Indexed In
- Open J Gate
- Genamics JournalSeek
- China National Knowledge Infrastructure (CNKI)
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Original Research Article - (2023) Volume 13, Issue 5
Management of Iron Deficiency Anemia with Fixed Dose Combination of Ferrous Gluconate, Multivitamins and Multi-mineral Capsule: A 12 Week, Open Label Single Arm Prospective, Non-Interventional (Observational) Study in Philippines
Christopher Joseph L. Soriano1, V Abalos2, CM Romero3, R. Manalastas4, G Villanueva5, Poonam Sule6*, Roger D Gibb7, Sue Aspley8 and NS Comia92Department of Cardiovascular Surgery, Chong Hua Hospital, Cebu, Philippines
3Department of Internal Medicine, South General Hospital, Cebu, Philippines
4Department of Obstetrics and Gynecology, Cardinal Santos Medical Center, Metro Manila, Philippines
5Department of Obstetrics and Gynecology, Healthserv Los Banos Medical Center, Laguna, Philippines
6Department of Clinical Development, Procter and Gamble Health Limited, Mumbai, India
7Department of Biostatistics, The Procter and Gamble Company, Mason, United States of America
8Department of Clinical Development, Procter and Gamble Technical Centres, Berkshire, United Kingdom
9Department of Internal Medicine, Mary Mediatrix Medical Center, Batangas, Philippines
Received: 11-Oct-2023, Manuscript No. CPECR-23-23537; Editor assigned: 13-Oct-2023, Pre QC No. CPECR-23-23537 (PQ); Reviewed: 27-Oct-2023, QC No. CPECR-23-23537; Revised: 03-Nov-2023, Manuscript No. CPECR-23-23537 (R); Published: 10-Nov-2023, DOI: 10.35248/2161-1459.23.13.385
Abstract
Aim: The study evaluated the efficacy and safety of an Iron+multivitamin and multi mineral capsule (SB+) capsule in subjects with mild to moderate Iron Deficiency Anemia (IDA).
Methods: This was a Phase IV/post-marketing, open label, single arm, prospective, non-interventional study to evaluate the SB+ capsule’s performance in real life scenario. The study enrolled 97 female subjects aged ≥ 15 years to ≤ 55 years (mean ± SD age: 34.9 ± 10.24 years) with mild to moderate IDA. The primary efficacy outcome was mean Hemoglobin (Hb) concentration after administering an SB+ capsule orally for up to 90 days. 66 subjects received 30 mg elemental iron once a day and 31 subjects received 60 mg elemental iron twice a day at baseline depending on their anemia status. The secondary efficacy outcomes were change in Hb concentration at days 14, 30, and 60; ferritin concentration at day 90; assessment of Quality of Life (QoL) with the EQ-5D-5L questionnaire and IDA symptoms; and safety outcomes. Statistical analysis was performed with SAS version 9.4.
Results: Of 97 subjects enrolled, 32 (33%) subjects had mild IDA and 64 (66%) had moderate IDA. One subject (1%) had no IDA at baseline. A total of 83 subjects completed the study. The mean Hb value was significantly increased at day 90 when compared to baseline visit for all subjects (12.6 g/dL vs. 10.1 g/dL, P<0.0001), mild IDA subjects (12.5 g/dL vs. 11.5 g/dL, P<0.0001) and moderate IDA subjects (12.6 g/dL vs. 9.4 g/dL, P<0.0001). The mean Hb value was significantly increased at day 30 (P=0.0054) and 60 (P=0.0002) for mild IDA subjects and at day 14, 30 and 60 (P<0.0001) for moderate IDA subjects. The median ferritin value was significantly elevated from 7.8 ng/mL to 28.8 ng/mL at day 90 (P<0.0001). The data also demonstrated significant improvements in QoL and IDA symptoms. Safety analysis revealed that the SB+ capsule was well-tolerated. The limitation of the current study is its single arm design.
Conclusion: SB+ capsule (containing Iron equivalent to 30 mg) is effective in treating mild to moderate IDA. Importantly, the safety analysis revealed that the SB+ capsule was well-tolerated by the subjects for the entire 90 days duration of the study.
Keywords
Iron+multivitamin multi mineral capsule; SB+; Iron deficiency anemia; Hemoglobin; Ferritin; Quality of life
Abbreviations
AEs: Adverse Events; BMI: Body Mass Index; CBC: Complete Blood Count; CFS: Chronic Fatigue Syndrome; CI: Confidence Interval; COVID-19: Coronavirus Disease 2019; dL: deciliter(s); EOS: End Of Study; G: Gram(s); GCP: Good Clinical Practice; Hb: Hemoglobin; i.e.: that is; IDA: Iron Deficiency Anemia; IEC/IRB: Institutional Ethics Committee or Institutional Review Board; ITT: Intent-To-Treat; Kg: Kilogram(s); LOCF: Last Observation Carry Forwarded; m2: Square meter; mcg: Microgram(s); Mg: Milligram(s); mL: Millilitre(s); Ng: Nanogram(s); P: Calculated probability; PP: Pre-protocol; PT: Preferred Term; QoL: Quality of Life; RBCs: Red Blood Cells; RLS: Restless Legs Syndrome; SAE: Serious Adverse Event; SD: Standard Deviation; SB+: Sangobion® Iron+; SOC: System Organ Class; VAS: Visual Analogue Scale; WHO: World Health Organization
Introduction
Iron Deficiency (ID) and Iron Deficiency Anemia (IDA) is caused by insufficient iron to build normal Red Blood Cells (RBCs). The condition occurs due to inadequate iron intake and/or increased demand for iron in the body (e. g. pregnancy), and/or increased iron loss (e. g. cases of inflammatory bowel diseases, hookworm infections, menstruation, and gastrointestinal bleeding [1,2]. According to the World Health Organization (WHO), iron deficiency causes microcytic and hypochromic red cells on the peripheral smear and is the most prevalent cause of anemia worldwide affecting approximately 30-50% of the population [2-4]. Anemia prevalence is consistently higher in females than males, mainly due to gynecological causes from menarche to menopause [2,5]. The South Asian region has a high prevalence of anemia, approximately 55% in 2016 and 52% in 2019 [6,7]. IDA is highly prevalent in the Philippine population. The prevalence of anemia in Philippine population is categorized in age groups in infants aged 6-11 months (40.1%), elderly men (23%), elderly women (19.1%), pregnant women (24.6%), and breastfeeding women (16.7%). Teenage pregnant women in Philippine are reported to have a higher prevalence of anemia (30.6%) than their adult counterparts (25.4%) [8,9].
Iron homeostasis is mainly controlled through dietary intake, intestinal absorption, and iron recycling [2,10]. The depletion of iron stores is progressive, with the absence of anemia in the initial phase. This is attributed to iron recycling from erythrocyte turnover that compensate for the deficiency. Depleted iron stores in pure iron deficiency result from an imbalance between iron uptake and utilization. Various factors such as blood loss, impeded absorption, reduced intake, or increased demand can also lead to a reduction of iron stores [4]. Initially, anemia may not be present as iron recycling from erythrocyte turnover can compensate for the deficiency [11]. The occurrence of negative iron balance for a prolonged time can lead to an advanced stage of iron deficiency or IDA. During this stage patients experience listlessness, fatigue, labored breathing, palpitations on exertion, and impaired work. Functional implications of IDA include diminished work capacity and compromised immunological function [10]. Other symptoms of IDA are feeling tired, pale skin, headache, rapid heartbeat, dizziness, and shortness of breath [2,4]. Additional complications of IDA include depression, cardiac problems, developmental delays in children, elevated risk of infections, and pregnancy difficulties, longer hospital stays, more cardiovascular events, and higher mortality rates. IDA significantly impairs the QoL for those affected, emphasizing the importance of timely diagnosis and treatment to mitigate these adverse outcomes [4]. The resolution is achieved through appropriate iron repletion and the effective management of the underlying cause of iron deficiency [11].
