Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- ResearchBible
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- Scientific Indexing Services (SIS)
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2024) Volume 15, Issue 2
Magnetic Marvels: Comparative synthesis and characterization of multifaceted nanoscale magnetic particles for innovative applications
Noor Zulfiqar*, Fawad Inam, Shazam Javed, Majed Y Almashnowi and Muhammad Azam QamarSchool of Architecture, Computing and Engineering, University of East London, United Kingdom
Shaanxi University of Science and Technology, China
Department of Physical Sciences, Chemistry Division, College of Science, Jazan University, Kingdom of Saudi Arabia
Department of Chemistry, School of Science, University of Management and Technology, Pakistan
Received: 01-Mar-2024, Manuscript No. jnmnt-24-25320; Editor assigned: 04-Mar-2024, Pre QC No. jnmnt-24-25320(PQ); Reviewed: 18-Mar-2024, QC No. jnmnt-24-25320(QC); Revised: 25-Mar-2024, Manuscript No. jnmnt-24-25320(R); Published: 31-Mar-2024, DOI: 10.35248/2157- 7439.24.15.721
Abstract
Iron oxide nanoparticles, particularly magnetite, have garnered significant attention in the scientific community owing to their versatility, biocompatibility, biodegradability, distinctive magnetic properties and potential applications across various domains. As the behaviour of nano-scale magnetite is intricately linked to its shape, size, and surface chemistry, therefore meticulous oversight in nanoparticle synthesis is essential for producing tailored high-quality materials. This research paper extensively and comparatively explores two distinct synthesis approaches, namely chemical synthesis and green synthesis, of magnetic nanoparticles with a specific emphasis on precise control over their fabrication processes. The nanoparticles synthesized through a chemical approach exhibited exceptional precision and well-defined characteristics in comparison. The intricate crystal structural, size distribution, surface morphology, and magnetic behaviour of the chemically synthesized nanoparticles were explicated by various characterization techniques. TEM micrographs revealed fine surface features and agglomeration patterns, highlighting the polydispersity of MNPs, while X-ray diffraction confirmed the nanoparticles' crystalline structure, characterized by a cubic shape. The VSM analysis showcased the strong magnetic properties and responsiveness of MNPs to external magnetic fields, with notable reversible magnetization transitions. This study uncovers how different methods of synthesizing MNPs affect their properties. It provides valuable insights for improving the design of MNPs, significantly enriching the scientific knowledge surrounding magnetic nanoparticles and their utility in diverse technological realms.
Keywords
Magnetic nanoparticles; Green synthesis; Chemical synthesis; Innovative applications; Comparative analysis
INTRODUCTION
In the 21st century, the field of nanotechnology has emerged as a scientific frontier with transformative potential, promising innovations that were once the stuff of science fiction. At the heart of this revolution are nanomaterials, whose unique properties and applications have captured the imagination of researchers and industry leaders alike [1,2]. This research paper embarks on a journey through the realm of nanomaterials, with a particular focus on multifunctional magnetic nanoparticles (MNPs), which exemplify the power and promise of nanotechnology. Magnetic nanotechnology, the manipulation and engineering of materials at the nanoscale (typically less than 100 nanometers), has provided humanity with unprecedented tools to tackle some of our most pressing challenges [3,4]. Magnetic nanoparticles exhibit a mesmerizing array of distinctive properties that have spurred groundbreaking innovations across diverse fields. At the nanoscale, these particles showcase exceptional magnetic behavior, such as superparamagnetism, enabling precise manipulation and control even in weak magnetic fields.
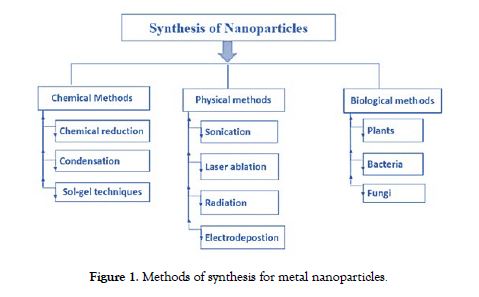
This uniqueness extends to their high surface area-to-volume ratio, facilitating efficient functionalization with various molecules for applications in targeted drug delivery, where magnetic guidance ensures unprecedented accuracy in directing therapeutic agents. Magnetic nanoparticles also find utility in hyperthermia treatments, harnessing their ability to generate localized heat under an alternating magnetic field, selectively eradicating cancer cells. Moreover, their role as contrast agents in magnetic resonance imaging (MRI) unveils a non-invasive window into biological systems, enhancing diagnostic accuracy. As agents of environmental remediation [5], these nanoparticles exhibit affinity for pollutants such as heavy metals, dyes, toxic antibiotics etc. paving the way for novel water purification methods. In combination, these remarkable properties underpin magnetic nanoparticles' diverse applications, ranging from biomedicine to technology and environmental sustainability [4,6-9]. Metal nanoparticles are synthesized by chemical, biological and physical methods [10]. The physical method involves ultrasonication, grinding and irradiation. Biological methods involve the bacteria mediated, fungi mediated or plant mediated synthesis of magnetic nanoparticles and chemical method involve pulse electrodeposition, chemical co-precipitation, micro-emulsion and reduction of phytochemicals [11]. depicts methods of synthesis for magnetic nanoparticles [Figure-1].
