Indexed In
- Open J Gate
- Academic Keys
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2021) Volume 9, Issue 4
Lung-Protective Ventilation for Adult Patients with ARDS in Intensive Care Unit: A Systematic Review and Evidence-Based Guideline
Yophtahe B. Woldegerima1*, Tikuneh A. Yetneberk1 and Habtamu K. Getinet22Department of Anesthesia, College of Medicine and Health Sciences, Debre-Tabor University, Debre-Tabor, Ethiopia
Received: 31-Aug-2021 Published: 21-Sep-2021, DOI: 10.35248/2329-9088.21.9.250
Abstract
Introduction: Mechanical ventilation has important role in ARDS management, despite harmful complications such as ventilator-associated lung injury (VALI). Although lung-protective ventilation (LPV) is considered to minimize VALI and improve outcomes, there are controversies on its effectiveness and ways of delivery. This article aimed to review current evidence and develop a clinical practice guideline; especially for limited human and material resource settings.
Methods: Current evidence was collected using reputable scientific search engines such as PubMed, Google Scholars, and Cochrane Library by setting appropriate filtering methods. Collected evidence was critically appraised by appropriate tools accordingly. Final conclusions and recommendations were made by comparing the benefits and downsides of the alternative strategies based on levels of evidence and classes of recommendation.
Discussion: LPV was found to decrease morbidity, mortality, hospital stay and improve long-term outcomes. It can be applied by limiting tidal volume (TV=4-7 ml/Kg), end-inspiratory plateau pressure (Pplat<30 cm H2O), and FiO2 and providing PEEP. Using PEEP/FiO2 protocol designed by ARDSnet and ARMA trials is favored to date. In contrast, ventilation with both low TV and PEEP is associated with mortality. Most literature inclined to use recruitment maneuvers, but cautiously or avoid in hemodynamic instability. No mode of ventilation was found superior over the others. Oxygenation, long- term outcomes, and mortality were found to improve with early and prolonged applications of prone positioning. Neuromuscular blocking agents (NMBAs) have equivocal outcomes. They can improve oxygenation despite increased risk of ICU-acquired myopathy. However, recent studies suggested routine and early initiation of NMBAs in moderate-sever ARDS, and Cis-atracurium is the drug of choice.
Conclusion: Patients with ARDS should be treated with LPV strategy; using lower tidal volume, limited end-inspiratory plateau pressure, PEEP:FiO2 titration protocol, recruitment maneuvers, longer prone positioning, and NMBAs. An algorithmic approach is prepared to simplify implementation.
Keywords
Acute respiratory distress syndrome; Lung-protective ventilation; Mechanical ventilation;Ventilator-associated lung injury; Evidence-based practice
Abbrevations
ALI: Acute Lung Injury; ARDS: Acute Respiratory Distress Syndrome; ATS: American Thoracic Society; ESICM: European Society of Intensive Care Medicine; ICU: Intensive Care Unit; LPV: Lung-protective Ventilation; MV: Mechanical Ventilation; NMBA: Neuro-Muscular Blocking Agents; PBW: Predicted Body Weight; PEEP: Positive End-Expiratory Pressure; PP: Prone Positioning; QALY: Quality Adjusted Life Years; RCT: Randomized Control Trail; RM: Recruitment Maneuver; SCCM: Society of Critical Care Medicine; SRM: Staircase Recruitment Maneuver; TV: Tidal Volume; VALI: Ventilatory Associated Lung Injury
Introduction
Acute Respiratory Distress Syndrome (ARDS) is a life-threating condition characterized by non-cardiogenic pulmonary edema, bilateral lung infiltrates and decreased lung compliance that leads to acute on-set of refractory hypoxemia. The two world wars brought evidences of edematous lungs after traumatic injuries and the condition with such features obtained the term “shocked lung.” Later on 1994, American-European Consensuses Conference has given the term “acute respiratory distress syndrome.” However, some debatable issues were persisted till the recent and current definition of ARDS was developed by international expert’s panel held in Berlin in 2011. Compared to other definitions, the Berlin definition has significant prediction ability for mortality (Table 1).
| Parameters | Definition | |
|---|---|---|
| Timing | In a week of a known insult followed by new or worsening respiratory symptoms | |
| Chest imaging | Bilateral opacities-not fully explained by effusion, lobar/lung collapse or nodule | |
| Origin of edema | Respiratory failure not fully explained by cardiac failure or fluid overload Need objective assessment (e.g. echocardiograph)to exclude hydrostatic |
|
| Oxygenation | Mild | 200 mmHg<PaO2/FiO2 ≤ 300 mmHg with PEEP ≥ 5 cmH2O |
| Moderate | 100 mmHg<PaO2/FiO2 ≤ 200 mmHg with PEEP ≥ 5 cmH2O | |
| Sever | PaO2/FiO2 ≤ 100 mmHg with PEEP ≥ 5 cmH2O | |
| Predisposition | If none identified,then need to rule out cardiogenic edema with additional data | |
Table 1: Global distribution of deep samples positive for Candida spp.
However, still critical issues are being raised on the definitions of ARDS [1]. Wide spread inflammation of the lungs, diffuse alveolar injury to alveolar cells, endothelial injury, increased pulmonary capillary permeability, atelectasis, activation of complex local and systemic inflammatory and immune responses, surfactant dysfunction and disturbed coagulation are common features of ARDS. Usually, ARDS follows predisposing conditions that can be broadly classified as extra-pulmonary origin (sepsis, burn, and pancreatitis) and pulmonary origin (pneumonia, contusion, aspiration) [2,3].