Anemia is defined by WHO as Hb level below 13.0 g/dL in men and 12.0 g/dL in women. Ferritin concentration is positively correlated with the magnitude of the total body iron stores in the absence of inflammation. Ferritin concentrations<15 ng/ml are indicative of iron stores depletion in both genders. In the presence of factors that affect ferritin levels such as age, inflammation, infection, or pregnancy, ferritin concentrations of<30 ng/ml are also indicative of iron stores depletion. Almost 40% of ID/IDA patients suffer in silence, with no signs and symptoms presented during diagnosis [12]. This could be attributed to underdiagnosis of common yet atypical non-hematological symptoms that appears over a continuum based on ferritin levels [13]. One of the earliest symptoms that occur in ID (serum ferritin levels<70 μg/L) is hair loss [13]. ID, even in the absence of IDA, is associated with fatigue, impaired physical performance, and decreased work productivity [14]. The functional and clinical implications of ID/IDA is debilitating and is estimated to be worse than major depression, musculoskeletal disorders and diabetes [15].
The treatment of IDA includes treating the underlying cause, such as gastrointestinal bleeding, and oral iron supplementation. The goal of treatment is to replenish iron stores while restoring Hemoglobin (Hb) concentrations and RBCs indices to normal which may improve Quality of Life (QoL), morbidity, and prognosis in chronic cases [2,16]. A beneficial approach could be the secondary prevention of iron insufficiency, increasing iron consumption and optimizing absorption by reducing iron inhibitors and increasing iron enhancers [17]. The rapid response to treatment is often seen in 14 days which is evident by the rise in Hb concentration followed by the correction of ferritin levels. Iron supplementation is required for at least 3 months to completely rebuild tissue iron stores [4]. Although elemental iron dosages normally range from 100 mg/ day to 200 mg/day, it is possible to achieve effective repletion with doses as low as 15 mg/day to 30 mg/day. Additionally, given that dose-dependent gastrointestinal problems are typical with iron ingestion, smaller doses might provide better tolerance [18,19].
Oral iron supplements such as, ferrous gluconate, and others are the preferred agents for the treatment of anemia due to their ease of availability and cost-effectiveness [20]. Multiple-Micronutrient (MMN) deficiencies is common among women of reproductive age increasing their risk for ID/IDA and are recommended to take iron supplementation to prevent ID/IDA. Iron with multivitamins is used to supplement insufficient dietary intake and have proven to have positive birth related outcomes [21]. However, there is limited scientific and clinical reports on the efficacy, safety, and benefits of ferrous gluconate in combination with multivitamins for the treatment of ID/IDA. The studied formulation Sangobion® Iron+ (SB+) formulation combines six important micronutrients in 1 capsule to prevent the progression of ID and to treat IDA by replenishing iron stores and increasing RBC/Hb levels in the body. Each SB+ capsule contains 30 mg elemental iron in the form of ferrous gluconate (250 mg): Manganese sulfate (0.2 mg), copper sulfate (0.2 mg), vitamin C (50 mg), folic acid (1 mg), vitamin B12 (7.5 mcg), the formulation also contains sorbitol (25 mg). Ferrous gluconate is associated with higher iron utilization due to better absorption of iron in the body and has better tolerability [22,23]. Vitamin C facilitates iron absorption. Folic acid and vitamin B12 are vital for RBC production and fetal development during pregnancy [24]. Copper sulphate and manganese sulphate are crucial in the process of iron production and metabolism [25,26]. In the present study, the effectiveness and safety of SB+ capsule formulation was evaluated in mild to moderate IDA patients together with QoL improvement.
Materials and Methods
Study design
This was a Phase IV/post-marketing, open label, single arm, prospective, non-interventional study conducted at 6 sites in Philippines to evaluate the SB+ capsule’s performance in real life scenario. PFDA approval was received prior to initiating the study. Each site also received Institutional Ethics Committee or Institutional Review Board (IEC/IRB) approval before initiation of the study, and informed consent was provided by each subject prior to participation. The study was conducted in compliance with Good Clinical Practice (GCP) guidelines and the Declaration of Helsinki and was registered on the local registry for clinical trials, PFDA-Registry.
Number: PHRR200413-002602 (http://www.registry.healthresearch.ph/index.php/registry?view=research&layout=details&cid=2602).
The study subjects with mild to moderate IDA, as defined by WHO and adapted by Philippines Obstetrical and Gynecological Society (Foundation) Inc. Clinical Practice Guidelines for IDA were recruited from the participating hospitals or clinics and reported to the centers for four visits: Visit 1 (baseline), visit 2 (day 30 ± 3 days), visit 3 (day 60 ± 3 days) and visit 4 (day 90 ± 3 days) [27,28] (Table 1). A Complete Blood Count (CBC) was recommended at day 14. A total of 97 subjects with mild to moderate IDA were enrolled in the study.
| Subject population | Mild anemia | Moderate anemia |
|---|---|---|
| Non-pregnant woman | 11.0 g/dL –11.9 g/dL | 8.0 g/dL –10.9 g/dL |
| Pregnant woman (2nd trimester: 13-28 weeks) | 9.5 g/dL –10.5 g/dL | 8.0 g/dL –9.4 g/dL |
| Pregnant woman (3rd trimester: 29 weeks to term) | 9.5 g/dL –11.0 g/dL | 8.0 g/dL –9.4 g/dL |
| Post-Partum | 11.0 g/dL –11.9 g/dL | 8.0 g/dL –10.9 g/dL |
| Men | 11.0 g/dL –12.9 g/dL | 8.0 g/dL –10.9 g/dL |
Table 1: Criteria for mild to moderate anemia expressed as hemoglobin levels in the blood.
Subjects
Female (including women of reproductive age, and pregnant women) subjects aged ≥ 15 years to ≤ 55 years with known mild to moderate IDA and a serum ferritin value of<30 ng/mL at baseline who were willing to comply with the prescribed treatment regimen for the duration of study participation and subjects who were vaccinated against Coronavirus Disease 2019 (COVID-19) were enrolled.
Subjects with co-morbidities and conditions that would confound the study objectives were excluded like subjects with severe anemia requiring blood transfusions; subjects with anemia due to conditions other than dietary deficiency with serious gastrointestinal disorders known autoimmune disease, severe or uncontrolled systemic or metabolic diseases, bleeding disorders, severe cardiovascular, renal, or hepatic illness; and subjects who could not take oral iron therapy or in whom oral therapy had failed per clinical practice were excluded.
Treatment
All subjects received SB+ capsule, depending on their IDA status at baseline (mild or moderate) they received either 1 (30 mg of elemental iron) or 2 (60 mg of elemental iron) capsules per day, the starting dose was decided by the prescribing physician. This dosing was in line with the product dosing instructions on the label. Tolerability of the doses was observed, and depending on the subject’s response, the dosing regimen was continued or changed accordingly by the investigator. A total of 66 (68%) subjects were prescribed SB+ capsule orally once a day and 31 (32%) subjects twice a day at baseline as per the product label to be consumed during or after meals, or as directed by the prescribing physician for a period of up to 90 days. The prescribed dose and/or dosing frequency was decreased at subsequent post baseline visits (day 30 and day 60) as compared to baseline visit at the prescribing physician’s discretion. The subjects were involved in the study for at least 30 days and up to 90 days. There was no follow-up of subject’s post-study. Treatment compliance was evaluated by comparing the number of capsules dispensed to the number of capsules returned in relation to the number of capsules subjects were instructed by their physician to take over the study period. Most of the subjects (≥ 80%) had 90%-110% compliance across all three visits (baseline to day 30, day 30 to day 60 and day 60 to day 90).