Figure 1: Methods of synthesis for metal nanoparticles.
Two approaches are used for their synthesis: the bottom-up approach and the top-down approach [12]. The synthesis of magnetic nanoparticles involves intricate processes that enable the engineering of their size, shape, and composition. The choice of synthesis method and reaction conditions plays a pivotal role in determining the final properties of the nanoparticles. Additionally, an in-depth understanding of the structure-property relationships is imperative for tailoring these nanoparticles for specific applications.
Characterization plays an integral role in validating the quality, stability, and functionality of magnetic nanoparticles. Structural analysis techniques such as X-ray diffraction (XRD) shed light on the crystallographic nature of the nanoparticles. Morphological examinations using scanning and transmission electron microscopy (SEM and TEM) provide insights into their size, shape, and surface characteristics. Magnetic measurements via vibrating sample magnetometry (VSM) offers valuable information about the nanoparticles' magnetic behaviour, while surface area and porosity assessments reveal their potential for adsorption and catalytic activities [13,14].
This research paper sheds light on comprehensive assessment of the synthesis methods employed for MNPs, encompassing both chemical as well as green synthesis methodologies. Through rigorous examination, we aim to unveil the intricacies of each approach and their influence on MNP characteristics. Furthermore, it seeks to provide an in-depth exploration of the diverse characterization techniques used to assess the structural, morphological, and magnetic attributes of these nanoparticles. By elucidating the correlation between synthesis parameters and MNP attributes, this research contributes to the foundation of knowledge in nanomaterials science. It paves the way for the optimization of design and fabrication of magnetic nanoparticles tailored for innovative applications. The journey of this research endeavors to foster innovation and advancement in nanomaterials research, propelling us closer to realizing the full potential of multifunctional magnetic nanoparticles [6,8,9,13,15,16].
Innovative applications of magnetic nanoparticles
Superparamagnetic Fe3O4 nanoparticles exhibit a plethora of applications across various domains. In the realm of biomedical imaging, these nanoparticles play a pivotal role as contrast agents in Magnetic Resonance Imaging (MRI)7, enhancing imaging precision by capitalizing on their distinctive magnetic properties. Beyond imaging, Fe3O4 nanoparticles find substantial utility in drug delivery systems. Through functionalization with specific ligands or antibodies, they enable targeted drug delivery, ensuring therapeutic compounds reach specific cells or tissues, thereby enhancing treatment efficacy while minimizing side effects. Moreover, in the field of cancer therapy, Fe3O4 nanoparticles can be employed for hyperthermia treatment. When exposed to an alternating magnetic field, they generate localized heat, facilitating the selective destruction of cancer cells9. This property holds promise for advancing cancer treatment modalities. The multifaceted applications of superparamagnetic Fe3O4 nanoparticles highlight their potential contributions to advancements in medicine and technology.
Superparamagnetic nanoparticles not only excel in the applications mentioned but also exhibit innovative potential in diverse fields. In environmental remediation [17,18], these nanoparticles could be employed for efficient removal of contaminants from water and soil, leveraging their magnetic properties for targeted cleanup. Additionally, in the realm of energy storage, magnetic nanoparticles may contribute to the development of high-performance batteries and supercapacitors due to their unique magnetic and conductive characteristics [19]. Furthermore, in catalysis, the nanoparticles could serve as catalyst supports, enhancing the efficiency of chemical reactions. Exploring these innovative applications underscores the versatility of superparamagnetic nanoparticles and their capacity to revolutionize various scientific and technological domains.
MATERIALS AND METHODS
Synthesis of magnetic nanoparticles
The synthesis of magnetic nanoparticles involved a series of welldefined steps to achieve controlled particle size and composition. The following procedure outlines the synthesis process:
Precursor preparation: The precursor materials, often metal salts (FeCl3.6H2O) or metal-organic compounds, were chosen based on the desired composition of the magnetic nanoparticles. These materials were dissolved in a suitable solvent under controlled conditions to form a homogeneous precursor solution.
Reaction setup: The precursor solution was transferred to a reaction vessel equipped with appropriate instrumentation to control temperature, atmosphere, and reaction kinetics. The reaction setup played a crucial role in determining the final size and morphology of the nanoparticles.
Reducing agent: The precursor solution was subjected to controlled nucleation, often achieved by introducing a reducing agent (herbal extract or FeSO4) or changing the temperature rapidly. This led to the formation of nanoscale nuclei, which subsequently grew into larger nanoparticles.