Acute Respiratory Distress Syndrome (ARDS) is one of the commonest clinical problem for admission to Intensive Care Units (ICU). The annual incidence of ARDS in the western world is estimated to be 7.2-34 per 100,000 people [4,5]. Approximately, 5% of hospitalized patients meet the diagnosis criteria for ARDS. Acute Respiratory Distress Syndrome (ARDS) is responsible for 9% ICU admissions, 39% of mechanically ventilated patients [1,4]. Multiple studies from different parts of the world are still identifying pneumonia and sepsis as the leading causes for ARDS. Hepatic cirrhosis, compromised immunity, aspiration, different cardiac events, trauma, pancreatitis and metastatic cancer also are the commonest concomitant conditions in ARDS [5-8]. Due to severity of the condition 75% of patients has moderate to severe form of ARDS and only the remaining 25% has mild form. Short-term mortality is 45% and survivors have significant longterm morbidities, low quality of life, cognitive dysfunction and disabilities. Many studies have disclosed that “life is hardly the same after ARDS.”
These figures are expected to differ in the developing and resource limited countries due to many factors such as higher rate infectious disease including retro-viral infection, trauma, underdiagnosis and limited level of hospital and ICU care [1,3,4].
Providing care for a patient with ARDS is really one of the challenging conditions in medical practice. It needs multidisciplinary approaches that involve timely and effective resuscitation, handling of the predisposing condition, antimicrobial therapy and source control in sepsis, optimal ventilatory strategy, hemodynamic monitoring and management, and supportive care [1,3]. Regardless of some improvements in the incidence and prognosis of ARDS in the last decades due to better understanding of the syndrome and advancements in critical care, mortality rate in ARDS remains unacceptably very high (>40%) [1,9]. The determinants of outcomes in ARDS were age, risk factor for ARDS, comorbidity, severity of illness, multiple organ dysfunction syndrome (MODS), oxygenation index (OI), and ventilation strategies [4,5,8,10-18].
Additional to poor quality of life, thereafter surviving ARDS, the financial implications of ARDS have enormous burden. Cost analysis studies done nearly two decades ago have showed that, health care costs of initial hospital/ICU admission, readmission and inpatient rehabilitation are very high. Especially, in high risk patients (prognostic estimate to survive at least 2 months is less than 50%), the cost per Quality Adjusted Life Years (QALY) was exaggeratedly very high (>100,000 USD). The median (IQR) of health cost of 5 years survivors after discharge was found to be 58,500 USD (19,700-157,800 USD, 90 percentile: 328,083 USD). These costs are supposed to be increased due to high inflation, expensiveness of medical services and currency manipulations. Survivors of ARDS had dependence on activities of daily life, exercise limitation and poor QALY. As a result, they cannot generate income comparable to pre-ARDS life years. On the second year of survival, only 65% survivors had returned to work with high incidence of physical limitations. Acute Respiratory Distress Syndrome (ARDS) and Acute Lung Injury (ALI) are widespread diseases with massive socioeconomic impacts comparable with the burden from breast cancer, acquired immune-deficiency syndrome, asthma or myocardial infarction [7,19-22].
Lung protective ventilation is a strategy of mechanical ventilation targeting the delivery of optimal ventilation support without causing Ventilator Associated Lung Injury (VALI) secondary to barotrauma (pressure overload), volutrauma (volume overload), atelectrauma (shearing of collapsed lung while opening and closing) and biotrauma (release of inflammatory mediators). These injuries worsen the conditions of the sick lung and worsen the outcome [23,24].
The rationales of this systematic review and guideline
Mechanical Ventilation (MV) has a central and invaluable role in the complex management of patient with ARDS. However, it is associated with many unwanted events including VALI, ventilator associated pneumonia and hemodynamic instability. Especially, in patients with ARDS, the incidence and the burden of VALI is higher due to preexisting damage and inflammatory processes in the lungs that result in diminished uneven compliance [25]. To prevent VALI, multiple studies advocated to implement LPV strategies for ARDS patients [4,6,12]. Even though, LPV was primarily introduced for patients with ARDS, patient without ARDS also can be benefited from it [26-28]. Despite these recommendations, Under-use of LPV was reported in developed countries for many reasons [29-31]. Furthermore, there are literature concluded that LPV did not improve the outcomes of ARDS; especially, in-relation to long-term survival [5]. Equi-vocal results also were obtained [17]. The burden of critical illness is very huge world-wide and extremely very huge in the developing countries with limited capacity, well-trained personnel and infrastructures [32].
Developing concise and practicable clinical guideline according to current evidence may help to improve use of LPV strategies; hence, care provision and outcome of patients with ARDS in settings with human and material resource limitations. One of the resource limitations is human-resource scarcity. The developing world has a small numbers of well-trained clinicians and specialists. So provision of practicable clinical guideline based on current evidences can improve the situations. This facilitates early discharge from ICU hence, free ICU beds and other resources will be available for other patients. All of these have cost implications; specifically in the low-income countries. Our objectives were to review systematically current evidence and develop achievable clinical practice guidelines; hence we aimed to provide a working document for better applications of LPV for patients with ARDS in ICU especially in areas with human and material resource limitations. For the simplicity of implementing the guideline, it is presented in algorithmic approach.
