Indexed In
- PUBMED
Useful Links
Share This Page
Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2024) Volume 9, Issue 3
Low-Level Laser Therapy: As a New Approach to Modulate Oxidative Stress in Diabetes Mellitus and its Complications
Mohammad Reza Ashoori1, Afsaneh Seyed Mikaeili2, Hossein Mirmiranpour3 and Saeedeh Abdolahpour3*2Department of Biochemistry, Islamic Azad University, Tehran, Iran
3Department of Endocrinology, Tehran University of Medical Sciences, Tehran, Iran
Received: 22-Feb-2021, Manuscript No. DCRS-24-8592; Editor assigned: 25-Feb-2021, Pre QC No. DCRS-24-8592 (PQ); Reviewed: 11-Mar-2021, QC No. DCRS-24-8592; Revised: 16-Aug-2024, Manuscript No. DCRS-24-8592 (R); Published: 13-Sep-2024, DOI: 10.35841/2572-5629.24.9.221
Abstract
As a chronic metabolic disease, Diabetes Mellitus (DM) can be strongly associated with a combination of heredity, environmental factors and lifestyle, demanding life-through control and management. This condition can also have irreparable consequences such as cardiovascular abnormalities, brain damage, nephropathy, Diabetic Peripheral Neuropathy (DPN) and diabetic wounds and even death. In today’s modern world, many individuals are at most risk of DM and its consequences. Accordingly, insulin injection and medications are being used to control type 1 and type 2 DM, respectively. However, DM can result in the production of free radicals and oxidative stress, as one of the most important consequences. In this respect, Low-Level Laser Therapy (LLLT) has been introduced as a novel form of phototherapy, which is being practiced as a non-invasive and safe method. Nowadays, oxidative stress reduction and cell proliferation in low-level laser exposed tissues have been further taken into consideration. Therefore, LLLT has been utilized as a complementary method for treatment of some DM consequences, such as skin wounds.
Keywords
Low-level laser therapy; Oxidative stress; Diabetes complications; Diabetes mellitus
Introduction
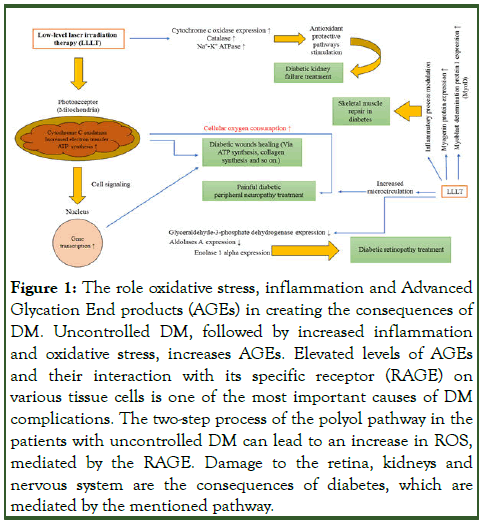
Nowadays, Diabetes Mellitus (DM), as a chronic and complex metabolic disease, is threatening different populations across the world. According to the report released by the World Health Organization (WHO), around 422 million people worldwide are affected with DM, mostly in undeveloped or less developed countries. As cited, DM has been classified into two main types, type 1 and type 2. In this sense, type 1 DM is caused by insufficient insulin secretion from the pancreatic beta cells and type 2 DM may arise due to insulin resistance, which is more commonly seen in obese individuals and it is significantly shaped by lifestyle and food consumption. Inflammation, oxidative stress and aging all play a significant role in the pathogenesis of type 2 DM [1]. The fundamental role of oxidative stress in the pathophysiology of diabetes is well known. Oxidative stress leads to the inactivation of enzymes such as Superoxide Dismutase (SOD), Catalase (CAT) and other antioxidant enzymes. Decreased activity of these enzymes is also effective in the development of oxidative stress. DM control and management is accordingly of utmost importance to prevent the consequences of this condition such as Diabetic Peripheral Neuropathy (DPN), nephropathy, cardiomyopathy, etc (Figure 1). Studies have shown that Diabetic Kidney Disease (DKD) takes place in more than 40% of patients with type 1 and type 2 DM. Oxidative stress and inflammation are factors that play an important role in the growth and promotion of DKD. Reactive Oxygen Species (ROSs) play an important function in signaling the proliferation, differentiation, apoptosis and immune defense of renal cells. DM can be also controlled by insulin injection as well as taking some medications [2].
