Indexed In
- Open J Gate
- Academic Keys
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- Google Scholar
- SHERPA ROMEO
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 11, Issue 2
Long-Term Prognosis of AL Amyloidosis in Patients Treated with Bortezomib or Thalidomide
Yuemeng Sun1, Xiaoyang Yu2, Ping Lan2, Wanhong Lu2, Jiping Sun2, Dan Niu2, Yaning Hao2, Dapeng Hao2, Jing Lv2, Liyi Xie2* and Jie Feng2*2Department of Nephrology, The First Affiliated Hospital of Xi’an Jiao Tong University, Shaanxi Xi’an, China
Received: 20-Feb-2023, Manuscript No. JVMS-23-19970; Editor assigned: 23-Feb-2023, Pre QC No. JVMS-23-19970 (PQ); Reviewed: 16-Mar-2023, QC No. JVMS-23-19970; Revised: 23-Mar-2023, Manuscript No. JVMS-23-19970 (R); Published: 30-Mar-2023
Abstract
Introduction: AL amyloidosis has become a common secondary cause in elderly male patients presenting with nephrotic syndrome. Daratumumab-CyBorD regimen is approved as the standard first-line regimen for AL-amyloidosis. We aim to analyze the prognosis of patients treated with bortezomib-based regimen and other alternative regimens.
Method: We retrospectively collected the baseline and follow up data of newly diagnosed AL amyloidosis patients. Hematological and renal response rate were compared among different regimens and overall survival and renal survival were analyzed. Subgroup analysis of Mayo stage III patients was also performed.
Results: 72 cases were included, 48.6% of whom had cardiac involvement. Overall response rate in patients treated with bortezomib-based regimen was 67.4%, including 27.9% Complete Response (CR). Renal outcomes such as proteinuria response rate and incidence of End Stage Renal Disease (ESRD) were not different between bortezomib and thalidomide-based therapy.Median follow up for whole cohort was 22 months,and 13 (18.1%) patients died at the end of follow-up, while 7 patients (9.7%) progressed to dialysis. Median Overall Survival (OS) was not reached in both regimens and 1 year survival rates were 90.4% and 80.0% respectively in bortezomib and thalidomide based therapy (P=0.127). Patients treated with bortezomib-based treatment had Major Organ Deterioration Progression-Free Survival (MOD-PFS).There was no difference in response rate and OS between patients treated with Cyclophosphamide, Bortezomib,Dexamethasone (CVD) and Bortezomib, Dexamethasone (VD). Mayo stage III patients who were treated with VD regimen had longer OS compared with TD or CTD regimen.
Conclusion: Bortezomib-based regimen had high efficacy in promoting rapid hematologic responses and reducing the risk of major organ deterioration in AL amyloidosis, which was also effective among patients with cardiac insufficiency. Addition of cyclophosphamide into VD regimen could not further improve the overall remission or survival of AL amyloidosis.
Keywords
AL amyloidosis; Prognosis; Overall survival; Bortezomib; Thalidomide
Introduction
AL amyloidosis is a disease resulting from abnormal proliferation of plasma cells which forms amyloid deposition in multi-organ and leads to organ impairment. The incidence of AL amyloidosis has rapidly increased in recent years with the improvement in diagnostic methods, which has become one of the common secondary causes in elderly male patients presenting with nephrotic syndrome. With emergence of effective therapies, the median survival of AL amyloidosis patients has greatly prolonged to 36 months [1]. ANDROMEDA trial proved high efficacy of Daratumumab-CyBorD regimen in the treatment of AL-amyloidosis, which has been approved as the standard first-line regimen for AL-amyloidosis [2]. Considering Daratumumab is not available in some region, bortezomib-based regimen becomes an optional choice for the treatment of AL amyloidosis. Bortezomib combined with cyclophosphamide and dexamethasone (VCD regimen) was effective in the treatment for AL amyloidosis with median OS being 72 months [3]. Thalidomide had the advantages of low price and convenient oral administration compared with bortezomib, thus becoming another major alternative regimen for ASCT ineligible patients in our center. We included 72 newly diagnosed AL amyloidosis patients in the department of nephrology from a single center in China, and retrospectively analyzed the prognosis of patients under bortezomib and thalidomide-based treatment, in order to provide guidance for clinical decisions in AL amyloidosis.