Study endpoints and assessments
In order to prove SB+ effectiveness in increasing the Hb, and ferritin levels, that impacts IDA symptoms and QoL, the following end points were assessed:
The primary efficacy goal of the study was the mean Hb concentration at day 90 after daily oral treatment with SB+ capsule. Compared to the mean Hb concentration at baseline.
The secondary efficacy endpoints were to measure change from baseline in Hb after 14, 30, and 60 days of treatment with SB+ capsule; change from baseline in serum ferritin concentration at the End Of Study (EOS); assessment of non-pregnant subject’s QoL with the EQ-5D-5L questionnaire and summary of IDA symptoms assessed via Visual Analogue Scale (VAS), respectively, at baseline, day 30, day 60, and day 90; and to assess the tolerability and safety of the SB+ capsule.
The results of the subject’s Hb and serum ferritin tests together with that of physical examination, vital signs, and clinical laboratory results were documented at baseline and post-baseline visits. The assessment of subject’s (non-pregnant) QoL was performed with the EQ-5D-5L questionnaire and IDA symptoms were assessed via VAS.
Statistical analysis
The descriptive statistics including means and Standard Deviation (SD) for continuous data and frequencies and percentages for categorical data were calculated. The primary efficacy analysis was statistically tested by the 2-sided hypothesis (null hypothesis, i. e. the mean change from baseline is equal to zero; and alternative hypothesis, i.e. the mean change from baseline is not equal to zero) with a paired-difference t-test (used to compare “before” and “after” scores in same sample to determine whether significant change has occurred) at a significance level of 5%. The 2-sided p-value for this test was reported together with a 95% Confidence Interval (CI).
The Last Observation Carry Forward (LOCF) is the simplest approach to account for missing data in the analysis of repeated measures over time. In a LOCF analysis, a missing follow-up visit value is imputed as that subject’s previously observed value, i.e. analyzed as though there were no missing data. Although, the resulting bias may occur because the values are missing. LOCF was chosen in this study because it is a real world research wherein a higher dropout was anticipated.
The LOCF approach was taken to impute missing data for the primary efficacy endpoint among subjects who have at least one post- baseline observation. To avoid biasness, a sensitivity analysis was performed to assess the impact of not using LOCF data imputation on Hb data. This included repeating the efficacy analyses for Hb at days 14, 30, 60 and 90 without any data imputation and comparing results to those when LOCF data imputation was used. Additionally, a sensitivity analysis was performed to determine the impact of protocol non-compliance and/or violations on the primary and some secondary efficacy results. This was done by repeating the efficacy analyses for Hb at days 14, 30, 60 and 90 and ferritin at day 90 for the Pre-Protocol (PP) population and then comparing the results to those of the Intent-to-Treat (ITT) population with no LOCF datasets.
The change from baseline in Hb was assessed with a paired difference t-test. The change from baseline in ferritin at the EOS, EQOL-5D-5L, and IDA symptoms on VAS scale were assessed with Wilcoxon signed rank 2-sided test at a significance level of 5%. Adverse Events (AEs) were summarized by System Organ Class (SOC), Preferred Term (PT), and causality. Statistical analysis was performed with SAS version 9.4.
Results
Study participants
A total of 97 subjects were enrolled in the study. A total of 32 (33%) subjects had mild IDA and 64 (66%) had moderate IDA. One subject (1%) had no IDA at baseline. Out of 97 subjects, 83 (85.6%) completed the study. The discontinuation timeframe was between day 0 and day 29 for 5 (35.7%) subjects, from day 30 to day 59 for 6 (42.9%) subjects and day 60 or later for 3 (21.4%) subjects. The reasons for discontinuation are summarized in Figure 1. All subjects took at least one dose of study drug and were included in the ITT population. The ITT population was used for the primary/ secondary efficacy and safety analysis. A total of 89 subjects were included in the PP population; 8 subjects, who were rendered non- evaluable due to protocol non-compliance, were excluded from the PP population. The PP population was used for the sensitivity analysis on the primary efficacy results and two elements of secondary efficacy results (Hb after 14, 30, and 60 days of treatment and serum ferritin concentration at EOS).
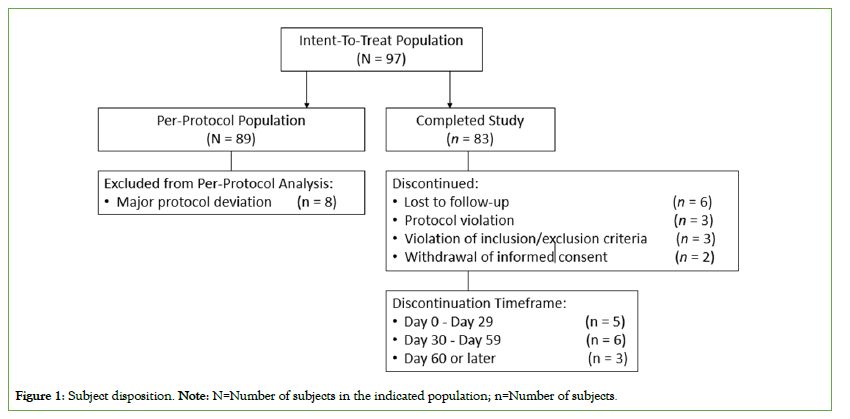
Figure 1: Subject disposition. Note: N=Number of subjects in the indicated population; n=Number of subjects.
Table 2 summarizes the demographics and baseline characteristics. The mean (SD) age was 34.9 (10.24) years, and the mean BMI was 24.0 (4.17) kg/m². All enrolled subjects were female. Of the 97 subjects, most subjects (88.7%) were non-pregnant. The majority of subjects (83.5%) were Hispanic and all 97 enrolled subjects were of Asian population. The mean duration of menses was 5 days; 49 (50.5%) subjects had normal blood flow, 41 (42.3%) subjects had heavy blood flow and 6 (6.2%) subjects had very heavy blood flow. Most of the subjects (>95%) consumed food containing iron absorption enhancers. The percentage of subjects who consumed food containing iron absorption inhibitor were 90.7% with phytic acid in bran products, 41.2% with soy protein, 93.8% with egg protein, 78.4% with calcium containing milk products, 66% with polyphenols, and 20.6% with consumption of herbs.