Figure 1: The role oxidative stress, inflammation and Advanced Glycation End products (AGEs) in creating the consequences of DM. Uncontrolled DM, followed by increased inflammation and oxidative stress, increases AGEs. Elevated levels of AGEs and their interaction with its specific receptor (RAGE) on various tissue cells is one of the most important causes of DM complications. The two-step process of the polyol pathway in the patients with uncontrolled DM can lead to an increase in ROS, mediated by the RAGE. Damage to the retina, kidneys and nervous system are the consequences of diabetes, which are mediated by the mentioned pathway.
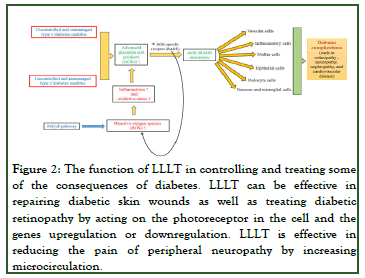
In this respect, Niels Ryberg Finsen won the Nobel Prize in 1903 for his effort to treat diseases via light therapy. Over recent years, light therapy, such as Low-Level Laser Therapy (LLLT) has been employed as a therapeutic method for a number of conditions. LLLT consists of a non-ionized, non-invasive and focused radiation, whose contact with various tissues of the body produces thermal, photochemical and nonlinear effects. LLLT can thus induce the proliferation of some cells and consequently aid in tissue repair (Figure 2). Many studies have been accordingly conducted on the impacts of LLLT on diabetic patients. In patients with DM, it is important to monitor glycated hemoglobin (HbA1c) levels during diabetes control. Photodynamic therapy with diode laser at a wavelength of 670 nm can be effective in reducing HbA1c levels. The main purpose of this review article, reflecting on recent literature, is to investigate the effectiveness of LLLT and phototherapy in patients with DM [3].
Figure 2: The function of LLLT in controlling and treating some of the consequences of diabetes. LLLT can be effective in repairing diabetic skin wounds as well as treating diabetic retinopathy by acting on the photoreceptor in the cell and the genes upregulation or downregulation. LLLT is effective in reducing the pain of peripheral neuropathy by increasing microcirculation.
Literature Review
LLLT and its biochemical mechanism
LLLT was first used by the National Aeronautics and Space Administration (NASA) to heal space wounds. LLLT, also known as bio-stimulation, photobiomodulation and cold or soft laser, is a new therapeutic aspect in which different cells and tissues are directly exposed to low levels of red and Infrared (IR) lasers or Light-Emitting Diodes (LEDs). Utilizing photons and non-thermal radiation, this method is able to alter biological activity in different cells and tissues. Additionally, LLLT utilizes high-density light and works locally. The wavelengths exploited in this method also range from 630 nm to 680 nm, 700 nm to 830 nm and 900 nm [4].
The photo-biological foundation of LLLT is not well specified. Karu investigated the molecular mechanism of the therapeutic effect of LLLT. In Smith's study, the effect of bio-stimulation of low-level radiation at a wavelength of 633 nm is explained. According to Caro's model, a chain of molecular events occurs when light is absorbed by a photoreceptor. Eventually these events lead to a photo-response. Light is absorbed by the components of the light chain, which activates the respiratory chain. Oxidation of NAD leads to changes in the redox state of both the mitochondria and the cytoplasm. This condition affects the permeability of the membrane to K+, Na+ and Ca2+ ions and the activity of membrane channels. The flow of Ca2+ ions into the cell can affect the levels of cyclic nucleotides. DNA and RNA synthesis are then modulated and cell proliferation occurs. The metabolic effects of light also occur at the levels of the mitochondrial respiratory chain [5].
Effects of LLLT on DM consequences
Effects of LLLT on oxidative stress: Hyperglycemia caused by DM is very effective in increasing ROSs. Inadequate detoxification of the ROSs or their excessive production can thus produce oxidative stress, inducing cellular damages. Besides, oxidative stress is the cause of inflammatory responses in the body. Due to the elevated production of ROSs and the absence or decline of antioxidants, ROSs can also interact with cellular molecules, resulting in cell membrane lipid peroxidation and Deoxyribonucleic Acid (DNA) damage. Oxidative stress is further assumed as an important pathogenic factor in diabetic foot ulcers and one of the complications of uncontrolled DM. Evidence suggests that hyperglycemia and the production of Advanced Glycation End-products (AGEs) are among factors restraining wound healing in patients with uncontrolled DM. Moreover, foot ulcers are one of the most common causes of amputations in individuals affect with chronic and untreated DM [6].