Literature Review
The research question was constructed in PICOS (Population, Interventions, Comparisons, Outcomes and Study designs) model. Studies done on adult human patients with ARDS on mechanical ventilation were included. The interventions and comparisons were applications of lung-protective strategies. The outcome was measured in terms of morbidity, mortality, hospital stay and long-term outcomes. Most of studies included in this review were prospective randomized controlled trials and meta-analyses. Current evidence in the last ten years (2009- 2019) was collected using appropriate search engines and databases such as PubMed through HINARI, Google Scholars and Cochrane Library by setting appropriate filtering method. Terms such as “ARDS” “ventilation,” “mechanical ventilation,” “lung protection,” “lung protection in ARDS,” “lung-protective ventilation strategies,” “lung protective ventilation in ARDS” “VALI” and combinations of terms with Boolean operators (AND, OR, NOR) were used. Keywords, mesh-terms, Boolean operators, time bound and English language were used to limit literature searching. Animal studies, articles published other than English language, articles on mechanical ventilation in non- ARDS patients were excluded from the current review. Authors independently performed literature searching and critical appraisal was done with appropriate appraisal tools, presented for coauthors and consensus reached on the eligibility of the included articles. Data was extracted from articles according to the research question. Final conclusions and recommendations were done by balancing the benefits and downsides of the alternative strategies for LPV in adult patients with ARDS based on levels of evidence and classes of recommendation (Tables 2 and 3).
| Levels | Evidence of the levels |
|---|---|
| A | Data derived from multiple randomized clinical trials or meta-analyses, systematic review, evidence based guideline |
| B | Data derived from a single randomized clinical trial (RCT) or large non-randomized studies |
| C | Consensus of opinion of the experts and/or small studies, retrospective studies, registries |
Table 2: Levels of evidence.
| Classes | Definitions |
|---|---|
| I | Evidence and/or general agreement that a given treatment or procedure is beneficial, useful, and effective |
| II | Conflicting evidence and/or a divergence of opinion about the usefulness/efficacy of the given treatment or procedure |
| IIa | Weight of evidence/opinion is in favor of usefulness/efficacy |
| IIb | Usefulness/efficacy is less well established by evidence/opinion |
| III | Evidence or general agreement that the given treatment or procedure is not useful/effective, and in some cases may be harmful |
Table 3: Classes of recommendations.
The characteristics of included studies are illustrated Table 4. This systematic review was reported using PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta- Analyses). The guidelines are preferred for reporting items for systematic reviews and meta-analyses and developed by Cochrane Collaboration.
| Author name | Year | Type | Patients involved | TV and Pplat | Mortality | |
|---|---|---|---|---|---|---|
| Petrucci N, De Feo C |
2013 | Cochrane review | 1297 | Pplat ≤ 30 cm H2O) TV of ≤ 7 ml/Kg Combination |
Decreased 28 day mortality | Highly recommended |
| Petrucci N. Iacovelli W. |
2009 | Cochrane review | 1297 | Pplat ≤ 30 cm H2O) TV of ≤ 7 ml/Kg Combination |
Decreased 28 day mortality | Highly recommended |
| Santa Cruz | 2013 | Cochrane review | 2565 | Higher vs. lower PEEP | Higher PEEP Improved oxygenation No difference in mortality |
Highly recommended |
| Briel et al. | 2010 | Systematic review and meta-analysis | 2299 | Higher vs. lower PEEP | Higher PEEP improved survival in moderate and sever ARDS | Highly Recommended |
| Goligher et al. | 2017 | Systematic review and meta-analysis | 1423 | Recruitment maneuver | Reduce mortality, especially when combined with Higher PEEP | Highly recommended |
| Hodgson C. et al. | 2016 | Cochrane review | 1658 | Recruitment maneuver | Improve ICU survival No difference in 28 day and hospital mortality |
Highly recommended |
| Chacko et al. | 2013 | Cochrane review | 1089 | Pressure-controlled ventilation vs. volume controlled ventilation | No difference | Highly recommended |
| Guerin et al. | 2013 | Randomized controlled trial | 466 | Prone vs. supine positioning | Prone positioning has reduced 28 and 90 day mortality | Recommended |
| Sarkis J. | 2018 | Systematic review | Not specified | Prone positioning | Reduce mortality especially when used longer and with other LPV strategies | Highly recommended |
| Papazian et al. | 2010 | Randomized controlled trial | 340 | Early Cis-atracurium infusion | Reduce duration of mechanical ventilation and mortality in moderate and sever ARDS without muscle weakness | Recommended |
| Hampton | 2012 | Systematic review | Not specified | Use of NMBAs | Decrease 90 day hospital mortality especial in cases of sever hypoxemia | Highly recommended |
| Sottile et al. | 2017 | Observational cohort | 13,436 | Cis-atracurium vs. Vecuronium | No difference in terms of mortality Cis-atracurium is preferred in other measurements |
Recommended |
| Sottile et al. | 2017 | Retrospective | 6065 | Cis-atracurium vs. Vecuronium | No significant difference in outcomes between the groups | Suggested |
| Hampton et al. | 2019 | Retrospective | 113 | Cis-atracurium vs. .Vecuronium | No difference in mortality and ventilator-free days Cis-atracurium has shortened ICU and hospital stay |
Suggested |
Table 4: Summary on characteristics of included studies.
Patient and public involvement statement
Patients and/or, the public were not involved in the design, conduct, reporting, or dissemination plans of this research.