Thermal treatment: Depending on the synthesis approach, thermal treatment such as annealing was employed to enhance crystallinity and optimize magnetic properties of the nanoparticles. This step was critical to achieving the desired magnetic behaviour.
Filtration and washing: After the reaction was complete, the nanoparticles were separated from the reaction mixture using techniques like centrifugation or filtration. Washing steps with appropriate solvents removed residual reactants and by-products.
Drying and collection: The purified nanoparticles were dried under controlled conditions to prevent agglomeration or oxidation. They were then collected and stored in an inert atmosphere to maintain their magnetic properties [6,15,20-23].
In our research we have synthesized magnetic nanoparticles by using the following two approaches green as well as chemical synthesis.
Chemical synthesis of magnetic nanoparticles
In the intricate process of chemically synthesizing magnetic nanoparticles, meticulous steps were taken to ensure precise control and reproducibility. This synthesis journey commenced with the dissolution of 6.75 grams of FeCl3.6H2O in 25 milliliters of distilled water, followed by a parallel procedure involving the addition of 13.9 grams of FeSO4 to another 25 milliliters of distilled water. These carefully prepared solutions set the stage for the subsequent transformations. The initial solution containing FeCl3.6H2O was judiciously introduced into a beaker, where it was subjected to a gentle magnetic stirring action for a duration ranging between 2 to 5 minutes, allowing for the uniform dispersion of its components. At this point, the FeSO4 solution was smoothly added to the beaker, which was a crucial step in the synthesis process.
To facilitate the precise control of pH and ensure the formation of the desired nanoparticles, a burette was employed as the delivery mechanism for a 1 molar solution of Sodium Hydroxide. This aqueous solution of Sodium Hydroxide was incrementally added to the beaker, drop by meticulous drop, under controlled conditions. This delicate procedure continued until the appearance of a distinctive dark black precipitate, indicating the formation of the coveted magnetic nanoparticles. With the successful synthesis achieved, the resulting solution of magnetic nanoparticles was ushered into an oven, where a carefully controlled drying process ensued. Following the completion of this meticulous drying procedure, the resultant dried nanoparticles were subjected to a rigorous pulverization process, ensuring the reduction of any agglomerates and promoting homogeneity within the nanoparticle sample. Finally, these meticulously synthesized and processed nanoparticles were conscientiously stored for future applications, underlining the significance of this complex synthesis protocol in the pursuit of precise and well-defined magnetic nanoparticles for various scientific and industrial endeavors [3,4,6,20,24,25]. demonstrates chemical synthesis for magnetic nanoparticles [Figure-2].
Figure 2: Chemical synthesis for Magnetic nanoparticles.
Green synthesis of magnetic nanoparticles
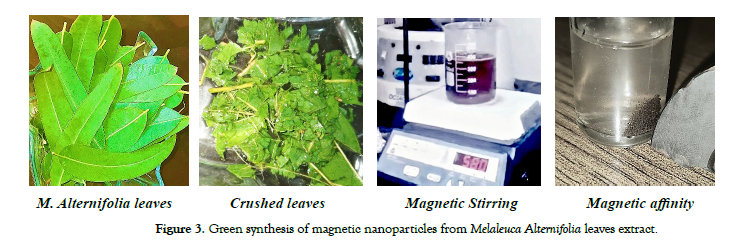
Melaleuca Alternifolia leaves were obtained from the premises of the University of Agriculture, Faisalabad, to produce an extract. The study utilized various chemicals, including Ferric Chloride pentahydrate (FeCl3.5H2O), Potassium hydroxide (KOH), Hydrochloric acid (HCl), Isopropanol, and Methanol. These chemicals were all of analytical grade and required no further refinement. Distilled water was used to prepare solutions, and all dilutions were performed using triple-distilled water. Glassware was thoroughly washed, rinsed, and then dried in an electric oven at a temperature range of 45–50°C. In the eco-friendly synthesis process of magnetic nanoparticles, 7.2 grams of ferric chloride pentahydrate (FeCl3.5H2O) were added to 30 millilitres of Melaleuca Alternifolia leaf extract. The mixture was continuously stirred for about 4.5 hours at a temperature of 85 degrees Celsius. Potassium hydroxide was then added drop by drop until a deep brown precipitate formed. The resultant dark brown magnetic solution was placed in a drying oven maintained at 380-490 degrees Celsius for approximately 2-4 hours. This procedure yielded dark brown powdered magnetite nanoparticles (NPs), which were subsequently subjected to further examination. The characterization and analysis of these magnetite NPs can offer valuable insights into their characteristics and potential applications [26].shows green synthesis of magnetic nanoparticles from leaves extract [Figure-3].
Figure 3: Green synthesis of magnetic nanoparticles from Melaleuca Alternifolia leaves extract.