Antioxidant enzymes, such as catalase and superoxide dismutase, play an important role in the immune system of the body to eliminate the deleterious effects of ROSs. Hyperglycemia and DM can also cause glycation of antioxidant enzymes and affect their proper functioning. Nowadays, various methods including microwave, light and heat radiation are being administered to increase the activity of these antioxidant enzymes and to reduce ROSs. In this line, Salam et al. had investigated the effects of a static magnetic field on the function of the liver enzymes in an animal model [7]. They had accordingly observed a significant rising trend in enzymatic activity after one week, every day for an hour, associated with magnetic field effect. In another study by Vojisavljevic et al., the impact of electromagnetic radiation on enzymatic kinetics had been explored, which had thus altered enzymatic functions in the IR, visible and Ultraviolet (UV) ranges. In a previous study on diabetic rats by the authors, the effects of intravenous laser irradiation on some enzymes, such as catalase antioxidant enzyme, had been evaluated and it had been concluded that short wavelength laser irradiation (i.e., LLLT) could be more effective in enhancing enzyme activity than long wavelength laser irradiation. In separate studies, Silva Macedo et al. and Denadai et al., had similarly reflected on the therapeutic effects of LLLT on changes in oxidative stress. Denadai et al. had correspondingly reported that the acute effects of LLLT on the reduced levels of oxidative stress could be beneficial in diabetic rats with cutaneous wounds.
The effects of laser phototherapy on the enzymatic activity of the salivary glands in hamsters treated with 5-Fluorouracil (5-FU) had been comparably investigated by Campos et al., revealing that laser phototherapy had potentially acted as a protective mechanism against oxidative stress. Studies had further suggested that augmented ROS could contribute to the formation of renal, hepatic, cardiac, pulmonary and skeletal muscle fibrosis. It is of note that the bulk of fibrosis in these organs can be among the consequences of DM. It has been also reported that cellular redox activity is promoted by LLLT [8]. Absorption of a specific light wavelength by a lightaccepting molecule is thus assumed as a photo-biological response. The redox features of electronic absorption and excitation centers can additionally change with regard to LLL irradiation. The biological effects of light also depend on wavelength, radiation duration, intensity, dose, as well as pulse parameters. Likewise, LLLT can prevent oxidative stress by escalating antioxidant activity. In this regard Lu et al. had investigated the effects of LLLT on ROS homeostasis in injured muscles. In the previous study, by the authors, on the antioxidant enzyme catalase, glycated in vitro, the impacts of laser irradiation at different wavelengths had been explored. The results had established that catalase activity had been increased by blue laser irradiation more than green and red types. A recent study, by the authors, on oxidant parameters such As Advanced Oxidant Protein Products (AOPP) and Oxidized Low-Density lipoprotein (OX-LDL) had demonstrated that LLLT with shorter wavelengths (i.e., blue light with a wavelength of 450 nm) could be more effective in mitigating the mentioned oxidant parameters. In contrast, longer wavelengths (such as red light with a wavelength of 638 nm and green light with a wavelength of 532 nm) had failed to be so. The results of the study by Ibuki et al.'s had correspondingly indicated that low-power laser irradiation had a protective effect against oxidative stress. Moreover, they had found that laser irradiation could encourage antioxidant activity to remove ROSs. In a recent study, the authors had reported that laser therapy could be effective in enhancing the levels of antioxidant enzymes such as catalase and superoxide dismutase as well as some minerals e.g. magnesium and zinc [9].
Effects of LLLT on diabetic nephropathy
As mentioned, hyperglycemia and consequently DM cause oxidative damages to tissues, somehow prone to the consequences of uncontrolled DM. One of the consequences of untreated DM is kidney disease and nephropathy. Producing ROS with chronic DM, ROSs affect the kidneys through the pathway of the formation of AGEs and other pathways. Previous studies have further shown that sodium-potassium adenosine triphosphatase, also known as the sodium-potassium pump (Na+- K+ ATPase pump) activity, decreases in chronic and uncontrolled DM. Disruption of the pump’s function and loss of energy as well as glycation of the protein may lead to impaired kidney function and impaired ion balance, resulting in diabetic nephropathy. Progressive diabetic nephropathy also plays a role in reduced concentration of superoxide dismutase antioxidant enzyme. In a study on the kidneys of diabetic rats, the therapeutics of LLLT on these organs, damaged by induction of DM, had been investigated. Lim et al. had further found that LLLT had improved kidney function and antioxidant defense in the kidney of type 1 diabetic rats. In general, they had concluded that LLLT at a wavelength of 670 nm could be widely used to improve the renal consequences of DM, in which the defense mechanism had been abolished [10]. Moreover Asghari et al. had further studied the effects of LLLT on induced renal injury in diabetic rats. They had used a 685 nm laser diode, in which it had been found that progression of renal injury in diabetic rats could drop following induction of Ischemia- Reperfusion Injury (IRI) by LLLT.