| Characteristics | Overall |
|---|---|
| Demographic characteristics | |
| Mean (SD) age, years | 34.9 (10.24) |
| Sex, n (%) | |
| Female | 97 (100.0%) |
| Mean (SD) height, cm | 155.5 (7.09) |
| Mean (SD) weight, kg | 58.0 (11.08) |
| Mean (SD) BMI, kg/m2 | 24.0 (4.17) |
| Race, n (%) | |
| Asian | 97 (100.0%) |
| Ethnicity, n (%) | |
| Hispanic | 81 (83.5%) |
| Not Reported | 12 (12.4%) |
| Unknown | 4 (4.1%) |
| Pregnancy status, n (%) | |
| Not pregnant | 86 (88.7%) |
| Pregnant, second trimester | 4 (4.1%) |
| Pregnant, third trimester | 7 (7.2%) |
| Number of pregnancies (inclusive of current)a, n (%) | |
| 1 | 6 (54.5%) |
| 2 | 3 (27.3%) |
| 3 | 2 (18.2%) |
| Mean (SD) pregnancy durationa, weeks | 28.2 (5.90) |
| Socio-economic status, n (%) | |
| Poor | 9 (9.3%) |
| Low income | 33 (34.0%) |
| Lower middle income | 25 (25.8%) |
| Middle income class | 24 (24.7%) |
| Upper middle income | 5 (5.2%) |
| Upper income | 1 (1.0%) |
| Baseline characteristics | |
| Iron deficiency anemia status, n (%) | |
| None | 1 (1.0%) |
| Mild | 32 (33.0%) |
| Moderate | 64 (66.0%) |
| Severe | 0 |
| Mean (SD) duration of menses (women only), days | 5.4 (1.47) |
| Amount of menses, n (%) | |
| Normal blood flow | 49 (50.5%) |
| Heavy blood flow | 41 (42.3%) |
| Very heavy blood flow | 6 (6.2%) |
| Consumes food containing iron absorption enhancersa, n (%) | |
| Vitamin C containing fruits and vegetables | 94 (96.9%) |
| Meat | 95 (97.9%) |
| Fish | 95 (97.9%) |
| Poultry | 92 (94.8%) |
| Consumes food containing iron absorption inhibitorsa, n (%) | |
| Phytic acid in bran products, bread, grains, cereals, oats, rice, legumes | 88 (90.7%) |
| Soy protein from soya products | 40 (41.2%) |
| Egg protein from whole eggs and yolk | 91 (93.8%) |
| Calcium containing milk products like milk, yogurt, cheese | 76 (78.4%) |
| Polyphenols in coffee, cocoa, tea and wine | 64 (66.0%) |
| Herbs like chamomile, peppermint, oregano | 20 (20.6%) |
| Level of activity, n (%) | |
| Inactive (no activity) | 45 (46.4%) |
| Low (some activity < 150 min/week) | 28 (28.9%) |
| Medium (150 - 300 min/week) | 21 (21.6%) |
| High (>300 min/week) | 3 (3.1%) |
Note: ITT=Intent-To-Treat, N=Number of subjects in the indicated population, n (%)=Number of subjects with non-missing values; SD=Standard Deviation; BMI=Body Mass Index; aSubjects were allowed to select one or more items.
Table 2: Demographic and baseline characteristics (ITT population, N=97).
The most frequent medical history (>10%) was surgical and medical procedures (38.1%) followed by pregnancy, puerperium, and perinatal conditions (28.6%) and vascular disorders (19%). Most subjects (≥ 80%) consumed the SB+ capsules between all three visits (baseline to day 30, day 30 to day 60 and day 60 to 90).
Efficacy
The mean Hb value was significantly increased at day 90 when compared to baseline visit (12.6 g/dL vs. 10.1 g/dL, P<0.0001) for all subjects. The primary efficacy endpoint met the alternative hypothesis (mean change from baseline is not equal to zero). Similarly, the mean value of Hb was significantly increased at day 90 for both mild and moderate IDA subjects when compared to baseline (mild IDA subjects: 12.5 g/dL vs. 11.5 g/dL, P<0.0001 and moderate IDA subjects: 12.6 g/dL vs. 9.4 g/dL, P<0.0001) (Table 3). The moderate IDA subjects started with a much lower baseline Hb levels than the subjects with mild IDA at baseline, however, both groups (mild and moderate IDA cases) had similar day 90 Hb values (12.5 g/dL) irrespective of baseline Hb levels (Figure 2). The odds of improvement in IDA at day 90 was 3.8 and 4.7 in mild and moderate IDA subjects, respectively. The IDA status was significantly improved at day 14, 30, 60 and 90 with 24%, 45%, 66% and 72% subjects with no IDA, respectively, when compared to 1% subjects with no IDA at baseline (P<0.0001) (Table 4).
| Anemia status | n | Baseline | Post-baseline | Change from baseline | ||
|---|---|---|---|---|---|---|
| Mean (SE) | Mean (SE) | Mean (SE) | Mean 95% CI | P-valuea | ||
| All subjects | ||||||
| Day 90 | 87 | 10.1 (0.16) | 12.6 (0.15) | 2.4 (0.21) | (2.0, 2.8) | <0.0001 |
| Mild iron deficiency anemia at baselineb | ||||||
| Day 90 | 30 | 11.5 (0.16) | 12.5 (0.21) | 1.1 (0.24) | (0.6, 1.6) | <0.0001 |
| Moderate iron deficiency anemia at baseline | ||||||
| Day 90 | 57 | 9.4 (0.16) | 12.6 (0.20) | 3.1 (0.24) | (2.7, 3.6) | <0.0001 |
| Note: ITT=Intent-To-Treat, LOCF=Last Observation Carried Forward, N=Number of subjects in the indicated population, n=Number of subjects with non-missing values, SE=Standard Error, CI=Confidence Interval, P=Calculated probability. aPaired t-test 2-sided p-value; bOne subject had no iron deficiency anemia at baseline. |
||||||
Table 3: Hemoglobin (g/dL) levels at day 90 (ITT population LOCF, N = 97).
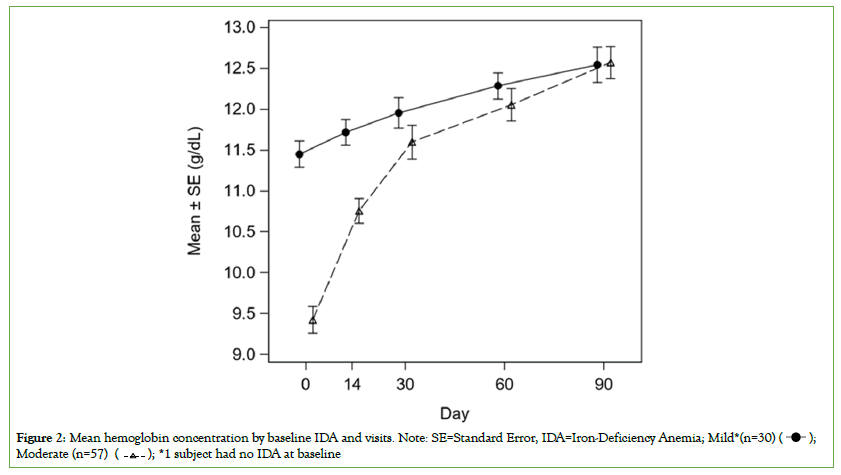
Figure 2: Mean hemoglobin concentration by baseline IDA and visits. Note: SE=Standard Error, IDA=Iron-Deficiency Anemia; Mild*(n=30)  Moderate (n=57)
Moderate (n=57)  *1 subject had no IDA at baseline.
*1 subject had no IDA at baseline.
| Iron Deficiency Anemia Status | Baseline | Day 14 | Day 30 | Day 60 | Day 90 |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| None | 1 (1.0%) | 21 (24%) | 39 (45%) | 57 (66%) | 63 (72%) |
| Mild | 32 (33%) | 27 (31%) | 31 (36%) | 17 (20%) | 14 (16%) |
| Moderate | 64 (66%) | 39 (45%) | 16 (18%) | 12 (14%) | 10 (11%) |
| Severe | 0 (0.0%) | 0 (0.0%) | 1 (1.1%) | 1 (1.1%) | 0 (0.0%) |
| Total | 97 (100%) | 87 (100%) | 87 (100%) | 87 (100%) | 87 (100%) |
| P-valuea | - | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Note: ITT = Intent-to-treat, N = Number of subjects in the indicated population, n (%)=Number and percent of subjects with non-missing data.
aBhapkar’s test p-value for comparison of iron deficiency anemia results at the indicated visit to baseline. Severe anemia cases were treated as moderate cases to facilitate calculations.