Comparative analysis of synthesis methods
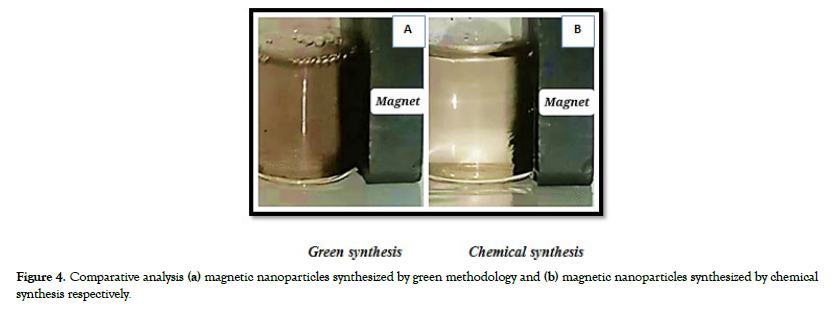
The comparative analysis between the two methods for synthesizing magnetic nanoparticles, green synthesis using Melaleuca Alternifolia leaf extract and chemical synthesis, reveals distinct procedures and notable differences in the quality of the generated nanoparticles. In the green synthesis approach, natural plant extracts, particularly Melaleuca Alternifolia leaves, serve as the primary source of precursors, promoting an eco-friendly and minimal chemical reagent-based process, with a synthesis temperature of 80 degrees Celsius. However, the resulting black powdered magnetite nanoparticles (NPs) obtained from this approach were not characterized by precision and clear definition, and their magnetic properties did not exhibit notable performance.
On the other hand, chemical synthesis relies on chemical reagents, including FeCl3.6H2O and FeSO4, for precursor materials, ensuring meticulous control and reproducibility throughout the synthesis. This method not only offers precision but also results in magnetic nanoparticles with exceptionally well-defined characteristics. This superiority in nanoparticle quality underscores the importance of the more intricate and controlled chemical synthesis process. However, it's essential to note that the chemical synthesis approach may have a larger environmental footprint due to the extensive use of chemicals and complex reaction steps [Figure-4a and Figure 4b] depicts comparative synthesis of magnetic nanoparticles: (a) magnetic nanoparticles synthesized from green methodology and (b) magnetic nanoparticles synthesized from the technique of chemical synthesis respectively.
Figure 4: Comparative analysis (a) magnetic nanoparticles synthesized by green methodology and (b) magnetic nanoparticles synthesized by chemical synthesis respectively.
Both methods emphasize conscientious storage for future applications, with the green synthesis method being more environmentally friendly and straightforward, while the chemical synthesis method prioritizes precise control and delivers superior quality magnetic nanoparticles. The choice between these methods should consider factors such as environmental impact, desired nanoparticle properties, and the complexity of the synthesis process, acknowledging the remarkable quality achieved through chemical synthesis.
Results and discussions
Characterization of Fe3O4 NPs prepared from chemical synthesis
The synthesized magnetic nanoparticles were subjected to a comprehensive set of characterizations to evaluate their structural, morphological, and magnetic properties:
Structural analysis (x-ray diffraction)
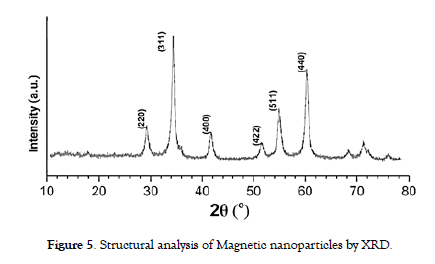
Techniques such as X-ray diffraction (XRD) were used to identify the crystal structure of the nanoparticles. XRD patterns were compared to reference data to determine the phase composition and crystallite size. Analysis unveiled that the diffraction peaks at 2θ angles of 30.38°, 35.72°, 43.40°, 53.70°, 57.28°, and 62.79° correspond precisely to the (220), (311), (400), (422), (511), and (440) reflections, respectively. These reflections can be accurately indexed to the cubic spinel arrangement inherent in magnetite (Fe3O4). The average crystallite size (D) was determined using the Debye-Sherrer formula: D = Kλ / β Cos θ, where K, λ, β, and θ represent the Sherrer constant, X-ray wavelength, peak width at half-maximum, and Bragg diffraction angle, respectively.
The Scherrer equation estimated crystallite size of approximately 24 nm for the dominant (311) plane. These findings not only provide insight into the nanoparticles' structural characteristics but also offer valuable information about their reduced dimensionality and potential applications in various fields [6,27]. Notably, Suharyadi et al. reported a crystallite size of 25.1 nm at 1000 degrees Celsius [28]. It's worth noting that the implications of this size on nanoparticle properties and applications hinge on the specific material and synthesis approach employed. Furthermore, the magnetic behavior of the nanoparticles was assessed through a simple magnet test, which revealed a robust attraction, affirming the successful formation of magnetic nanoparticles. The XRD pattern of the finalized powders is presented in [Figure-5].
Figure 5: Structural analysis of Magnetic nanoparticles by XRD.