One of the consequences of hyperglycemia is metabolic syndrome. Hyperglycemia and hypertension in the metabolic syndrome can also cause Chronic Kidney Disease (CKD). In the study by Ucero et al., the kidneys of rats with diabetic nephropathy had been subjected to LLLT for eight weeks and the findings had revealed that the given treatment had been effective in lowering blood pressure and maintaining glomerular filtration rate. Ucero et al. had further reported that laser therapy could be effective in modulating the progression of CKD, since it could provide a non-invasive and painless therapeutic method. In addition to the impact of LLLT on diabetic nephropathy, numerous studies have been performed on the impact of LLLT on renal abnormalities and dysfunction. Suryani Dyah et al. had also studied photodynamic therapy with diode laser to cell activation in renal dysfunction. They had further reported that renal tubular cells would have the power to proliferate, if they had been exposed to LLLT at a wavelength of 650 nm.
Nephrotoxicity induced by cisplatin can also cause severe kidney damage. Cell proliferation also occurs with concomitant use of exogenous Epidermal Growth Factor (EGF) and laser therapy. Replacing healthy cells with damaged ones can help heal the injured kidneys. Overall, according to the above-mentioned studies, renal cell injury due to DM, medications and induction of kidney injury in animal models can be improved by noninvasive and painless LLLT [11].
Effect of LLLT on DPN
DPN is the most common complication in patients with DM. It is also known as the leading cause of death in individuals with DM. DPN is often the most common progressive and irreversible complication in both type 1 and type 2 DM, which is often the result of damage to peripheral nerves, loss of axons and axonal atrophy. Both the functions of the Schwann cells and their interactions with axons, playing a vital role in energy metabolism and ion conduction, are two processes affected in DPN. If neuropathy is not controlled for a long time, microcirculatory damage may develop diabetic gangrene as well as wounds and infections of the skin and bones. In 30% of people with DPN, pain can be observed as a sign. In addition to drug therapy in patients with DPN, non-pharmacological sympathomimetic therapies e.g. acupuncture, IR phototherapy, laser therapy and electrotherapy have been also suggested. Among nonpharmacological treatments, LLLT may potentially have a biological stimulatory effect on the nervous system. To date, many studies have been conducted on the effect of laser therapy on the control of neuropathic pain, as discussed by Andrade et al. in a systematic review article. Accordingly, it has been concluded that the application of laser therapy in the treatment of neuropathic pain can have positive effects on pain control and relief [12].
In this regard, Yamany et al. had studied the effect of laser therapy on neuropathic function of DPN in patients with type 2 DM, who had demonstrated painful symptoms of DPN in both legs lasting more than six months. They had also used an IR laser helium-neon at a wavelength of 850 nm as treatment, suggesting that laser therapy could be effective in caring for painful DPN. In the study by Shashi, the effectiveness of laser therapy on DPN had been further investigated. Symptoms of DPN had been thus detected in the legs of 19 patients with type 2 DM and they had been subsequently treated with two types of lasers at different wavelengths. Accordingly Shashi Kumar et al. had reported that laser therapy could be effective in easing pain in patients. Various studies had correspondingly reflected on oxidative stress as one of the causes of DPN development. In this sense, Abdel-Wahhab et al. had examined the benefits of concomitant use of gabapentin and laser therapy in DPN management. They had studied diabetic rats affected with DPN and had concluded that laser therapy could be effective in managing this condition and its consequences. Laser therapy could be also used as an adjunct to medications such as gabapentin. Photobiomodulation could further influence the survival of cultured neurons with glucose toxicity in a laboratory model of DM.