Table 4: Iron deficiency anemia (IDA) status by visit (ITT population, N=97).
Additionally, sensitivity analysis was performed to assess the impact of no LOCF data imputation and protocol non-compliance and/ or violations on the primary and secondary efficacy results. Results of Hb efficacy analysis with no LOCF were comparable to the LOCF data. The mean Hb value was significantly increased at day 90 when compared to baseline with no LOCF data in all subjects (12.6 g/dL vs. 10 g/dL, P<0.0001), mild IDA subjects (12.5 g/dL vs. 11.4 g/dL, P=0.0003) and moderate IDA subjects (12.6 g/dL vs. 9.4 g/dL, P<0.0001). Results of Hb efficacy analysis with the PP population were comparable to the results obtained in ITT population. The mean Hb value was significantly increased at day 90 when compared to baseline with PP population in all subjects (12.6 g/dL vs. 10.1 g/dL, P<0.0001), mild IDA subjects (12.5 g/dL vs. 11.4 g/dL, P=0.0002) and moderate IDA subjects (12.7 g/dL vs. 9.4 g/dL, P<0.0001). The results of both sensitivity analyses (no LOCF data and PP population) were comparable to the LOCF data and ITT population, respectively.
The mean Hb concentrations at days 14, 30, and 60 were statistically compared to the mean Hb concentration at baseline. The mean Hb at baseline was 10.1 g/dL, and it significantly increased by 1.0, 1.6 and 2.0 g/dL at day 14, 30 and 60, respectively (P<0.0001). As shown in Figure 2, the mean value of Hb was significantly increased at day 30 (P=0.0054) and day 60 (P=0.0002) for mild IDA subjects and at day 14, day 30 and day 60 (P<0.0001) for moderate IDA subjects when compared to baseline. The mean value of Hb at day 14 for mild IDA subjects was comparable to that at the baseline visit (11.7 g/dL at day 14 vs. 11.5 g/dL at baseline, P=0.1033). The results of both analysis (no LOCF data and PP population) were comparable to the LOCF data and ITT population, respectively.
The median ferritin concentration at EOS was statistically compared to the median ferritin concentration at baseline. The median ferritin value was significantly increased at day 90 when compared to baseline visit (28.8 ng/mL vs. 7.8 ng/mL, P<0.0001) for all subjects mild IDA subjects (39.7 ng/mL vs. 9.1 ng/mL, P<0.0001), and moderate IDA subjects (22.4 ng/mL vs. 6.4 ng/mL, P<0.0001) (Figure 3). Results of ferritin efficacy analysis in the PP population were comparable to those observed in ITT population. There was no impact of not applying LOCF data imputation, protocol non-compliance and/or violations on the primary and secondary efficacy analysis results.
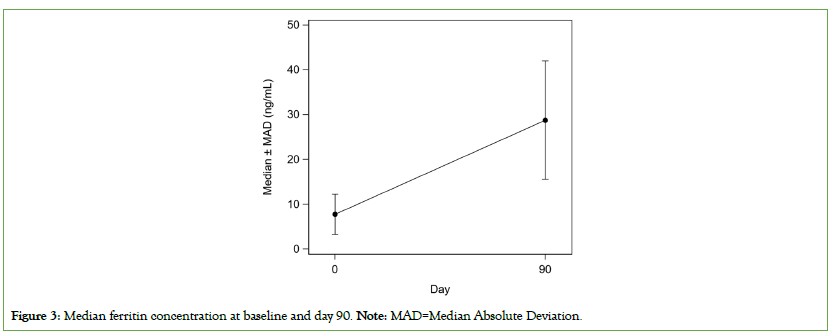
Figure 3: Median ferritin concentration at baseline and day 90. Note: MAD=Median Absolute Deviation.
The percentage of subjects with any deficiency in EQ-5D-5L measures (mobility, usual activities, pain/discomfort, and anxiety/ depression) was 48.8% at baseline which significantly decreased to 29.1%, 19.5% and 16% at day 30, 60 and 90, respectively (all P<0.05) (Figure 4). The mean EQ-5D-5L Overall Health VAS score (0=worst overall health and 100=Best overall health) at baseline was 82.1, which increased to 87.9 at day 30 and further increased to 90.1 and 93.3 at day 60 and 90, respectively (P<0.0001). The IDA symptoms by visits are presented in Figure 5. The number of IDA symptoms per subject were more at baseline and the numbers were lower at day 30, 60 and 90. Among subjects who had IDA symptoms (tiredness, shortness of breath, dizziness, headache, swelling, fast heartbeats, hair fall, restless leg, brittle nails, cold hands or feet, and pica) at baseline, the mean baseline VAS (0=symptom absent and 100=worst symptom) ranged from 39 to 54. The mean VAS was statistically significantly lower (P<0.05) at day 30 and each successive visit for 10 of the 11 symptoms. At day 90, the mean VAS ranged from 0 to 9.
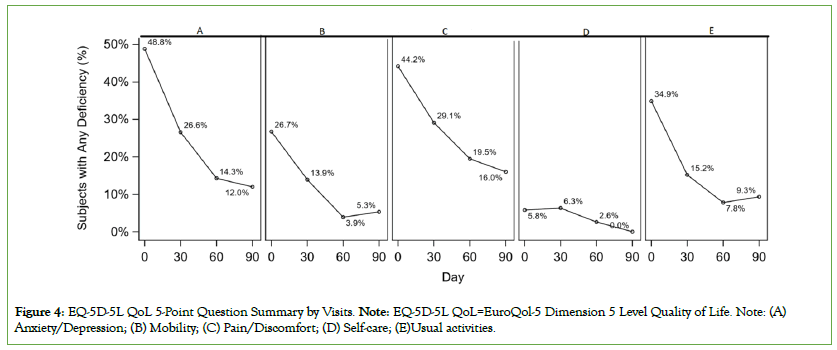
Figure 4: EQ-5D-5L QoL 5-Point Question Summary by Visits. Note: EQ-5D-5L QoL=EuroQol-5 Dimension 5 Level Quality of Life. Note: (A) Anxiety/Depression; (B) Mobility; (C) Pain/Discomfort; (D) Self-care; (E)Usual activities.