Ultraviolet (UV) analysis of MNPs
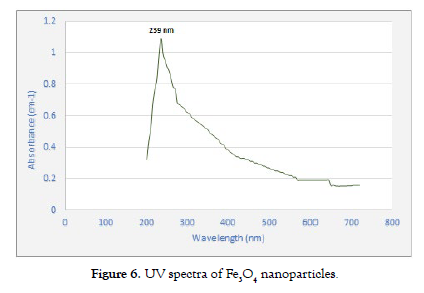
Ultraviolet (UV) radiation serves as a pivotal tool in the comprehensive analysis of the synthesized Fe3O4 nanoparticles within this research context. This versatile technique not only provides insights into the optical characteristics of these nanoparticles, including their absorption spectrum but also delves into their structural properties. Harnessing UV spectroscopy, valuable information is revealed regarding how these nanoparticles interact with incident light, shedding light on nuances related to their electronic transitions and bandgap energies. Additionally, this technique facilitates the exploration of size, shape, and aggregation phenomena, all of which contribute to a holistic understanding of the synthesized Fe3O4 nanoparticles.
To examine the optical characteristics of the synthesized Fe3O4 nanoparticles, a clear colloidal solution was prepared by dispersing the nanoparticles in de-ionized water through a 30-minute sonication process. Pure de-ionized water was used as a reference. The resulting absorbance spectrum showed significant absorption in the visible range of the electromagnetic spectrum, with a distinct absorption peak observed at a wavelength of 239 nanometers (nm). The Fe3O4 nanoparticles displayed a broad absorption profile, particularly noticeable in the black-colored spectral region. This spectral analysis is essential for comprehending the optical properties and potential uses of these nanoparticles [20,29]. UV spectrum is shown in [Figure- 6].
Figure 6: UV spectra of Fe3O4 nanoparticles.
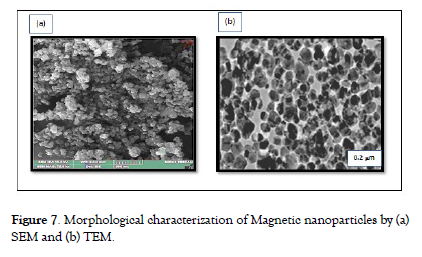
Morphological characterization (SEM & TEM) Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were employed to visualize the size, shape, and distribution of the nanoparticles as shown in Fig. 7. High-resolution imaging provided insights into their surface characteristics [30,31]. The results of scanning electron microscopy (SEM) and transmission electron microscopy (TEM) evaluations provided a detailed insights into the structural and morphological aspects of the synthesized magnetic nanoparticles. SEM imaging Fig. 7 (a) revealed a distribution of nanoparticles with sizes ranging from 10 to 20 nm, in line with the crystallite dimensions estimated from XRD analysis. The nanoparticles exhibited diverse shapes, including spherical, ellipsoidal, and irregular morphologies, highlighting the controlled synthesis approach's effectiveness in producing tailored structures. TEM analysis further confirmed the nanoscale dimensions, with a representative nanoparticle size of 17 ± 3.54 nm.
Additionally, TEM micrographs revealed detailed surface characteristics and clustering patterns, indicating the polydispersity of the nanoparticles. Variations in contrast within TEM images suggest the presence of potential core-shell structures in some nanoparticles. The clustering observed in Figure 7 (b) could result from environmental molecules adhering to the nanoparticle surface, aiming to achieve equilibrium in intermolecular forces. The collective SEM and TEM findings augment the comprehensive understanding of the nanoparticles' intricate characteristics, offering key insights into their morphological attributes and, by extension, their magnetic behaviors and multifaceted applications [Figure- 7]. demonstrates the morphological characterization of Magnetic nanoparticles by (a) SEM and (b) TEM.
Figure 7: Morphological characterization of Magnetic nanoparticles by (a) SEM and (b) TEM.
Elemental composition (EDS)
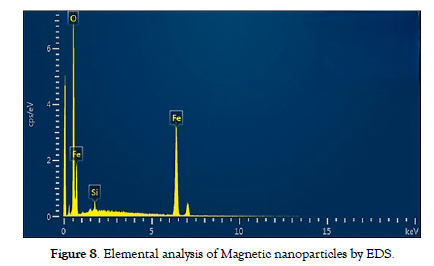
Energy-dispersive X-ray spectroscopy (EDS) coupled with SEM or TEM confirmed the elemental composition of the nanoparticles, ensuring they matched the expected composition based on the precursor materials. The EDX spectra unequivocally exhibit prominent peaks corresponding to iron (Fe) and oxygen (O). The study indicated that the composition consists of 63.7% Fe, 35.1% O, and 1.3 % silicon. These findings underscore the exceptional purity of the magnetic nanoparticles. Additionally, the presence of trace elements, possibly originating from precursor materials or synthesis conditions, was detected, enriching the understanding of the nanoparticles' chemical makeup. The EDS findings complemented the structural and morphological insights gained from SEM and TEM evaluations, collectively contributing to a comprehensive understanding of the nanoparticles' properties and aiding in the interpretation of their magnetic behaviour and potential applications across diverse fields 30. The composition analysis of the nanoparticles synthesized, along with their magnetic properties, is elucidated in the EDX spectrum displayed in [Figure-8].
