Indexed In
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Proquest Summons
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Case Report - (2023) Volume 14, Issue 6
Manual Exchange Transfusion: Practical Challenges and Overcomes
Pruthviraj Guduri1*, Balwant Kumar2, Ipsita Nag1, Mekhala Paul3, Thejasri Vishnubhatla4 and Sanjana Sai Makanaboyina52Department of General Medicine, The Mission Hospital, West Bengal, India
3Department of Emergency Medicine, The Mission Hospital, West Bengal, India
4Department of Dentistry, The Mission Hospital, West Bengal, India
5Department of Medical Sciences, Rajarajeswari Medical college and Hospital, Karnataka, India
Received: 21-Apr-2023, Manuscript No. JBDT-23-21347; Editor assigned: 24-Apr-2023, Pre QC No. JBDT-23-21347 (PQ); Reviewed: 15-May-2023, QC No. JBDT-23-21347; Revised: 22-May-2023, Manuscript No. JBDT-23-21347 (R); Published: 29-May-2023, DOI: 10.4172/2155-9864.23.14.569
Abstract
This case report describes the successful use of manual exchange transfusion in the management of a 17-year-old female patient with dapsone-induced methemoglobinemia, which did not respond to conventional therapy with methylene blue and high-dose vitamin C. The patient underwent three cycle of manual exchange transfusion at 12- hour intervals to remove the excess methemoglobin, resulting in significant clinical improvement with the gradual reduction of methemoglobin levels. The manual exchange transfusion each cycle was performed using two units of Anti Hemophilic Globulin (AHG) phase crossmatch compatible Packed Red Blood Cells (PRBC), two units of Random donor platelet (RDP), and one unit of Fresh Frozen Plasma (FFP). The protocol for manual exchange transfusion was followed through a literature review, and the patient was discharged after 15 days of hospitalization with no cyanosis and neurologically intact. This case report highlights the importance of manual exchange transfusion as a potential lifesaving procedure in the acute setting where automated services are unavoidably delayed.
Keywords
Exchange transfusion; Methemoglobinemia; Dapsone poisoning; Red cell exchange
Introduction
Exchange transfusion is a potentially lifesaving procedure. Red blood cell exchange is a major treatment for removing abnormal Sickle Red Blood Cells (RBC) in Sickle Cell Disease (SCD), poisoning and severe methemoglobinemia refractory to conventional therapy [1]. The treatment can be performed manually via a combination of bloodletting and transfusion [2]. Exchange transfusion may be performed as manual exchange transfusion or Automated Red cell exchange. Manual exchanges are performed using repeated alternating isovolumetric phlebotomy and blood transfusion [3,4]. Manual exchange is only to be used in the acute setting where an automated service is unavoidably delayed. As this is an uncommon emergency procedure, we will describe the protocol followed through a literature review.
Case Presentation
A 17-year-old female patient presented to the emergency department with a headache, vertigo, and multiple episodes of vomiting for the past day. Upon taking a detailed history, it was revealed that she had ingested 28 tablets of Dapsone in an attempt to commit suicide. Furthermore, she shared that her mother's demise one month prior which had traumatized her mentally. Family members also reported a recent history of self-inflicted injuries.
During examination, the patient appeared cyanotic with decreased level of consciousness. Her oxygen saturation, as measured by pulse oximetry, was 77%. A co-oximeter was requested, which revealed a methemoglobin level of 36.3%. Arterial Blood Gases (ABGs) were also significant, showing a pH of 7.385, partial pressure of Carbon dioxide (pCO2) of 17, partial pressure of Oxygen (PaO2) of 99.6, alveolar to Arterial Oxygen (a/AO2) of 78%, and oxygen saturation of arterial blood (SaO2) of 94.9%. Due to the discrepancy between her low oxygen saturation as detected by the pulse oximeter (SpO2) and normal SaO2 and PaO2 on ABG analysis, a diagnosis of dapsone-induced acquired methemoglobinemia was considered. The patient was treated with methylene blue 1 mg/kg intravenously daily and high-dose vitamin C 5 g intravenously every six hours. ABGs were monitored every 12 hours to track PaO2 and methemoglobin levels. However, despite treatment with methylene blue, she showed no signs of improvement with progressively increasing oxygen requirements.
In view of refractory methemoglobinemia, manual single volume exchange transfusions were performed thrice at an interval of 12 hrs. Following the procedure the SpO2 increased to 92%, sensorium improved and she was extubated the next day (Table 1). The Methhemoglobin (MetHb) level gradually decreased to 11.0% (Figure 1). She was discharged on the 15th day of hospitalization. Follow-up after 1 week revealed a neurologically intact patient with no cyanosis.