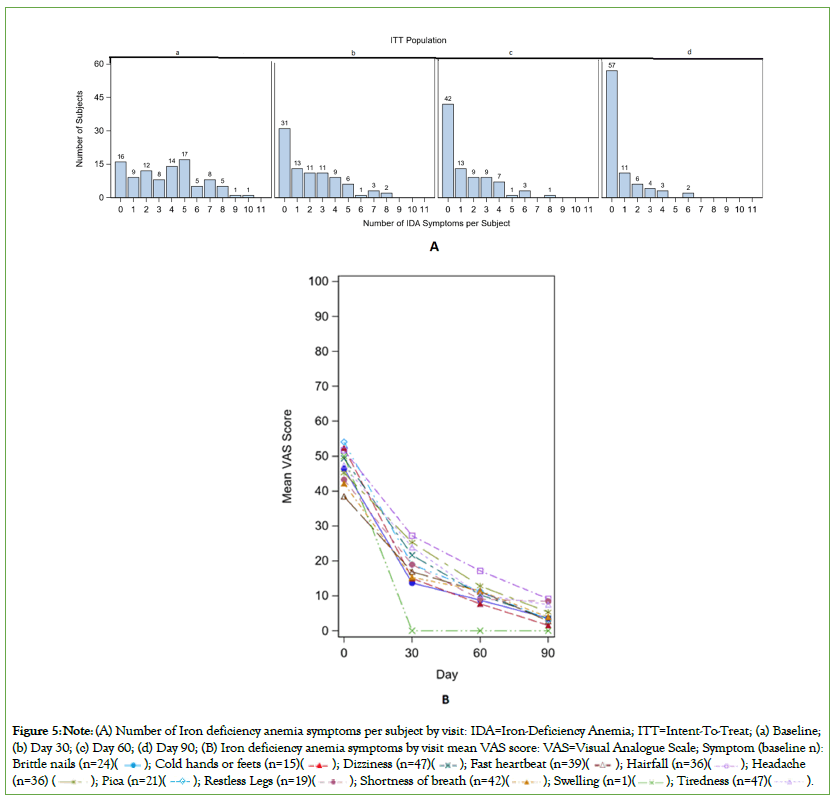
Figure 5: Note: (A) Number of Iron deficiency anemia symptoms per subject by visit: IDA=Iron-Deficiency Anemia; ITT=Intent-To-Treat; (a) Baseline;
(b) Day 30; (c) Day 60; (d) Day 90; (B) Iron deficiency anemia symptoms by visit mean VAS score: VAS=Visual Analogue Scale; Symptom (baseline n):
Brittle nails (n=24)  (n=36)
(n=36) 
Safety
The safety analysis was performed on the ITT population. A total of 20 (20.6%) subjects reported 25 AEs and 1 Serious Adverse Event (SAE) was reported by one (1%) subject in the study. The SAE was not related to the study drug and resolved without sequelae. The majority of AEs were mild (8 AEs, 32%) to moderate (16 AEs, 64%) in nature. Most of the AEs (22 AEs, 88%) were not suspected to be related to the study drug. None of the subjects were withdrawn from the study due to AEs. Out of 25 AEs, only 3 (12%) AEs (abdominal pain, hyperchlorhydria, and dizziness) were suspected to be related to the study drug. The most common AEs reported by the subjects (≥ 10%) in the study were COVID-19, dizziness, vaginal hemorrhage, and heavy menstrual bleeding (Table 5). The mean number of AEs per enrolled subject was 0.3 and the mean number of AEs per subject with AEs was 1.3.
| System Organ Class (SOC) / Preferred term | Suspected | Not Suspected | Unknown |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Eye disorders | |||
| Overall | 0 | 1 (5.6%) | 0 |
| Vision blurred | 0 | 1 (5.6%) | 0 |
| Gastrointestinal disorders | |||
| Overall | 2 (100%) | 0 | 0 |
| Abdominal pain | 1 (50%) | 0 | 0 |
| Hyperchlorhydria | 1 (50%) | 0 | 0 |
| Infections and infestations | |||
| Overall | 0 | 3 (16.7%) | 0 |
| COVID-19 | 0 | 2 (11.1%) | 0 |
| Gastroenteritis | 0 | 1 (5.6%) | 0 |
| Injury, poisoning and procedural complications | |||
| Overall | 0 | 1 (5.6%) | 0 |
| Exposure to SARS-CoV-2 | 0 | 1 (5.6%) | 0 |
| Nervous system disorders | |||
| Overall | 1 (50%) | 2 (11.1%) | 0 |
| Dizziness | 1 (50%) | 1 (5.6%) | 0 |
| Cerebral venous sinus thrombosis | 0 | 1 (5.6%) | 0 |
| Reproductive system and breast disorders | |||
| Overall | 0 | 11 (61.1%) | 0 |
| Heavy menstrual bleeding | 0 | 9 (50%) | 0 |
| Vaginal hemorrhage | 0 | 2 (11.1%) | 0 |
| Vascular disorders | |||
| Overall | 0 | 1 (5.6%) | 0 |
| Hypertension | 0 | 1 (5.6%) | 0 |
Note: ITT=Intent-To-Treat, N=Number of subjects randomized to specified treatment group, n=Number of subjects who reported the adverse event, SOC=System Organ Class; COVID-19=Coronavirus Disease 2019; SARS-CoV-2=Severe acute respiratory syndrome coronavirus 2; %=Percentage of subjects who reported the adverse event out of the total who had any adverse event.
Table 5: Adverse events by system organ class, preferred term and causality (ITT population, N=97).
There were no clinically significant changes in vital signs (i.e. body temperature, diastolic blood pressure, pulse rate, respiration rate and systolic blood pressure) at days 30, 60 and 90 when compared to baseline. The mean value of vital signs at post baseline visit was comparable with baseline visit. There were no clinically relevant trends observed in most CBC laboratory parameters at days 14, 30, 60 and 90 when compared to baseline, however, the mean value of RBCs and hematocrit was increased at post baseline visits as compared to baseline visit which may be due to the folic acid and vitamin B12 content of SB+ capsule. The increase in RBCs and hematocrit levels were aligned with the increase in Hb levels after SB+ capsule treatment. This observation is of clinical significance favoring SB+ capsule treatment, as low RBC and hematocrit levels are also the typical predictors of IDA, and their levels are expected increase post iron supplementation. The physical examination outcome was “normal” for majority of the subjects at days 30, 60 and 90 and comparable with baseline visit.
Discussion
Anemia, primarily IDA, has been reported in 46% of South-East Asian women of reproductive age [29]. A critical risk factor for IDA among nonpregnant women of childbearing age is menstrual blood loss [30]. This establishes a necessity for iron supplementation in the female population globally. Replenishing iron stores and returning the Hb to normal levels is the main aim in the management of patients with IDA [2]. The treatment modality of IDA includes iron replenishment via oral, parenteral, and transfusion routes. Oral iron administration has been the preferred treatment in patients with anemia [16]. Various reports support the combination of iron with multivitamins and multimineral for the treatment of IDA. Vitamin C plays a crucial role in the absorption of non-heme iron reversing the effect of dietary inhibitors. It is reported that changes in peripheral blood and bone marrow found in iron deficiency states are due to a relative lack of folate or vitamin B12 that results in hyperplasia [31-33].
As per the WHO recommendations, the Hb levels in non-anemic pregnant and non-pregnant women should be atleast 11.0 g/dL and 12.0 g/dL or higher, respectively [27]. In the present study, SB+ capsule, combining six important micronutrients, administered to mild to moderate IDA patients was associated with a clinically meaningful and statistically significant increase in mean Hb levels at day 90 compared to baseline (mild IDA subjects: 12.5 g/dL vs. 11.5 g/dL, and moderate IDA subjects: 12.6 g/dL vs. 9.4 g/dL); and Hb improvement as early as 14 days (1 g/dL P<0.001); Ferrous gluconate is a ferrous (Fe2+) salt which is the reduced form of iron that is favourable for absorption in the duodenum and proximal jejunum [34]. The Hb levels were restored to normal levels recommended by the WHO. The positive results also signify the importance of adding vitamins and minerals to iron supplements for better treatment outcomes. The moderate IDA subjects started with much lower baseline Hb levels compared to mild IDA subjects, however both groups by day 90 were at similar Hb levels. Therefore, it can be inferred that the subjects who needed more therapeutic benefit received greater therapeutic benefit with SB+ capsule. The improvement in Hb levels have also been observed in other trials with the combination of iron and multivitamins supplementation. A randomized, controlled trial in 175 non-pregnant Asian females reported that supplementation of iron (200 mg), folic acid (0.5 mg) and vitamin C (l00 mg) was associated with a significant improvement in mean Hb level at day 30 and days 100 in comparison to the supplementation of iron (200 mg) and folic acid (0.5 mg) [35]. An interventional study was conducted in adolescent girls, wherein weekly supplementation of vitamin C along with iron and folate was associated with significant increase in Hb concentration compared to weekly iron and folate supplementation alone (0.76g/dL vs. 0.71 g/dL) after three months [36]. Further, it is reported that supplementation of 50 mg of vitamin C along with iron and folate increased the iron absorption to 7.7% as compared to the iron absorption to 1.6% with iron and folate supplementation alone [37].