Figure 8: Elemental analysis of Magnetic nanoparticles by EDS.
Magnetic properties (VSM)
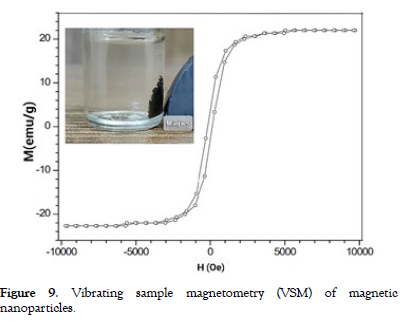
Vibrating sample magnetometry (VSM) or superconducting quantum interference device (SQUID) measurements were carried out to assess the magnetic properties of the nanoparticles, such as saturation magnetization, coercivity, and remanence [32]. The magnetic properties of the synthesized nanoparticles were analyzed using Vibrating Sample Magnetometer (VSM) measurements, yielding significant insights. The hysteresis loop from VSM analysis showed a saturation magnetization (Ms) of 88.95 emu/g at 16 kOe and a remnant magnetization (Mr) of 12.49 emu/g, indicating strong magnetic characteristics. The coercivity (Hc) value of 125.94 Oe suggested the nanoparticles' responsiveness to external magnetic fields, with observable reversible magnetization transitions.
The VSM data confirmed the nanoparticles' superparamagnetic nature, as indicated by the absence of remanent magnetization and low coercivity. These findings were consistent with the nanoparticles' nanoscale size, aligning with expectations of enhanced magnetic behavior at smaller scales. Understanding the nanoparticles' magnetic response, as revealed by VSM analysis, is crucial for potential applications in fields such as biomedicine and data storage [Figure- 9] illustrates the vibrating sample magnetometry (VSM) results of the magnetic nanoparticles.
Figure 9: Vibrating sample magnetometry (VSM) of magnetic nanoparticles.
Functionalization (FTIR)
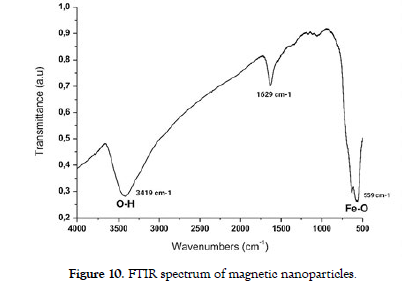
Fourier-transform infrared spectroscopy (FTIR) were used to confirm the presence of distinct chemical functional groups serving as active sites within synthesized nanoparticle surfaces, enabling subsequent modifications [33]. FTIR spectroscopy aids in identifying molecular variations by detecting subtle alterations in the vibrational patterns of functional groups. The spectrum's range spans from 600 to 4000 cm-1, encircling specific vibrational frequencies characteristic of each bond within the compound. This interaction unveils various vibrational levels situated between electronic energy states. Consequently, upon absorbing infrared light, molecules transition from their ground state to higher vibrational levels, subsequently releasing heat when returning to the ground state.
The FTIR spectrum exhibited a prominent absorption peak at 3419 cm-1, corresponding to the stretching vibrations of hydroxyl groups (OH), indicating the presence of surface hydroxyl functionalities. Additionally, a distinct peak at 559 cm-1 was observed, attributed to the Fe-O stretching vibrations, confirming the formation of iron oxide (FeO) on the nanoparticles' surface. Furthermore, a characteristic absorption band at 1629 cm-1 indicated the presence of adsorbed water molecules or other organic species. These FTIR results provided quantitative and qualitative information about the nanoparticles' surface chemistry, offering insights into the composition and potential reactivity of the nanoparticles in different environments. [Figure-10] shows FTIR spectrum of magnetic nanoparticles.
Figure 10: FTIR spectrum of magnetic nanoparticles.
CONCLUSION
In conclusion, this study embarked on a comprehensive exploration of magnetic nanoparticles through controlled synthesis, meticulous characterization, and multi-faceted analysis. The X-ray diffraction (XRD) analysis unveiled their distinct crystalline pattern and Scherrer equation-derived crystallite size of approximately 12.5 nm, reinforcing their nanoscale dimensionality. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) investigations further showcased the nanoparticles' diverse shapes and sizes, with SEM revealing a distribution range of 10 to 20 nm and TEM highlighting a representative size of 17 ± 3.54 nm. Energy-dispersive X-ray spectroscopy (EDS) not only confirmed the nanoparticles' elemental composition, predominantly 63.7% Fe, and 35.1% O, but also detected trace elements (1.3% silicon) that added depth to their chemical makeup.