In the study by da Silva Oliveira et al., the survival of cultured neurons had been also investigated. Accordingly, they had reported that neurogenesis could occur under the influence of photobiomodulation and through the activation of Protein Kinase B (PKB), also known as Akt signaling pathways in these cells. The reproductive potential of these cells had been further enhanced in DPN. Moreover, the activation of the Akt signaling pathway had directly indicated increased cell survival through inhibition of pre-apoptotic factors and intracellular oxidation. This method could be administered as an adjunct in the treatment of DM and its consequences.
It should be noted that Carpal Tunnel Syndrome (CTS) associated with DPN is common in individuals with DM. The therapeutic effect of ultrasound and laser therapy, i.e., an IR laser at a wavelength of 904 nm, had been accordingly explored by Ahmed et al. in CTS in type 2 diabetic patients with neuropathy. The study of finding had revealed that laser and ultrasound therapy could be both effective in treating these patients with mild-to-moderate CTS. After surgery, peripheral polyneuropathy could also occur in patients. The analgesic effect of laser therapy (660 nm) and LED therapy is thus confirmed for pain control in patients with high blood glucose (namely, type 1 DM) and normal blood glucose undergoing Coronary Artery Bypass Graft (CABG) through intrauterine vascular transplantation. Laser and LED therapy have further shown similar analgesic effects in patients with high blood glucose and normal blood glucose compared with controls.
Besides, nerve inflammation plays an important role in development and progression of neuropathic pain following Chronic Contraction Injury (CCI). For example, the study by Yueh-Ling Hsieh et al., had concluded that subcutaneous laser therapy on a damaged nerve could suppress inflammation induced by Tumor Necrosis Factor-alpha (TNF), Interleukin 1 beta (IL-1β) and Hypoxia-Inducible Factor 1-alpha (HIF-α) and consequently control neuropathic pain. Constant laser energy at a wavelength of 450 nm could be further effective in reducing the levels of ILs in diabetic rats. Laser therapy could also boost the levels of Vascular Endothelial Growth Factor (VEGF) and Nerve Growth Factor (NGF) in damaged nerves. Moreover, it could be a new strategy to improve neural tissue hypoxia by regulating and modulating HIF-1α activity. Laser therapy could thus mitigate the expression of the Epidermal Growth Factor Receptor (EGFR) gene and help develop neuropathy in patients with DM. In this sense, laser therapy could aid reduce some effects of DM via regulating the expression of the EGFR gene and the enzyme arginase.
Discussion
LLLT and diabetic wounds
Another common and significant consequence of uncontrolled DM is the development of diabetic ulcers, which typically occur in the legs and the toes and lead to amputations if left untreated. Nearly 15% of individuals with DM experience diabetic foot ulcers. In this respect, diabetic foot is defined as a complication of neuropathy or ischemia that induces chemical changes at the ends of the lower limbs, the legs and the toes. Diabetic wounds can additionally become infected as a result long-term loss of touch and pain. Therefore, success in treating such wounds is very important. One way to care for these wounds is to practice laser and LED treatment. The use of laser therapy is painless and it is considered as a kind of biological stimulant, that is, it accelerates tissue repair. Studies have further shown that laser therapy is effective in reducing edema and hypertension in the inflammatory process. Laser therapy also stimulates the proliferation of epithelial cells, osteoblasts and fibroblasts. It further induces collagen synthesis. As an example, in the study by Fonescs Santos et al., the effects of laser therapy on the healing process of chronic wound tissue in diabetic feet had been investigated, reporting that the effectiveness of laser therapy in short-term repair of chronic wound tissues in diabetic feet could be significant.
In the study by Dadpay et al., IR LLL radiation on open skin wound healing in diabetic rats with streptozotocin had been considered through biomechanical evaluation. In this respect, wound healing with this type of treatment had been significantly quickened by a pulsed IR laser in healthy and diabetic rats. Therefore, laser treatment might improve wounds caused by DM through increasing fibroblasts, enhancing endothelium of blood vessels, improving tensile strength, inducing collagen synthesis and reducing inflammatory response.
Likewise, the effect of laser therapy on pain and inflammation in a diabetic case with type 2 DM with diabetic dermopathy had been further studied by Hazari et al. and it had been observed that diabetic dermopathy could be more common in men aged over 50. In their study, laser treatment (at a wavelength of 830 nm for 9 minutes and 3 weeks) had been performed on the legs of a 62-year-old man and it had been concluded that laser therapy could be an effective treatment for diabetic wounds in dermopathy.