Discussion
The four major components of LPV are limiting tidal volume (TV), end-inspiratory plateau pressure (Pplat) and FiOTsub, then providing optimal PEEP to prevent lung collapse [24]. Lungprotective ventilation (LPV) was found to decrease mortality by 22% (IB) [33]. A Cochrane review of RCTs that compared ventilation with TV ≤ 7 ml/Kg, Pplat ≤ 30 cm H2O or combination versus TV 10-15 ml/Kg found use of low TV and Pplat has reduced hospital and 28-days mortality (relative risk=0.86). However, it had evidence insufficiency to conclude on morbidity and long-term outcomes [4] (IA). A prospective cohort showed that ventilation with TV ≤ 6.5 ml/Kg and Pplat ≤ 30 cm H2O had benefit on two years survival [12] (IIC). The joint American Thoracic Society (ATS), European Society of Intensive Care Medicine (ESICM) and Society of Critical Care Medicine (SCCM); strongly recommended that ARDS patients should be ventilated with strategies that limit the tidal volume (4-8 ml/ Kg PBW) and inspiratory pressures (Pplat<30 cm H2O) [23] (IA). Avoiding over-distention while MV can attenuate release of inflammatory mediators [34] (IB). A double-blind RCT in China concluded that ventilation with 6 ml/Kg of TV and 10 cm H2O of PEEP could protective of VALI with less hemodynamic, stress and inflammatory effects [35] (IB). The low TV ventilation is associated with higher PaCO2 that has potential benefits by increasing O2 delivery (right-ward shift of O2-hemoglobin dissociation curve), micro-circulatory vasodilatation and cardiac index. To achieve these, PaCO2 as high as 66 mmHg and рH as low as 7.15 can be tolerable (or buffer with bi-carbonate) unless there are strong contra-indications such as raised intracranial pressure [3,36] (IA). In addition, low TV ventilation can result in patient-ventilator asynchrony and stacked breaths [37] (IIaC).
Applying PEEP is considered as a typical treatment for hypoxia by reopening the collapsed lung units. Despite improved oxygenation, the optimal settings of PEEP and its effect on the outcome remained controversial [24]. Adjustment of PEEP should balance alveolar recruitment and avoiding overdistention in consideration of hemodynamic adverse events [38]. Multiple studies including ALVEOLI trial have not found significant difference between use of higher and lower PEEP in terms of mortality [23,39-44] (IA). A Cochrane review found no difference in mortality and barotrauma between the groups. However, oxygenation was improved in higher PEEP group [43] (IA). A meta-analysis by Briel et al. showed that patients with moderate/sever ARDS had lower mortality and more ventilator free days with higher PEEP. Whereas, patients with mild ARDS tends to have non-significantly worsened outcome [45] (IA). An RCT by Mercat et al. suggested that higher PEEP can improve lung function, duration of MV and organ failure rather than mortality [40] (IB). Another meta-analysis has concluded higher PEEP ventilation combined with RM has reduced mortality [46] (IA). In contrast, decremental PEEP titration with Staircase Recruitment Maneuver (SRM) has improved lung compliance, shunt fraction, oxygenation and PaO2/FiO2 in 80% of patients [47] (IIaC). Ventilation with lower TV and PEEP is associated with increased mortality; especially, when PEEP is lower than 5 cm H20 [48,49] (IB). The joint of ATS, ESICM and SCCM; recommended that ARDS patients should receive higher rather than lower levels of PEEP [23] (IA). In unilateral lung diseases; higher PEEP may over-distend the healthy lung then worsen shunt and oxygenation [24] (IIaB). Regarding method of PEEP delivery, PEEP/FiO2 protocols designed by ARDSnet and ARMA trials are favored till date [33,41,50] (IB). Whereas according to severity of the ARDS, Hess recommended to use PEEP of 5-10 cm H2O in mild, 10-15 cm H2O in moderate and 15-20 cm H2O in severe cases [38] (IIaA). Inverse ratio ventilation (I:E>1:1) can increase airway pressure and oxygenation, but can result in high intrinsic PEEP; then worsen gas exchange, volutrauma and hemodynamic instability [3] (IA).
Recruitment maneuver is transient use of high trans-pulmonary pressure to open atelectatic units and increase lung volume. It is expected to prevent atelectrauma and VALI. Like other LPV strategies, there are no consensuses on the techniques of RM and impacts on outcomes of ARDS [3,38,50,51]. A small size study by Villagar et al. has concluded that RM has no shortterm benefit on oxygenation; whereas it can result in regional alveolar over-distention and worsen intrapulmonary shunt [52-69] (IIbB). In contrast, other studies reported that RM can improve oxygenation and reduce need for rescue therapy and mortality [23,46,50] (IA). However, higher PEEP may be required to maintain the recruitment and increase the benefit [38,46,50] (IA). The commonest technique of RM is applying continuous positive airway pressure 30-40 cm H2O for 20-40 seconds [38]. Another technique recently gaining popularity is SRM or step-wise increment of pressure up to 60 cm H2O [3]. In a small size study, SRM with decremental PEEP titration had improved response in 80% of patients with transient reduction in mean arterial pressure and heart rate and increased central venous pressure but no barotrauma [47] (IIaB). The ARDSnet did not recommend the routine use of RM [38] (IB). But the recent article by the joint of ATS, ESICM and SCCM advised to use RM in ARDS patients [23] (IA). Supporting this, there is a Cochrane review that has found those ventilation strategies incorporated RM has improved oxygenation and reduced ICU mortality without risk of barotrauma [15] (IA). Close monitoring is highly recommended since transient hypotension (12%), desaturation (8%), barotrauma (1%) and arrhythmias (1%) can occur (IA), and use in caution or avoid RM in patients with circulatory compromises [47,50] (IB).
Volume assist-control is the most popular mode for lung protective ventilation. However till date there is no high quality evidence that proved its superiority over other modes [50] (IA). In accordance to this, a Cochrane review of RCTs with a total of 1089 patients failed to confirm or disprove whether pressurecontrolled or volume-controlled ventilation has benefit for ARDS patients [14] (IIA). Non-invasive ventilation modalities can be optionally use in patients with mild ARDS and immunecompromised with no other organ failure [50] (IA).