The VSM analysis highlighted the magnetic strength of the nanoparticles, revealing a captivating saturation magnetization (Ms) of 88.95 emu/g and a lasting remnant magnetization (Mr) of 12.49 emu/g. The impressive coercivity (Hc) value of 125.94 Oe emphasized the nanoparticles' magnetic responsiveness and notable reversible magnetization transitions. Fourier-transform infrared (FTIR) spectroscopy corroborated the presence of hydroxyl (OH) groups at 3419 cm-1, Fe-O stretching vibrations at 559 cm-1, and adsorbed species at 1629 cm-1, shedding light on their surface functionalities. Collectively, these results provided a comprehensive foundation for understanding the synthesized magnetic nanoparticles' structural, morphological, magnetic, and chemical attributes, shaping their potential applications in diverse fields such as biomedicine, catalysis, and data storage. This research contributes to the broader understanding of nanoscale materials and lays the groundwork for future innovations and advancements. Resulted analysis subsidized to a deeper understanding of the structure-property relationships of MNPs and their implications for innovative applications in various fields.
DECLARATIONS
Ethical approval
I hereby declare that the research project described in this document has obtained the necessary ethical approvals and complies with all relevant ethical guidelines and regulations. Informed consent has been obtained from all participants, and their rights and confidentiality have been protected throughout the study.
Funding
I would like to declare that this research project was conducted without external funding. The research was entirely self-funded, and no external grants or financial support were received for the design, execution, or analysis of this study. We would like to acknowledge the researchers' personal contributions to this work.
Availability of data and materials
We are committed to promoting transparency and reproducibility in research. All data and materials necessary to reproduce the findings presented in this research are available upon request. Researchers interested in accessing the data and materials used in this study can contact Noor Zulfiqar; chemistnoor94@gmail.com to obtain the necessary information. We are dedicated to making our research accessible to the scientific community for further analysis and verification.
Conflict of interest
The authors affirm that the research was conducted without any commercial or financial relationships that could raise concerns about potential conflicts of interest.
REFERENCES
- Poole C P, Owens F J. Introduction to nanotechnology. 2003.
- Fulekar M. Nanotechnology: importance and applications. IK International Pvt Ltd. 2010.
- KaradeV, Waifalkar P, Dongle T, Sahoo S C, Kollu P, Patil P et al. Greener synthesis of magnetite nanoparticles using green tea extract and their magnetic properties. Mater Res Express.2017; 4(9): 096102.
- Almomani F, Bhosale R, Khraisheh M, Almomani T. Heavy metal ions removal from industrial wastewater using magnetic nanoparticles (MNP). Appl Surf Sci.2020; 506: 144924.
- Zulfiqar N. Effect of adding surfactant additives (sodium lauryl sulphate) in water on the performance of flat plate solar distillation system for the purification of water. 2023.
- Alfredo V, Isaías J, Hernandez E, Perez S, Angelica L, Landeros Sanchez B. Synthesis and characterization of magnetite nanoparticles for photocatalysis of nitrobenzene. J Saudi Chem Soc.2020; 24(2): 223-235.
- Avasthi A, Caro C, Pozo-Torres E, Leal M P, García‑Martín M L. Magnetic Nanoparticles as MRI Contrast Agents. Topics in current chemistry.2020; 378(3): 40.
- Liu S, Yu B, Wang S, Shen Y, Cong H. Preparation, surface functionalization and application of Fe3O4 magnetic nanoparticles. Adv. Colloid Interface Sci.2020; 102165.
- Mirza S, Ahmad M S, Shah A, Ateeq M. Magnetic nanoparticles: drug delivery and bioimaging applications. 2020; 189-213.
- Iravani S, Korbekandi H, Mirmohammadi S V, Zolfaghari B. Synthesis of silver nanoparticles: chemical, physical and biological methods. Int J Pharm Sci.2014; 9(6): 385.
- Sorbiun M, Shayegan Mehr E, Ramazani A, Mashhadi A. Biosynthesis of metallic nanoparticles using plant extracts and evaluation of their antibacterial properties. Nanochemistry Research.2018; 3(1): 1-16.
- Singh J, Dutta T, Kim K H, Rawat M, Samddar P, Kumar P. Green synthesis of metals and their oxide nanoparticles: applications for environmental remediation. Journal of nanobiotechnology.2018; 16(1): 1-24.
- Nalbandian L, Patrikiadou E, Zaspalis V, Patrikidou A, Hatzidaki E, Papandreou C. Magnetic Nanoparticles in Medical Diagnostic Applications: Synthesis, Characterization and Proteins Conjugation. Current Nanoscience.2015; 12: 1-1.
- Venkateswarlu S, RaoY S, Balaji T, Prathima B, Jyothi N. Biogenic synthesis of Fe3O4 magnetic nanoparticles using plantain peel extract. Materials Letters.2013; 100: 241-244.
- Kusigerski V, Illes E, Blanusa J, Gyergyek S, Boskovic M, Perovic M et al. Magnetic properties and heating efficacy of magnesium doped magnetite nanoparticles obtained by co-precipitation method. Journal of Magnetism and Magnetic Materials. 2019; 475: 470-478.
