.
- Tanaka H. Progression in artificial seeding production of Japanese eel Anguilla japonica. Fish Sci. 2015;81:11-19.
- Tesch FW. The eel biology and management of Anguillid eel. Chapman & Hall. 1977.
- Okamura A, Yamada Y, Horie N, Mikawa N, Tanaka S, Kobayashi H, et al. Hen egg yolk and skinned krill as possible foods for rearing leptocephalus larvae of Anguilla japonica Temminck & Schlegel. Aquaculture. 2013;44:1531-1538.
- Masuda Y, Imaizumi H, Oda K, Hashimoto H, Teruya K, Usuki H. Artificial completion of the Japanese eel, Anguilla japonica, life cycle: Challenge to mass production. Bull Fish Res Agency. 2012;35:111-117.
- Galton VA. The role of thyroid hormone in amphibian metamorphosis. Tr Endocrinol Metabol.1992;3:96-100.
[Crossref] [Google Scholar] [PubMed]
- Tata J. Amphibian metamorphosis as a model for the developmental actions of thyroid hormone. Mol Cell Endocrinol.2006;246:10-20.
[Crossref] [Google Scholar] [PubMed]
- Power DM, Llewellyn L, Faustino M, Nowell MA, Bjornsson B Th, Einarsdottir IE, et al. Thyroid hormones in growth and developmental of fish. Comp Biochem Physiol C. 2001;130:447-459.
[Crossref] [Google Scholar] [PubMed]
- Trijuno DD, Yoseda K, Hirokawa JUN, Tagawa M, Tanaka M. Effects of thyroxine and thiourea on the metamorphosis of coral trout grouper Plectropomus leopardus. Fish Sci. 2002; 68: 282–289.
- Kawakami Y, Yokoi K, Kumai H, Ohta H. The role of thyroid hormones during the development of eye pigmentation in the Pacific bluefin tuna (Thummus orientalis). Comp Biochem Physiol B 2008;150:112-116.
[Crossref] [Google Scholar] [PubMed]
- Kawakami Y. Sensitivity of Anguilliformes leptocephali to metamorphosis stimulated by thyroid hormone depends on larval size and metamorphic stage. Comp Biochem Physiol A. 2023; 276. 111339.
[Crossref] [Google Scholar] [PubMed]
- Kawakami Y, Nomura K, Tanaka H. Growth promoting effect of hyaluronan synthesis promoting substances on Japanese eel leptocephali. Plos One.2014;96:e98688.
[Crossref] [Google Scholar] [PubMed]
- Sudo R, Kawakami Y, Nomura K, Tanaka H, Kazeto Y. Production of recombinant Japanese eel (Anguilla japonica) growth hormones and their effects on early-stage larvae. Gen Comp Endocrinol. 2023;317:113977.
[Crossref] [Google Scholar] [PubMed]
- Ohta H, Sato Y, Imaizumi H, Kazeto Y. Changes in milt volume and sperm quality with time after an injection of recombinant Japanese eel luteinizing hormone in male Japanese eels. Aquaculture. 2017;479:150-154.
- Ohta H, Kagawa H, Tanaka H, Okuzawa K, linuma N, Hirose K. Artificial induction of maturation and fertilization in the Japanese eel, Anguilla japonica. Fish Physiol Biochem. 1997;17:163-169.
- Kazeto Y, Ito R, Tanaka T, Suzuki H, Ozaki Y, Okuzawa K, Gen K. Establishment of cell-lines stably expressing recombinant Japanese eel follicle-stimulating hormone and luteinizing hormone using CHO-DG44 cells: Fully induced ovarian development and different modes. Front Endocrinol. 2023;10:3389.
[Crossref] [Google Scholar] [PubMed]
- Inaba H, Hara S, Horiuchi M, Ijiri S, Kitano T. Gonadal expression profiles of sex-specific genes during early sexual differentiation in Japanese eel Anguilla japonica. Fish Sci. 2021;87:203-209.
- Yoshikawa M. Relationships between body weight, age and sex ratio in natural and cultivated large Japanese eel Anguilla japonica. Bull Shizuoka Pref Fish Exp Stn. 1995;30:29-34.
- Matsumoto H, Akiyama T, Okuzono H, Mochioka N. Recapture of brackish and freshwater pond reared eels (Anguilla japonica) released into brackish water river system. Sci Bull Fac Agr Kyushu Univ. 2021.76:1-6.
Citation: Kawakami Y (2024) Maturation and Larval Production of Japanese Female Eel (Anguilla japonica) Produced by Induction of Early Metamorphosis for Long-Term Breeding. J Aquac Res Dev. 15:858.
Copyright: © 2024 Kawakami Y. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.