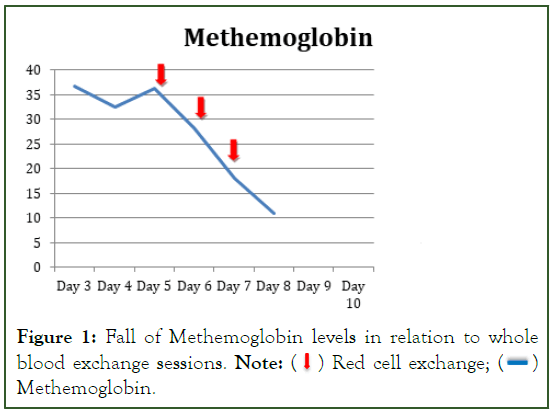
Figure 1: Fall of Methemoglobin levels in relation to whole blood exchange sessions. Note:  Red cell exchange;
Red cell exchange;  Methemoglobin.
Methemoglobin.
| Day of Hospitalization | Blood | Heart Rate | Sp O2 | Respiratory | Meth Hb |
|---|---|---|---|---|---|
| Pressure | rate | levels | |||
| Day 3 | 120/70 | 82 | 77% | 20 | 36.7 |
| Day 4 | 120/70 | 112 | 80% | 23 | 32.6 |
| Day 5 | 120/80 | 108 | 78% | 18 | 36.3 |
| Day 6 | 100/70 | 130 | 98% | 14 | 28.2 |
| Day 7 | 110/60 | 110 | 94% | 17 | 18 |
| Day 8 | 110/70 | 120 | 93% | 20 | 11 |
| Day 9 | 100/60 | 96 | 98% | 18 | 10 |
Table 1: Methhemoglobin levels and vitals of the patient.
Manual exchange transfusion
Pretransfusion workup of the patient revealed his blood group to be AB Rh (D) positive. Phenotype of the patient was found to be R1R1 (DCe/DCe) (Diamed Biorad, Switzerland). Direct antiglobulin test, autocontrol was negative. Due to unavailability of automated red cell exchange protocol in the apheresis machine at our centre manual exchange transfusion was planned [5]. The amount of blood to be exchanged was calculated by the formula as follows:
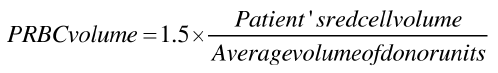
The characteristics of PRBCs used were AHG phase crossmatch compatible, sickle-negative, PRBCs <7 days old. The phelebotomy was performed via femoral line in a sterile closed procedure using tube sealer and sterile connecting device whereas the transfusions were given simultaneously through a peripheral line. The temperature, pulse, blood pressure, and oxygen saturation were monitored prior/post venesection as well as before, 15 min, and 1 hr after starting blood transfusions.
Preparation
Blood tests: Complete blood tests, Prothrombin Time (PT), activated Partial Thromboplastin Clotting Time (aPTT), International Normalised Ratio (INR), Serum electrolytes and Methemoglobin levels.
Blood products: Two units of AHG (Anti Human Globulin) phase cross match compatible PRBC (Packed Red Blood Cells), two units RDP (Random Donor Platelets) and one unit FFP (Fresh Frozen Plasma).
High flow venous access: Either via standard femoral line or vascath (apheresis line), or large vein cannula if patient has two large bore veins accessible. (Minimum grey or orange venflon)
Equipment: Sterile connecting device and Tube sealer.
Consumables: Sterile gloves, sterile dressing kit, syringes and injection heplock.
Procedure
Step 1: The patients’ vitals were noted and consent for the procedure has been obtained. A sterile Blood bag is connected to the femoral line after removal and discard of Heparin. Withdrawal of 500 ml of whole blood is done over 25 min but controlling the rate by tube compression. One the required volume has been obtained (measured by blood collection monitor at bed side) the blood bag tube has been sealed with a tube sealer. The sealed blood bag is discarded; the remnant tube connected to the femoral line is left intact for further phlebotomies. The patient’s vitals have been noted after the procedure (Figure 2).
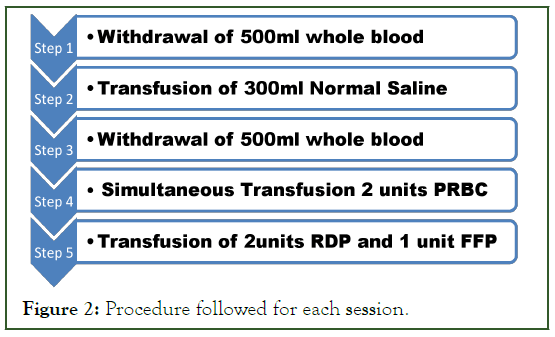
Figure 2: Procedure followed for each session.