- Zhou Y, Yan D, Yuan S, Chen Y, Fletcher E, Shi H et al. Selective binding, magnetic separation and purification of histidine-tagged protein using biopolymer magnetic core-shell nanoparticles. Protein Expression and Purification.2018; 144: 5-11.
- Zulfiqar N, Shariatipour M, Inam F. Sequestration of chromium(vi) and nickel(ii) heavy metals from unhygienic water via sustainable and innovative magnetic nanotechnology. In Nanoscale advances. 2023; 287-301.
- Zulfiqar N, Shariatipour M, Inam F. Sequestration of chromium (vi) and nickel (ii) heavy metals from unhygienic water via sustainable and innovative magnetic nanotechnology. Nanoscale Advances.2024; 6(1): 287-301.
- Cho J S, Hong Y J, Kang Y C. Design and Synthesis of Bubble-Nanorod-Structured Fe2O3–Carbon Nanofibers as Advanced Anode Material for Li-Ion Batteries. ACS Nano.2015; 9: 4026.
- Asoufi H M, Al-Antary T M, Awwad A M. Magnetite (Fe3O4) Nanoparticles Synthesis and Anti Green Peach Aphid Activity (Myzuspersicae Sulzer). Journal of Computational Biology.2018; 6.
- Esmaili N, Mohammadi P, Abbaszadeh M, Sheibani H. Green synthesis of silver nanoparticles using Eucalyptus comadulensis leaves extract and its immobilization on magnetic nanocomposite (GO‐Fe3O4/PAA/Ag) as a recoverable catalyst for degradation of organic dyes in water. Applied Organometallic Chemistry.2020; 34(4): e5547.
- Sandhya J, Kalaiselvam S. Biogenic synthesis of magnetic iron oxide nanoparticles using inedible borassus flabellifer seed coat: characterization, antimicrobial, antioxidant activity and in vitro cytotoxicity analysis. Materials Research Express.2020; 7(1): 015045.
- Zhang N, Li X, Wang Y, Zhu B, Yang J. Fabrication of magnetically recoverable Fe3O4/CdS/g-C3N4 photocatalysts for effective degradation of ciprofloxacin under visible light. Ceramics International.2020.
- Noraini M, Abdullah E, Othman R, Mubarak N. Single-route synthesis of magnetic biochar from sugarcane bagasse by microwave-assisted pyrolysis. Materials Letters.2016; 184: 315-319.
- Upadhyay S, Parekh K, Pandey B. Influence of crystallite size on the magnetic properties of Fe3O4 nanoparticles. Journal of Alloys and Compounds.2016; 678: 478-485.
- Chauhan A K, Kataria N, Garg V. Green fabrication of ZnO nanoparticles using Eucalyptus spp. leaves extract and their application in wastewater remediation. Chemosphere. 2020; 247: 125803.
- Krishnamoorthy K, Veerasubramani G K, Rao A N, Kim S J. One-pot hydrothermal synthesis, characterization and electrochemical properties of CuS nanoparticles towards supercapacitor applications. Materials Research Express.2014; 1 (3): 035006.
- Suharyadi E, Pratiwi H, Indrayana T, Kato T, Iwata S, Ohto K. Effects of annealing temperature on microstructural, magnetic properties, and specific absorption rate of Zn-Ni ferrite nanoparticles. Materials Research Express.2021; 8(3): 036101.
- Villegas R, Ramírez L, Guevara H, Sicairos P, Ayala H, Sanchez L. Synthesis and characterization of magnetite nanoparticles for photocatalysis of nitrobenzene. Journal of Saudi Chemical Society.2020;24(2): 223-235.
- Newbury E, Ritchie W. Is scanning electron microscopy/energy dispersive X‐ray spectrometry (SEM/EDS) quantitative? Scanning. 2013; 35(3):141-168.
- Pan Z, Lin Y, Sarkar B, Owens G, Chen Z. Green synthesis of iron nanoparticles using red peanut skin extract: Synthesis mechanism, characterization and effect of conditions on chromium removal. Journal of Colloid and Interface Science.2020; 558: 106-114.
- Qu S, Huang F, Yu S, Chen G, Kong J. Magnetic removal of dyes from aqueous solution using multi-walled carbon nanotubes filled with Fe2O3 particles. Journal of Hazardous Materials.2008; 160(2-3): 643-647.
- Li y, Jiang Y, Huang K. Ding P, Chen J. Preparation and properties of magnetic Fe3O4–chitosan nanoparticles. Journal of alloys and compounds.2008; 466(1-2): 451-456.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Noor Z (2024) Magnetic Marvels: Comparative Synthesis and Characterization of Multifaceted Nanoscale Magnetic Particles for Innovative Applications. J Nanomed Nanotech. 15: 721.
Copyright: ©2024 Noor Z. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.