It is of note that inflammatory factors, such as TNF-α are produced in DM. These factors can promote destructive processes by generating inflammation. In addition to creating pre-inflammatory cytokines such as IL-6, TNF-α also plays a part in oxidative stress through the production of ROS. Cytokine IL-6 can correspondingly hinder the development of DM, causing damage to Endothelial Cells (ECs), which can be accompanied by the secretion of inflammatory factors. According to the study by Goralczyk et al. on the effect of LLL radiation at wavelengths of 635 nm and 830 nm in ECs isolated from the human umbilical cord, under conditions of hyperglycemia, the secretion of inflammatory factors such as TNF-α and IL-6 had been also investigated. In their study, the inverse effects of hyperglycemia on ECs had been observed following the application of laser therapy at a wavelength of 830 nm. Decreased TNF-α concentration had also accelerated cell proliferation. Diabetic wound healing could be justified by the positive effect of laser therapy on the production and proliferation of ECs.
Some patients with DM may be ill with aphthous stomatitis ulcers on their mouths and lips, whose causes are unknown. Laser therapy at a wavelength of 635 nm is thus an effective and non-invasive method in reducing the signs and symptoms of oral aphthous stomatitis. This method can be of importance and even effective in improving and treating recurrent oral aphthous stomatitis.
Macrophages in inflammatory tissues also produce nitric oxide gas through the nitric oxide synthase enzyme. Accordingly, synthesis of large amounts of this gas can play a role in creating nitrosative stress. Mitochondrial cytochrome oxidase is additionally involved in the mechanism of significant effects of laser therapy by absorbing red and IR light emitted by the laser. Several factors including energy duration, wavelength and power density, are thus of utmost importance in laser therapy. Laser therapy with a wavelength of 904 nm could also increase collagen synthesis and it has been proven effective in moderating oxidative and nitrosative stress in the injured skin of diabetic mice. It is noteworthy that optimal wavelengths are normally between 620 nm and 830 nm and LEDs are significant for irradiating surface wounds. These remarks are the results of the study by Tatmatsu- Rocha et al., reporting that the antioxidant effects of laser therapy, as a treatment for wounds such as foot ulcers and other abnormalities, could be beneficial.
LLLT and diabetic retinopathy
Diabetic retinopathy is a major complication of uncontrolled DM. This complication is an important cause of visual impairment and even blindness in people affected with DM worldwide. Retinopathy is thus a complication that influences about 10% of people with DM. It has been further reported that obesity and elevated lipid levels in the blood can be among risk factors for this condition. Increased oxidative stress in the retina also occurs due to DM. The role of oxidative stress has been accordingly reported in the progression of apoptosis in retinal capillary cells. In this line, Kowluru et al. had stated that mitochondrial superoxide dismutase could play an important protective role in the development of diabetic retinopathy. Reducing this antioxidant enzyme due to DM could thus contribute to the development of diabetic retinopathy.
There have been numerous investigations on the effects of laser therapy on retinopathy. In the study by Tang et al., the far-red light inhibitory effect on diabetic neuropathy in diabetic rats and cultured retinal cells had been researched. In their study, the beneficial effects of photobiomodulation (670 nm) on vascular and neural elements in the retina had been also observed. The results of their investigation had further revealed that photobiomodulation could reduce the damages of diabetic retinopathy in diabetic rats. In the retinopathy cell model, the effect of the photobiomodulation on those cells had also reduced oxidative stress and cell death. It should be noted that photobiomodulation does not alter the activity of cytochrome oxidase in the retina, but it inhibits the production of superoxide in diabetic rats and maintains the expression of the superoxide dismutase enzyme. This method can be used as a low-risk and inexpensive treatment to reduce the development of diabetic retinopathy.
It has been reported that laser therapy can be also helpful in managing diabetic macular edema. In the study on 64 patients with diabetic retinopathy, the effects of anti-VEGF drugs combined with laser therapy (532 nm) on retinopathy as well as their impacts on choroid thickness had been investigated. The efficacy of anti-VEGF drugs with laser therapy on diabetic retinopathy could be thus better than laser therapy alone. Evaluation of changes in patients with diabetic retinopathy following laser therapy and taking medications had been also evaluated based on the variations in the thickness of the retinal nerve endothelium in the macular area as well as the thickness of the choroid (Table 1).