In our study, a significant increase in the serum ferritin value at day 90 from baseline was observed in mild (39.7 ng/mL vs. 9.1 ng/mL, P<0.0001) and moderate (22.4 ng/mL vs. 6.4 ng/mL, P<0.0001) IDA subjects. Similar results were reported in two other trials where adolescent females treated with iron, folic acid and vitamin C had an increase iron absorption and ferritin levels compared to females treated with iron and folic acid: Supporting the role of vitamin C in iron repletion [38,39]. In our study, median ferritin value at day 90 was comparatively higher in mild IDA subjects as compared to moderate IDA subjects. This is per normal body physiology as the body replenishes the iron stores preferentially first to Hb and secondarily to ferritin [40]. Though Hb stores are replenished in those with moderate IDA at baseline, they have insufficient time to fully replenish ferritin stores to optimum level, this reinforces the requirement for further research on iron therapy beyond 90 days and suggests that longer treatment may be required for moderate and severe IDA cases to fully replenish iron stores.
IDA also impairs overall health and is associated with symptoms such as Restless Legs Syndrome (RLS), Chronic Fatigue Syndrome (CFS) or decreased physical and cognitive performance, especially in children and premenopausal women, sleeping disorders, trophic changes in fingernails and hair, symptoms of pica and obstetric complications including low birth weight, preterm delivery, and stillbirth, which impacts the QoL [41]. In the present study the EQOL-5D-5L Questionnaire health survey data was summarized for non-pregnant subjects for each visit and the changes in overall health assessment based on VAS at day 30, 60 and 90 were significantly improved compared to baseline. A cross-sectional analysis substantiated a positive correlation between all vitamin intake and physical component score, and between vitamin B6, folic acid, vitamin C, and mental component score in women [42]. These data further suggest that intake of iron supplements with multivitamins and multimineral improves QoL.
The product was overall was well-tolerated with a low AE incidence rate. The majority of AEs (96%) reported in this study were mild to moderate. Although, oral iron is the most common and acceptable route, there are few limitations with it, the most common being gastrointestinal side effects such as gastritis and constipation [16]. In our study only two subjects reported gastrointestinal disorders (abdominal pain, hyperchlorhydria) which were suspected to be related to the study drug. The lower number of subjects associated with gastrointestinal events in our study could possibly be due to the combination of iron with multi-vitamins and multi-minerals. Co-formulation with sorbitol could potentially explain the absence of constipation reported as AEs in the study. Sorbitol is a well- known polyol that has been used as an excipient in formulations of various drug products. Sorbitol is used as a laxative to relieve constipation [43]. Sorbitol is also reported to improve the iron absorption in human [44].
Based on the study results, supplementation of Iron+multivitamin and multi mineral capsule (SB+) capsule for treatment of IDA in different patient populations will help in improving the health of patients with IDA there by reducing the global health burden due to IDA. Nevertheless, appropriately sized randomized control trials are needed to confirm the promising results obtained with SB+ supplementation in different clinical settings.
Conclusion
In conclusion, Sangobion® Iron+capsule demonstrates effectiveness and safety in treating of mild to moderate IDA resulting in significant improvement in the Hb levels, ferritin, QoL, and IDA symptoms. Notably, the increase in Hb level began as early as day 14 for moderate IDA subjects. This rapid, and sustained efficacy, observed over the of 90 day study period was accompanied by minimal adverse events. Furthermore the findings suggest that extended treatment beyond 90 days may be required for moderate IDA patients to replenish iron stores to optimum levels. The study paves the way for further investigations into the role of iron+ multivitamins, multi-minerals in the prevention and management of IDA across different life stages of women.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical Approval
The study was approved by Institutional Ethics Committee or Institutional Review Board (IEC/IRB), and informed consent was obtained from each subject prior to their participation in the study. This study was conducted in compliance with Good Clinical Practice (GCP) guidelines and the Declaration of Helsinki.
Acknowledgements
1. Licensor of QoL questionnaire EQ 5D5L, EuroQoL Group Association
2. PharmaLex India Pvt. Ltd. for providing the manuscript writing support
Sources of Funding
This study was funded by the Research & Development department of Personal Healthcare Division of Procter and Gamble, the participating investigators were paid an honorarium for their time and efforts.
Conflict of Interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- Johnson-Wimbley TD, Graham DY. Diagnosis and management of iron deficiency anemia in the 21st century. Therap Adv Gastroenterol. 2011;4(3):177-84.
[Crossref] [Google Scholar] [PubMed]
- Kumar A, Sharma E, Marley A, Samaan MA, Brookes MJ. Iron deficiency anaemia: Pathophysiology, assessment, practical management. BMJ Open Gastroenterol. 2022;9(1):e000759.
[Crossref] [Google Scholar] [PubMed]
- Long B, Koyfman A. Emergency medicine evaluation and management of anemia. Emerg Med Clin North Am. 2018 Aug;36(3):609-630.
[Crossref] [Google Scholar] [PubMed]
- Warner MJ, Kamran MT. Iron deficiency anemia. Treasure Island (FL): StatPearls Publishing. 2023.
- Wawer AA, Jennings A, Fairweather-Tait SJ. Iron status in the elderly: A review of recent evidence. Mech Ageing Dev. 2018;175:55-73.
[Crossref] [Google Scholar] [PubMed]
- Stevens GA, Paciorek CJ, Flores-Urrutia MC, Borghi E, Namaste S, Wirth JP, et al. National, regional, and global estimates of anaemia by severity in women and children for 2000-19: A pooled analysis of population-representative data. Lancet Glob Health. 2022;10(5):e627-e639.
[Crossref] [Google Scholar] [PubMed]
- Sunuwar DR, Singh DR, Pradhan PMS, Shrestha V, Rai P, Shah SK, et al. Factors associated with anemia among children in South and Southeast Asia: A multilevel analysis. BMC Public Health. 2023;23(1):343.
[Crossref] [Google Scholar] [PubMed]
- Palanog AD, Calayugan MIC, Descalsota-Empleo GI, Amparado A, Inabangan-Asilo MA, Arocena EC, et al. Zinc and iron nutrition status in the Philippines population and local soils. Front Nutr. 2019;6:81.
[Crossref] [Google Scholar] [PubMed]
- Herrin AN. Putting prevention of childhood stunting into the forefront of the nutrition agenda: A nutrition sector review. PIDS Discussion Paper Series; 2016.
- McDermid JM, Lonnerdal B. Iron. Adv Nutr. 2012;3(4):532-3.
- Jimenez K, Kulnigg-Dabsch S, Gasche C. Management of iron deficiency anemia. Gastroenterol Hepatol (N Y). 2015;11(4):241-50.
[Google Scholar] [PubMed]
- Fernandez-Jimenez MC, Moreno G, Wright I, Shih PC, Vaquero MP, Remacha AF. Iron deficiency in menstruating adult women: Much more than anemia. Womens Health Rep (New Rochelle). 2020;1(1):26-35.