Step 2: 300 ml 0.9% Normal Saline has been infused as a bolus through the peripheral line.
Step 3: A new blood bag is connected to the remnant tube by using a sterile connecting device. Once the blood bag is connected the vitals are noted down and step 1 is repeated. After completion the femoral line is sterilized and heparin is infused to maintain patency.
Step 4: Transfusion of the compatible PRBC through the peripheral line is started in concordance to step 3 so that the phlebotomized volume does not go above 10.5 ml/kg. PRBC transfusion is done in according to the SOP (Standard Operating Protocol).
Step 5: Two units RDP (Random Donor Platelets) and one unit FFP (Fresh Frozen Plasma) are transfused to replace the loss of platelets and coagulation factors.
A total of 3 such sessions were required in this patient. All transfusions were uneventful. To compensate the loss of platelets and coagulation factors RDP (Random Donor Platelets) and FFP (Fresh Frozen Plasma) were also transfused [5].
Post procedure
1. Remove access needle but leave cannula in, flush with normal saline.
2. Monitor vital signs at 15 and 30 minutes post procedure and then as clinically indicated
3. Take bloods 30 minutes post procedure for:
• CBC
• Met-Hb levels
• Serum electrolytes, calcium and magnesium
• PT, aPTT and INR
Discussion
To our knowledge, this report is the first to describe the use of manual whole blood exchange in the treatment of a patient with high-risk acquired methemoglobinemia. Exchange transfusion has traditionally been recommended for treatment of patients whose acquired methemoglobinemia is refractory to methylene blue [6]. Any patient who ingests an agent that may beget severe or prolonged methemoglobinemia should be considered a high threat of failure of methylene blue treatment [7]. Aniline dyes and nitroethane are two similar agents [7]. The volumes removed and infused with each cycle were limited to under 10% of the patient’s whole blood volume [8].
In cases such as ours wherein the patient is refractory to methylene blue treatment and automated red cell exchange is unavailable, manual whole blood exchange may be performed. The most important hurdles in performing this were maintaining sterility and volume calculations. We overcame the sterility issue by utilizing sterile connecting device and tube sealer and precise volume and reconstitution calculations were done with the help of review of literature previous cases of manual exchange.
Conclusion
Patients with clinically significant acquired methemoglobinemia, who are G6PD-deficient or who have ingested strong oxidants such as aniline dyes, are at high risk of failure of conventional treatment with methylene blue infusion. Automated RBC exchange should be considered a viable management option for these patients but unfortunately is not available at all centers. The current case highlights Manual red cell exchange could be considered as an effective alternative to Erythrocytapheresis in centers without automated red cell exchange facility and unaffordable patients.
Disclosure
The authors report no conflicts of interest in this work.
References
- Golden PJ, Weinstein R. Treatment of high-risk, refractory acquired methemoglobinemia with automated red blood cell exchange. J Clin Apher. 1998; 13(1): 28-31.
[Crossref] [Google Scholar] [PubMed]
- Driss F, Hequet O. Red blood cell exchange techniques and methods. Transfus Apher Sci. 2019;58(2):132-135.
[Crossref] [Google Scholar] [PubMed]
- Swerdlow PS. Red cell exchange in sickle cell disease. Hematology Am Soc Hematol Educ Program. 2006; 2006(1):48-53.
[Crossref] [Google Scholar] [PubMed]
- Howard J. Sickle cell disease: When and how to Transfuse. Hematology Am Soc Hematol Educ Program. 2016; 2016(1):625-631.
[Crossref] [Google Scholar] [PubMed]
- Mehra R, Gupta S, Borkar D. Manual red cell exchange transfusion to avert sickle cell related complications. Asian J Transfus Sci. 2018;12(2):157.
[Crossref] [Google Scholar] [PubMed]
- Mier RJ. Treatment of aniline poisoning with exchange transfusion. J Toxicol Clin Toxicol. 1988;26(56):357-364.
[Crossref] [Google Scholar] [PubMed]
- ROSEN PJ. Failure of methylene blue treatment in toxic methemoglobinemia. Ann Intern Med. 1971;75(1):83.
[Crossref] [Google Scholar] [PubMed]
- Weinstein R. Basic principles of therapeutic plasma exchange. Transfus Apher Sci. 2023;62(2):103675.
Citation: Guduri P, Kumar B, Nag I, Paul M, Vishnubhatla T, Makanaboyina SS (2023) Manual Exchange Transfusion: Practical Challenges and Overcomes. J Blood Disord Transfus. 14:569.
Copyright: © 2023 Guduri P, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