After introduced in 1970s, prone positioning (PP) has been constantly proven for its effectiveness to improve oxygenation in ARDS patients; particularly, when conventional measures have failed in sever hypoxemia. However, its impact on outcomes found to be inconsistent [3]. In the PROSEVA trial, mortality was reduced by 50% at 28th and 90th days (16% vs.32% and 23.6% vs. 41%, p<0.001) by PP compared with supine positioning [53] (IB). However, secondary analysis of this trial found that VAP (associated with higher mortality) was not minimized by PP [54] (IB). Two recent systematic reviews supported that PP can reduce mortality especially when combined with other LPV strategies, applied early and for longer duration (>12-16 hrs.) [55,56] (IA). A meta-analysis of RCTs also claimed PP can reduce mortality by 10% and prevent occurrence of sever ARDS in mechanically ventilated patients [57] (IA). Benefits of PP in ARDS have been explained by increased lung volumes, homogeneity of ventilation, providing regional recruitment, decreasing driving pressure and lung straining and displacing cardiac weight from the lungs [3,55,58,59] (IA). In addition to respiratory benefits, PP was known for hemodynamic advantages (reduce right ventricle pressure overload, incidence of cardiac arrest and stress response and increase cardiac index) [53,55,60,61] (IA). Complication rate such as skin necrosis, dislodgement of tubings and others were not significant; even lower in some studies [3,53,59] (IA). Despite efficiency and simplicity of PP, under-use was commonly reported [57,59] (IA).
There are no well-established agreements concerning the use of Neuromuscular Blocking Agent (NMBA) in ARDS patients [3,24,50,62-64] (IA). Spontaneous breathing may worsen ARDS, patient-ventilator asynchrony, breath stacking, lung straining,negative pressure pulmonary edema, and pendelluft (flow of gases between lung regions) especially in sever ARDS [64] (IB). In contrast, cessation of spontaneous breathing is criticized for ICU-acquired myopathy (dis-use and loss of muscles of respiration including the diaphragm) and increased time of recover from paralysis. So a review by Grawe et al. Recommended to avoid routine early use of NMBAs in ARDS patients [62] (IA). Use of NMBAs increases chest wall compliance; reduce oxygen consumption by skeletal muscles. Paralysis with NMBAs was reserved for refractory hypoxemia. Muscle weakness after NMBAs seems over-emphasized, even NMBAs have sedativesparing effect. Recent evidences suggested routine and early initiation in moderate-sever ARDS [3,50] (IA). As a relaxant of choice, the effects of Cis-atracurium are promising. In multiple studies including ACURASYS trial, early infusion (within 48 hrs.) of Cis-atracurium has increased oxygenation, ventilator-free days, patient-ventilator synchrony and reduced mortality without muscle weakness; especially if steroids and hyperglycemia are avoided. Its advantageous features are; it does not accumulate, no histamine release, no active metabolite, organ independency and non-steroid linked, but it is expensive [65-69] (IA). The cheaper drugs, Vecuronium was compared with Cis-atracurium and found to have no significant difference in terms of mortality; but ventilator days, ICU days and total hospital stay were less when Cis-atracurium used [65,69,70] (IB). Vecuronium had comparable effect with Cisatracurium when comorbidities and severity of illness were adjusted [65,70] (IB). With available equivocal evidences, we can recommend (moderate confidence in effect estimates) precautious intermittent use of Vecuronium at early-phase of ARDS as alternative to Cis-atracurium with close monitoring of peripheral neuromuscular function, circuit disconnection, malfunction and avoidance of steroids and hyperglycemia (Table 4, Figure 1).
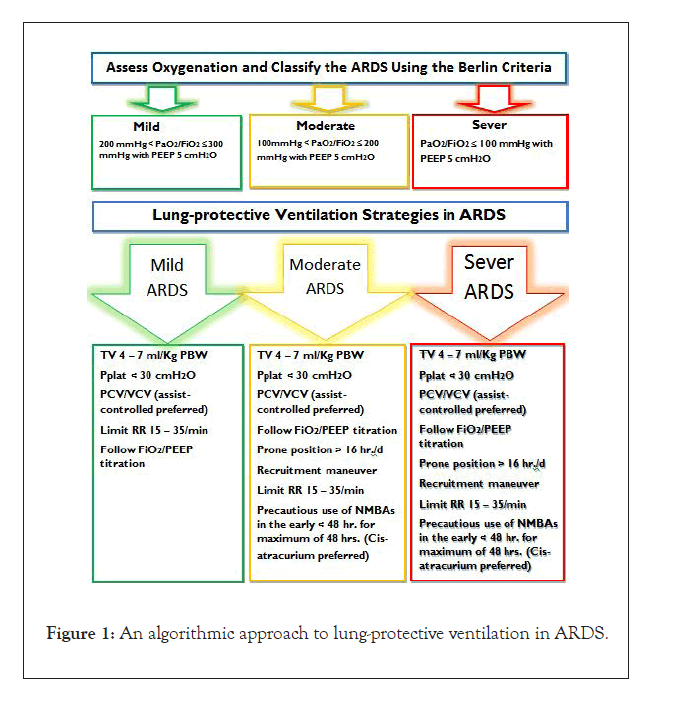
Figure 1:An algorithmic approach to lung-protective ventilation in ARDS.
Key-points
• Apply Lung Protective Ventilation (LPV) strategies as early as possible.
• Maintain patient-ventilator synchrony (sedation and analgesia).
• Apply and follow with standard monitoring.
• Routinely use peripheral neuromuscular monitoring. Especially when NMBAs are used.
• Monitor for ventilator disconnections and malfunctions.
• High tidal volumes and high plateau pressures need to be avoided.
• Tidal volume should be calculated based on predicted body weight (PBW) rather than on actual body weight, according to gender and height.
• Predicted body weight can be calculated as 50+0.91 × (height in cm-152.4) for men and 45.5+0.91 × (height in cm-152.4) for women.
• Tidal volumes should be watchfully adjusted (from 4 to 7 mL/ Kg of PBW) and maintain a plateau pressure of 30 cm H2O or less.