| Laser type | Study model | Type of diabetes mellitus | Tissue or organ |
|---|---|---|---|
| He-Ne laser (632.8 nm, 10 mW) | Animal (rat) | Type 1 | Tibia bone |
| Infrared 890-nm | Animal (rat) | Type 1 | Skin |
| Helium-neon laser | Animal (rat) | Type 1 | Skin |
| Laser with multidiode cluster probe | Human | Type 2 | Foot |
| Laser at a wavelength of 660 ± 20 nm | Human | Type 2 | Foot |
| Laser at a wavelength of 904 nm | Animal (mouse) | Type 1 | Skin |
| Helium-neon (He-Ne) laser | Animal (rat) | Type 1 | Achilles tendon |
| Laser at a wavelength of 890 nm | Animal (rat) | Type 1 | Bone |
| Diode laser (50 mW, 660 nm, 4 J/cm2 | Animal (rat) | Type 1 | Skin |
| Gallium-Aluminum-Arsenium (GaAlAs) diode laser (635 nm) | Animal (rat) | Type 1 | Skin |
| Infrared diode laser (890 nm) | Animal (rat) | Type 1 | Skin |
| Laser at a wavelength of 632.8 nm | Human | Type 2 | Foot |
| Low-energy Ga-Al-As laser | Animal (rat) | Type 1 | Bone |
| Laser at a wavelength of 632.8 nm | Cell culture | Normal and high glucose mediums | Skin fibroblasts |
| Laser at a wavelength of 810 nm | Animal (rat) | Type 1 | Skin |
| GaAlAs laser (940 nm) | Animal (rat) | Type 1 | Skin |
| Laser at a wavelength of 890 nm | Animal (rat) | Type 1 | Skin |
| AlGaAlP laser (635 nm) and GaAlAs laser (830) | Cell culture | Hyperglycemia conditions | Endothelial cells (HUVEC line) |
| Helium-Neon Laser (632.8 nm) | Cell culture | Hyperglycemia conditions | Fibroblast cells |
| Laser at a wavelength of 830 nm | Human | Type 2 | Skin |
| Low-level laser (the wavelength was not expressed) | Human | Type 2 | Muscle |
| GaAlAs diode laser (980 nm) | Animal (rat) | Type 1 | Bone |
| Diode lasers (670 and 810 nm) | Animal (rat) | Type 1 | Skin |
| Laser at a wavelength of 810 nm | Human | Type 1 | Blood |
| Laser at a wavelength of 830 nm | Animal (mouse) | Type 1 | Skin |
| Diode laser (532, 633, 810 and 980 nm) | Animal (rat) | Type 1 | Skin |
| HeNe laser at 632.8 nm | Animal (rat) | Type 1 | Skin |
| Argon dye laser at a wavelength of 630 nm | Animal (mouse) | Type 1 | Skin |
| Visible to far infrared at wavelengths of 532, 633, 810, 980 and 10,600 nm and polychromatic LED clusters (510-872 nm, visible to infrared) | Animal (rat) | Type 1 | Skin |
| Laser at a wavelength of 685 nm | Human | Type 2 | Foot |
| Diode laser at a wavelength of 670 nm | Human | Type 1 | Foot |
| Laser at a wavelength of 790 nm | Animal (rat) | Type 1 | Skin |
| He-Ne laser (632.8 nm wavelength) | Animal (rat) | Type 1 | Skin |
| InGaAlP type diode laser at a wavelength of 660 nm | Animal (rat) | Type 1 | Skin |
| Laser at a wavelength of 632 nm | Animal (rat) | Type 2 | Skin |
| Low-level laser (the wavelength was not expressed) | Human | Type 1 and Type 2 | Eye |
| GaAlAs diode laser at a wavelength of 808 nm | Human | Type 2 | Tooth |
| N-YAG laser at a wavelength of 1064 nm | Human | Type 1 and Type 2 | Foot |
| Slit-lamp-based green laser at a wavelength of 532 nm | Human | Type 1 | Eye |
Table 1: A number of studies based on LLLT method on DM complications.
Conclusion
In this literature review, findings on the effects of LLLT in patients with DM were considered. Due to the effects of oxidative stress on the DM consequences, in this study, this issue was more focused. Aspects of light bio-stimulation and LLLT in the treatment and control of complications of DM have been considered in the studies. Given the precise manifestation of the molecular and biochemical mechanisms of LLLT, it is hoped that this treatment method will receive more attention in the future. The results of the study and review of various studies showed that LLLT as an adjunctive can be used in control and treatment of DM complications.