[Crossref] [Google Scholar] [ PubMed]
- Park SY, Na SY, Kim JH, Cho S, Lee JH. Iron plays a certain role in patterned hair loss. J Korean Med Sci. 2013;28(6):934-8.
[Crossref] [Google Scholar] [PubMed]
- Diagnosis and management of iron deficiency anemia in the 21st century
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211-1259.
[Crossref] [Google Scholar] [PubMed]
- Goddard AF, James MW, McIntyre AS, Scott BB; British Society of Gastroenterology. Guidelines for the management of iron deficiency anaemia. Gut. 2011;60(10):1309-16.
[Crossref] [Google Scholar] [ PubMed]
- Pasricha SR, Flecknoe-Brown SC, Allen KJ, Gibson PR, McMahon LP, Olynyk JK, et al. Diagnosis and management of iron deficiency anaemia: A clinical update. Med J Aust. 2010;193(9):525-32.
[Crossref] [Google Scholar] [PubMed]
- Makrides M, Crowther CA, Gibson RA, Gibson RS, Skeaff CM. Efficacy and tolerability of low-dose iron supplements during pregnancy: A randomized controlled trial. Am J Clin Nutr. 2003;78(1):145-53.
[Crossref] [Google Scholar] [PubMed]
- Zhou SJ, Gibson RA, Crowther CA, Makrides M. Should we lower the dose of iron when treating anaemia in pregnancy? A randomized dose-response trial. Eur J Clin Nutr. 2009;63(2):183-90.
[Crossref] [Google Scholar] [PubMed]
- Nguyen M, Tadi P. Iron supplementation. Treasure Island (FL): StatPearls Publishing; 2023.
- Keats EC, Haider BA, Tam E, Bhutta ZA. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst Rev. 2019;3(3):CD004905.
[Crossref] [Google Scholar] [ PubMed]
- Falahati V, Ghasemi A, Ghaffari K, Eghbali A, Khodabakhshi S, Almasi-Hashiani A, et al. Comparison of the effect of ferrous sulfate and ferrous gluconate on prophylaxis of iron deficiency in toddlers 6-24 months old: A randomized clinical trial. J Educ Health Promot. 2022;11:368.
[Crossref] [Google Scholar] [PubMed]
- Casparis D, Del Carlo P, Branconi F, Grossi A, Merante D, Gafforio L. Effectiveness and tolerability of oral liquid ferrous gluconate in iron-deficiency anemia in pregnancy and in the immediate post-partum period: Comparison with other liquid or solid formulations containing bivalent or trivalent iron. Minerva ginecologica. 1996;48(11):511-8.
[Google Scholar] [PubMed]
- National Rural Health Mission. Guidelines for control of iron deficiency anemia. 2013.
- Myint ZW, Oo TH, Thein KZ, Tun AM, Saeed H. Copper deficiency anemia: Review article. Ann Hematol. 2018;97(9):1527-1534.
[Crossref] [Google Scholar] [PubMed]
- Bjorklund G, Aaseth J, Skalny AV, Suliburska J, Skalnaya MG, Nikonorov AA, et al. Interactions of iron with manganese, zinc, chromium, and selenium as related to prophylaxis and treatment of iron deficiency. J Trace Elem Med Biol. 2017;41:41-53.
[Crossref] [Google Scholar] [ PubMed]
- World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. WHO.2011.
- Philippine Obstetrical and Gynecological Society (Foundation), Inc. Clinical Practice Guidelines on Iron Deficiency Anemia. Second Edition November, 2015. 2015
- Milman N. Anemi--still a major health problem in many parts of the world! Ann Hematol. 2011;90(4):369-77.
[Crossref] [Google Scholar] [PubMed]
- Center for Disease Control. Recommendations to prevent and control iron deficiency in the United States. 2001.
- Barbieri RL. Recognize and treat iron deficiency anemia in pregnant women. OBG Management. 2017;29(12) 8-11.
- Vossough P, Leikin S, Purugganan G. Evaluation of parameters of folic acid and vitamin B12 deficiency in patients with iron deficiency anemia. Pediatr Res. 1968;2(3):179-86.
[Crossref] [Google Scholar] [PubMed]
- Kaur S. Iron Deficiency Anemia (IDA): A review. Int J Sci Res. 2016;5(4):1999-2003.
- Anderson GJ, Frazer DM. Current understanding of iron homeostasis. Am J Clin Nutr. 2017;106(Suppl 6):1559S-1566S.
[Crossref] [Google Scholar] [PubMed]
- Mehnaz S, Afzal S, Khalil S, Khan Z. Impact of iron, folate and vitamin C supplementation on the prevalence of iron deficiency anemia in non-pregnant females of peri urban areas of aligarh. Indian J Comm Med. 2006;31(3):201.
- Sharma A, Prasad K, Rao KV. Identification of an appropriate strategy to control anemia in adolescent girls of poor communities. Indian Pediatr. 2000;37(3):261-7.
[Google Scholar] [PubMed]
- Davidsson L, Walczyk T, Morris A, Hurrell RF. Influence of ascorbic acid on iron absorption from an iron-fortified, chocolate-flavored milk drink in Jamaican children. Am J Clin Nutr. 1998;67(5):873-7.
[Crossref] [Google Scholar] [PubMed]
- Astuti ND, Wirjatmadi B, Adriani M. The role of addition of vitamin C in iron supplementation on ferritin serum levels in anemia adolescent females. Health Notions. 2018;2(3):332-8.
- Katelhut A, Schultink W, Angeles I, Gross R, Pietrzik K. The effects of weekly iron supplementation with folic acid, vitamin A, vitamin C on iron status of Indonesian adolescents. Asia Pac J Clin Nutr. 1996;5(3):181-5.
[Google Scholar] [PubMed]
- Wheby MS. Effect of iron therapy on serum ferritin levels in iron-deficiency anemia. Blood. 1980;56(1):138-40.
[Crossref] [Google Scholar] [PubMed]
- Macher S, Herster C, Holter M, Moritz M, Matzhold EM, Stojakovic T, et al. The effect of parenteral or oral iron supplementation on fatigue, sleep, quality of life and restless legs syndrome in iron-deficient blood donors: A secondary analysis of the IronWoMan RCT. Nutrients. 2020;12(5):1313.
[Crossref] [Google Scholar] [PubMed]
- Narukawa N, Tsujiguchi H, Hara A, Miyagi S, Kannon T, Suzuki K, et al. Relationship between vitamin intake and health-related quality of life in a Japanese Population: A cross-sectional analysis of the shika study. Nutrients. 2021;13(3):1023.
[Crossref] [Google Scholar] [PubMed]
- Izzy M, Malieckal A, Little E, Anand S. Review of efficacy and safety of laxatives use in geriatrics. World J Gastrointest Pharmacol Ther. 2016;7(2):334-42.
[Crossref] [Google Scholar] [PubMed]
- Loria A, Medal LS, Elizondo J.n Effect of sorbitol on iron absorption in man. Am J Clin Nutr. 1962;10(2):124-7.
[Crossref] [Google Scholar] [PubMed]
Citation: Soriano CJL, Abalos V, Romero CM, Manalastas R, Villanueva G, Sule P, et al (2023) Management of Iron Deficiency Anemia with Fixed Dose Combination of Ferrous Gluconate, Multivitamins and Multi-mineral Capsule: A 12 Week, Open Label Single Arm Prospective, Non- Interventional (Observational) Study in Philippines. J Clin Exp Pharmacol. 13:385.
Copyright: © 2023 Soriano CJL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