• The respiratory rate should be titrated as needed (in a range of 15 to 35/min) to maintain a pH of 7.15 to 7.35 and PaCO2=55– 65 mmHg unless contraindicated (e.g. raised ICP).
• Apply recruitment maneuver by increasing the pressure to 30–50 cm H2O for 30–40 seconds, whilst monitoring for signs of hemodynamic adverse events.
• An appropriate combination of FiO2 and PEEP should be used to achieve adequate oxygenation (PaO2 of 55 to 85 mmHg, or SpO2 of 90% or greater).
• Prone positioning and returning should be done with enough numbers of trained personnel.
• Pad and lubricate the eyes and pressure point
• With available equivocal evidences, we recommend precautious intermittent use of neuro-muscular blocking agents (NMBAs) at early-phase of ARDS.
• Avoid hyperglycemia and limit use of steroids since increase the risks of ICU-induced muscle weakness. Especially when steroid linked NMBAs (e.g. Vecuronium) are used (Table 5).
| Fio2 | PEEP |
|---|---|
| 0.3 | 5 |
| 0.4 | 5 |
| 0.4 | 8 |
| 0.5 | 8 |
| 0.5 | 10 |
| 0.6 | 10 |
| 0.7 | 10 |
| 0.7 | 12 |
| 0.7 | 14 |
| 0.8 | 14 |
| 0.9 | 14 |
| 0.9 | 16 |
| 0.9 | 18 |
| 1.0 | 20 |
Table 5: FiO2/PEEP combinations to achieve oxygenation goal (UMC Health System Respiratory ARDSNet Protocol, 2017).
Conclusion
Acute respiratory distress syndrome is a life-threatening condition with very high mortality>40%. Implementation of lung protective ventilation strategies may improve the outcomes in ARDS patients. With till date evidences, mechanical ventilation strategies in ARDS patients should include use of low tidal volume, limited plateau pressure, PEEP: FiO2 titration protocols, recruitment maneuvers, prone positioning and neuromuscular blocking agents.
Acknowledgement
We would like to thank University of Gondar.
Conflict of Interest
The authors declared that they have no conflict of interests regarding this article.
REFERENCES
- Umbrello M, Formenti P, Bolgiaghi L, Chiumello D. Current concepts of ARDS: A narrative review. Int J Mol Sci. 2016;18(1): 64.
- Pfeilsticker FJDA, Neto AS. ‘Lung-protective’ventilation in acute respiratory distress syndrome: Still a challenge? J Thorac Dis. 2017;9(8): 2238.
- Silversides JA, Ferguson ND. Clinical review: Acute respiratory distress syndrome-clinical ventilator management and adjunct therapy. Crit Care.. 2013;17(2): 225.
- Petrucci N, Iacovelli W. Lung protective ventilation strategy for the acute respiratory distress syndrome. Cochrane Database Syst Rev. 2013(2).
- Villar J, Blanco J, Añón JM, Santos-Bouza A, Blanch L, Ambrós A, et al. The ALIEN study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med. 2011;37(12): 1932-41.
- Bein T, Weber-Carstens S, Goldmann A, Müller T, Staudinger T, Brederlau J, et al. Lower tidal volume strategy (≈ 3 ml/kg) combined with extracorporeal CO 2 removal versus ‘conventional’protective ventilation (6 ml/kg) in severe ARDS. Intensive Care Med. 2013;39(5): 847-56.
- Cheung AM, Tansey CM, Tomlinson G, Diaz-Granados N, Matté A, Barr A, et al. Two-year outcomes, health care use, and costs of survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006;174(5): 538-44.
- Seeley E, McAuley DF, Eisner M, Miletin M, Matthay MA, Kallet RH. Predictors of mortality in acute lung injury during the era of lung protective ventilation. Thorax. 2008;63(11): 994-8.
- Villar J, Blanco J, Kacmarek RM. Current incidence and outcome of the acute respiratory distress syndrome. Curr Opin Crit Care. 2016;22(1): 1-6.
- Davidson TA, Rubenfeld GD, Caldwell ES, Hudson LD, Steinberg KP. The effect of acute respiratory distress syndrome on long-term survival. Am J Respir Crit Care Med. 1999;160(6): 1838-42.
- Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14): 1293-304.
- Needham DM, Colantuoni E, Mendez-Tellez PA, Dinglas VD, Sevransky JE, Himmelfarb CRD, et al. Lung protective mechanical ventilation and two year survival in patients with acute lung injury: prospective cohort study. Bmj. 2012;344: e2124.
- Brower RG, Rubenfeld GD. Lung-protective ventilation strategies in acute lung injury. Crit Care Med. 2003;31(4): S312-S6.
- Chacko B, Peter JV, Tharyan P, John G, Jeyaseelan L. Pressure‐controlled versus volume‐controlled ventilation for acute respiratory failure due to acute lung injury (ALI) or acute respiratory distress syndrome (ARDS). Cochrane Database Syst Rev. 2015(1).
- Hodgson C, Goligher EC, Young ME, Keating JL, Holland AE, Romero L, et al. Recruitment manoeuvres for adults with acute respiratory distress syndrome receiving mechanical ventilation. Cochrane Database Syst Rev. 2016(11).
- Nieman GF, Gatto LA, Habashi NM. Impact of mechanical ventilation on the pathophysiology of progressive acute lung injury. J Appl Physiol. 2015;119(11): 1245-61.
- Petrucci N, De Feo C. Lung protective ventilation strategy for the acute respiratory distress syndrome. The Cochrane Library. 2013.