Acknowledgement
The authors have no acknowledgements to report.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Conflict of Interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
References
- Tatmatsu-Rocha JC, Ferraresi C, Hamblin MR, Maia FD, do Nascimento NR, Driusso P, et al. Low-level laser therapy (904 nm) can increase collagen and reduce oxidative and nitrosative stress in diabetic wounded mouse skin. J Photochem Photobiol B. 2016;164:96-102.
[Crossref] [Google Scholar] [PubMed]
- Ahmed OM, Mohamed T, Moustafa H, Hamdy H, Ahmed RR, Aboud E. Quercetin and low level laser therapy promote wound healing process in diabetic rats via structural reorganization and modulatory effects on inflammation and oxidative stress. Biomed Pharmacother. 2018;101:58-73.
[Crossref][Google Scholar] [PubMed]
- da Silva Tonetto L, da Silva CC, Gonzatti N, Guex CG, Hartmann DD, Boschi ES, et al. Effects of photobiomodulation on oxidative stress in rats with type 2 diabetes mellitus. Lasers Med Sci. 2023;38(1):90.
[Crossref] [Google Scholar] [PubMed]
- Ahmed SA, Ghoneim DF, Morsy ME, Hassan AA, Mahmoud AR. Low-level laser therapy with 670 nm alleviates diabetic retinopathy in an experimental model. J Curr Ophthalmol. 2021;33(2):143-151.
[Crossref] [Google Scholar] [PubMed]
- Martirosyan DM, Ashoori MR, Rezaeinezhad A, Jahanbakhshi F, Pezeshki S, Mikaeili AS, et al. Effects of quercetin and low-level laser on oxidative and inflammatory factors among patients with type 2 diabetes mellitus and mild cognitive impairment. Bioact Compounds Health Dis- Print. 2012;5(1):1-2.
- Frigero M, Dos Santos SA, Serra AJ, dos Santos Monteiro Machado C, Portes LA, Tucci PJ, et al. Effect of photobiomodulation therapy on oxidative stress markers of gastrocnemius muscle of diabetic rats subjected to high-intensity exercise. Lasers Med Sci. 2018;33:1781-1790.
[Crossref] [Google Scholar] [PubMed]
- Servetto N, Cremonezzi D, Simes JC, Moya M, Soriano F, Palma JA, et al. Evaluation of inflammatory biomarkers associated with oxidative stress and histological assessment of low‐level laser therapy in experimental myopathy. Lasers Surg Med. 2010;42(6):577-583.
[Crossref] [Google Scholar] [PubMed]
- Fillipin LI, Mauriz JL, Vedovelli K, Moreira AJ, Zettler CG, Lech O, et al. Low‐Level Laser Therapy (LLLT) prevents oxidative stress and reduces fibrosis in rat traumatized Achilles tendon. Lasers Surg Med. 2005;37(4):293-300.
[Crossref] [Google Scholar] [PubMed]
- Zhang H, Zhang C, Pan L, Chen Y, Bian Z, Yang Y, et al. Low-level Nd: YAG laser inhibiting inflammation and oxidative stress in human gingival fibroblasts via AMPK/SIRT3 axis. J Photochem Photobiol B Biology. 2024;251:112845.
[Crossref] [Google Scholar] [PubMed]
- Mansouri V, Arjmand B, Tavirani MR, Razzaghi M, Rostami-Nejad M, Hamdieh M. Evaluation of efficacy of low-level laser therapy. J Lasers Med Sci. 2020;11(4):369.
[Crossref] [Google Scholar] [PubMed]
- Anju M, Maiya AG, Hande M. Low level laser therapy for the patients with painful diabetic peripheral neuropathy-A systematic review. Diabetes Metab Syndr. 2019;13(4):2667-2670.
[Crossref] [Google Scholar] [PubMed]
- Piva JA, Abreu EM, Silva VD, Nicolau RA. Effect of low-level laser therapy on the initial stages of tissue repair: Basic principles. An Bras Dermatol. 2011;86:947-954.
[Crossref] [Google Scholar] [PubMed]
Citation: Ashoori MR, Mikaeili AS, Mirmiranpour H, Abdolahpour S (2024) Low-Level Laser Therapy: As a New Approach to Modulate Oxidative Stress in Diabetes Mellitus and its Complications. Diabetes Case Rep. 9:221.
Copyright: © 2024 Ashoori MR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.