- Zhou Y, Jin X, Lv Y, Wang P, Yang Y, Liang G, et al. Early application of airway pressure release ventilation may reduce the duration of mechanical ventilation in acute respiratory distress syndrome. Intensive Care Med. 2017;43(11): 1648-59.
- Angus DC, Musthafa AA, Clermont G, Griffin MF, Linde-Zwirble WT, Dremsizov TT, et al. Quality-adjusted survival in the first year after the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163(6): 1389-94.
- Hamel MB, Phillips RS, Davis RB, Teno J, Connors Jr AF, Desbiens N, et al. Outcomes and cost-effectiveness of ventilator support and aggressive care for patients with acute respiratory failure due to pneumonia or acute respiratory distress syndrome. Am J Med. 2000;109(8): 614-20.
- Lewandowski K, Lewandowski M. Epidemiology of ARDS. Minerva Anestesiol. 2006;72(6): 473-7.
- Ruhl AP, Huang M, Colantuoni E, Lord RK, Dinglas VD, Chong A, et al. Health care resource use and costs in long-term survivors of ARDS: a 5-year longitudinal cohort study. Crit Care Med. 2017;45(2): 196.
- Fan E, Del Sorbo L, Goligher EC, Hodgson CL, Munshi L, Walkey AJ, et al. An official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;195(9): 1253-63.
- Wright BJ. Lung-protective ventilation strategies and adjunctive treatments for the emergency medicine patient with acute respiratory failure. Emerg Med Clin North Am. 2014;32(4): 871-87.
- Pinhu L, Whitehead T, Evans T, Griffiths M. Ventilator-associated lung injury. The Lancet. 2003;361(9354): 332-40.
- Neto AS, Alencar R, D'Angelo V, Carmona R, Schultz MJX. Patients with uninjured lungs may also benefit from lung-protective ventilator settings. 2017;6.
- Neto AS, Nagtzaam L, Schultz MJ. Ventilation with lower tidal volumes for critically ill patients without the acute respiratory distress syndrome: a systematic translational review and meta-analysis. Curr Opin Crit Care. 2014;20(1): 25-32.
- Neto AS, Simonis FD, Barbas CS, Biehl M, Determann RM, Elmer J, et al. Lung-protective ventilation with low tidal volumes and the occurrence of pulmonary complications in patients without acute respiratory distress syndrome: a systematic review and individual patient data analysis. Crit Care Med. 2015;43(10): 2155-63.
- Kalhan R, Mikkelsen M, Dedhiya P, Christie J, Gaughan C, Lanken PN, et al. Underuse of lung protective ventilation: analysis of potential factors to explain physician behavior. Crit Care Med. 2006;34(2): 300-6.
- Newell CP, Martin MJ, Richardson N, Bourdeaux CP. Protective mechanical ventilation in United Kingdom critical care units: a multicentre audit. J Intensive Care Soc. 2017;18(2): 106-12.
- Rubenfeld GD, Cooper C, Carter G, Thompson BT, Hudson LD. Barriers to providing lung-protective ventilation to patients with acute lung injury. Crit Care Med. 2004;32(6): 1289-93.
- Adhikari NK, Fowler RA, Bhagwanjee S, Rubenfeld GD. Critical care and the global burden of critical illness in adults. The Lancet. 2010;376(9749): 1339-46.
- Network ARDS, Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18): 1301-8.
- Ranieri VM, Suter PM, Tortorella C, De Tullio R, Dayer JM, Brienza A, et al. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. Jama. 1999;282(1): 54-61.
- Tang C, Li J, Lei S, Zhao B, Zhang Z, Huang W, et al. Lung-Protective Ventilation Strategies for Relief from Ventilator-Associated Lung Injury in Patients Undergoing Craniotomy: A Bicenter Randomized, Parallel, and Controlled Trial. Oxid Med Cell Longev. 2017.
- Natalini G, Minelli C, Rosano A, Ferretti P, Militano CR, De Feo C, et al. Cardiac index and oxygen delivery during low and high tidal volume ventilation strategies in patients with acute respiratory distress syndrome: a crossover randomized clinical trial. Critical Care. 2013;17(4): R146.
- Pohlman MC, McCallister KE, Schweickert WD, Pohlman AS, Nigos CP, Krishnan JA, et al. Excessive tidal volume from breath stacking during lung-protective ventilation for acute lung injury. Crit Care Med. 2008;36(11): 3019-23.
- Hess DR. Recruitment maneuvers and PEEP titration. Resp Care. 2015;60(11): 1688-704.
- Dasenbrook EC, Needham DM, Brower RG, Fan E. Higher PEEP in patients with acute lung injury: a systematic review and meta-analysis. Resp Care. 2011;56(5): 568-75.
- Mercat A, Richard J-CM, Vielle B, Jaber S, Osman D, Diehl J-L, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. Jama. 2008;299(6): 646-55.
- National Heart L, Network BIACT. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351(4): 327-36.
- Sahetya SK, Brower RG. Lung recruitment and titrated PEEP in moderate to severe ARDS: Is the door closing on the open lung? Jama. 2017;318(14): 1327-9.
- Santa Cruz R, Rojas JI, Nervi R, Heredia R, Ciapponi A. High versus low positive end‐expiratory pressure (PEEP) levels for mechanically ventilated adult patients with acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst Rev. 2013(6).
- Walkey AJ, Del Sorbo L, Hodgson CL, Adhikari NK, Wunsch H, Meade MO, et al. Higher PEEP versus lower PEEP strategies for patients with acute respiratory distress syndrome. A systematic review and meta-analysis. Annals of the American Thoracic Society. 2017;14(Supplement 4): S297-S303.
- Briel M, Meade M, Mercat A, Brower RG, Talmor D, Walter SD, et al. Higher vs. . lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. Jama. 2010;303(9): 865-73.
- Goligher EC, Hodgson CL, Adhikari NK, Meade MO, Wunsch H, Uleryk E, et al. Lung recruitment maneuvers for adult patients with acute respiratory distress syndrome. A systematic review and meta-analysis. Ann Am Thorac Soc. 2017;14(Supplement 4): S304-S11.
- Hodgson CL, Tuxen DV, Bailey MJ, Holland AE, Keating JL, Pilcher D, et al. A positive response to a recruitment maneuver with PEEP titration in patients with ARDS, regardless of transient oxygen desaturation during the maneuver. J Intensive Care Med. 2011;26(1): 41-9.
- Ferguson ND, Frutos-Vivar F, Esteban A, Anzueto A, Alía I, Brower RG, et al. Airway pressures, tidal volumes, and mortality in patients with acute respiratory distress syndrome. Crit Care Med. 2005;33(1): 21-30.
- Levin MA, McCormick PJ, Lin HM, Hosseinian L, Fischer GW. Low intraoperative tidal volume ventilation with minimal PEEP is associated with increased mortality. Br J Anaesth. 2014;113(1): 97-108.
- Luks AM. Ventilatory strategies and supportive care in acute respiratory distress syndrome. Influenza Other Resp Viruses. 2013;7: 8-17.
- Pelosi P, de Abreu MG, Rocco PR. New and conventional strategies for lung recruitment in acute respiratory distress syndrome. Crit Care. 2010;14(2): 210.
- Villagra A, Ochagavia A, Vatua S, Murias G, DEL MAR FERNÁNDEZ M, Aguilar JL, et al. Recruitment maneuvers during lung protective ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med 2002;165(2): 165-70.
- Guérin C, Reignier J, Richard J-C, Beuret P, Gacouin A, Boulain T, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23): 2159-68.
- Ayzac L, Girard R, Baboi L, Beuret P, Rabilloud M, Richard J, et al. Ventilator-associated pneumonia in ARDS patients: the impact of prone positioning. A secondary analysis of the PROSEVA trial. Intensive Care Med. 2016;42(5): 871-8.
- Sarkis J. Does Prone Positioning Decrease Mortality Rate in ARDS? A Systematic Review. 2018.
- Scholten EL, Beitler JR, Prisk GK, Malhotra A. Treatment of ARDS with prone positioning. Chest. 2017;151(1): 215-24.
- Gattinoni L, Carlesso E, Taccone P, Polli F, Guerin C, Mancebo J. Prone positioning improves survival in severe ARDS: a pathophysiologic review and individual patient meta-analysis. Minerva Anestesiol. 2010.
- Aguirre-Bermeo H, Turella M, Bitondo M, Grandjean J, Italiano S, Festa O, et al. Lung volumes and lung volume recruitment in ARDS: a comparison between supine and prone position. Ann Intensive Care. 2018;8(1): 25.
- Guerin C, Beuret P, Constantin J, Bellani G, Garcia-Olivares P, Roca O, et al. A prospective international observational prevalence study on prone positioning of ARDS patients: the APRONET (ARDS Prone Position Network) study. Intensive care medicine. 2018;44(1): 22-37.
- Ruste M, Bitker L, Yonis H, Riad Z, Louf-Durier A, Lissonde F, et al. Hemodynamic effects of extended prone position sessions in ARDS. Ann Intensive Care. 2018;8(1): 120.
- Vieillard-Baron A, Charron C, Caille V, Belliard G, Page B, Jardin F. Prone positioning unloads the right ventricle in severe ARDS. Chest. 2007;132(5): 1440-6.
- Grawe ES, Bennett S, Hurford WE. Early Paralysis for the Management of ARDS. Respir Care. 2016;61(6): 830-8.
- Hraiech S, Forel J-M, Papazian L. The role of neuromuscular blockers in ARDS: benefits and risks. Current Opin in Crit Care. 2012;18(5): 495-502.
- Yoshida T, Fujino Y, Amato MB, Kavanagh BP. Fifty years of research in ARDS. Spontaneous breathing during mechanical ventilation. Risks, mechanisms, and management. Am J Respir Crit Care Med. 2017;195(8): 985-92.
- Hampton S, Shu J, Chu F, Huang W-T. Effect Of Cisatracurium Versus Vecuronium On Outcomes In Acute Respiratory Distress Syndrome. Critical Care Medicine. 2019;47(1): 535.
- Hraiech S, Yoshida T, Papazian L. Balancing neuromuscular blockade versus preserved muscle activity. Curr Opin in Crit Care. 2015;21(1): 26-33.
- Moore L, Kramer CJ, Delcoix-Lopes S, Modrykamien AM. Comparison of cisatracurium versus atracurium in early ARDS. Respir Care. 2017;62(7): 947-52.
- Papazian L, Forel J-M, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, et al. ACURASYS Study Investigators: Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363: 1107-16.
- Sottile PD, Kiser TH, Burnham EL, Ho PM, Allen RR, Vandivier RW, et al. An observational study of the efficacy of cisatracurium compared with vecuronium in patients with or at risk for acute respiratory distress syndrome. Am J Respir Crit Care Med. 2018;197(7): 897-904.
- Sottile P, Kiser TH, Burnham EL, Ho M, Allen R, Vandivier RW, et al. Mortality in intubated patients treated with vecuronium or cisatracurium. critical care: Improving prognostication in critical illness: Am Th Soc. 2017; 195: A2779.
Citation: Woldegerima YB, Yetneberk TA, Getinet HK (2021) Lung-Protective Ventilation for Adult Patients with ARDS in Intensive Care Unit: A Systematic Review and Evidence-Based Guideline. Trop Med Surg. 9: 250.
Copyright: © Woldegerima YB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