Materials and Methods
Patients
The primary cohort was a retrospective series of 72 patients who were newly diagnosed with AL amyloidosis in the department of nephrology of a single center from January 2017 to December 2021. All patients included were diagnosed as kidney AL amyloidosis by kidney biopsy and other kidney diseases contributing proteinuria or kidney dysfunction were excluded. Diagnosis of AL amyloidosis and evaluation of involved organs was defined by the 2004 guideline [4]. Exclusion criteria include: 1) Secondary causes such as multiple myeloma and Waldenstrom macroglobulinemia; 2) AA amyloidosis or hereditary amyloidosis; 3) Limited amyloidosis with skin or carpal tunnel syndrome as the only involvement; 4) Presence of uncontrollable infection or active neoplastic disease.
Treatment
Patients were treated with bortezomib-based therapy in forms of VD or VCD regimen. VCD regimen was given as following: bortezomib 1.3 mg/m2 subcutaneously on days 1,8,15,21 (reduced to 1.0 mg/m2 in fragile patients); cyclophosphamide 200 mg intravenously every week (increased to 400 mg if tolerated); and dexamethasone 20 mg intravenously every week (increased to 40 mg if tolerated). Patients in thalidomide-based therapy were treated with CTD or TD regimen. CTD regimen was given as: Thalidomide with the starting dose of 50 mg/d gradually increased to 100 mg/d-200 mg/d, orally twice daily, dexamethasone 20-40 mg, and cyclophosphamide 200-400 mg, intravenously.
The choice between VD and VCD regimen was based on physician’s decisions, and similar supportive treatment was applied in each group. Patients received at least 6-8 treatment cycles until the achievement of CR or VGPR. Maintenance therapy for thalidomide was based on patient’s responses to therapy. For patients with persistent no remission or who had hematologic or organ progression, a second-line regimen was considered.
Prognosis evaluation
Patients were prospectively followed up every 3 months until end. The primary endpoint was to compare Overall Survival (OS) and Major Organ Deterioration Progression-Free Survival (MOD-PFS). Overall survival refers to the date of diagnosis until the date of death or last follow-up. Last follow-up time was May 2022. MOD-PFS refers to the composite endpoint from diagnosis of disease to the occurrence of any of the following events: death, development of heart failure, renal failure, or hematologic progression. Heart failure was defined as cardiac failure requiring repeated hospitalization due to worsening cardiac amyloidosis alone, or requiring a left ventricular assist device, intra-aortic balloon pump, or cardiac transplantation. Renal failure is defined as end-stage renal disease requiring maintenance renal replacement therapy or renal transplantation. Secondary endpoints include hematologic responses at 3 and 6 months and organ response. Hematologic and organ responses were evaluated by validated criteria [5]. Complete Remission (CR) required negative serum and urine immune fixation and a normal FLC ratio. Very Good Partial Response (VGPR) required dFLCs<40 mg/L, and Partial Remission (PR) defined as a decrease of dFLC ≥ 50%. Renal response is defined as 30% decrease in urine protein or a decrease to <0.5 g/d with <25% worsening of creatinine clearance compared to baseline. Cardiac response defined as serum NT-proBNP decreased >30% and >300 ng/L. Adverse events were evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 [6].
Statistic analysis
Continuous data were presented as mean ± SD (normally distributed data) or median with inter-quartile range (non-normally distributed data) and categorical data as frequency (%). Differences between groups were evaluated with Student t-test or ANOVA for normally distributed data, with Mann-Whitney U test or Kruskal-Wallis test for non-normally distributed data, and with chi-square (χ2)-test or Fisher exact for categorical data. Kaplan–Meiercurves were plotted and log-rank test was applied to make comparisons. Overall survival was calculated from the date of diagnosis tothe date of last follow-up or the date of death. Time to hematologic response was calculated from the date of diagnosis to the date of first response. Time to event was analyzed by Kaplan Meier analysis. Multivariate analysis was performed using Cox proportional hazards (“LR” method was applied). Cut off value was determined by the ROC curve. A two-sides value of P<0.05 was considered statistically significant. All statistical analysis was performed by SPSS for Windows version 25.0 (IBM SPSS Statistic 25.0, September 2017. IBM Corporation. Chicago, USA).
Results
72 patients were included with the average age of 60.3 years and 61.1% were male, of which all had renal involvement and 48.6% had cardiac amyloidosis, with 38.8% were at mayo stage III. 59 patients (81.9%) were alive at the last follow-up, with the median follow-up duration being 22 months. Median overall survival by Kaplan-Meier analysis for the whole cohort was not reached.1-year OS rate was 87.5%, and 79.3% of patients did not progress to MOD at 1 year (Figure 1).
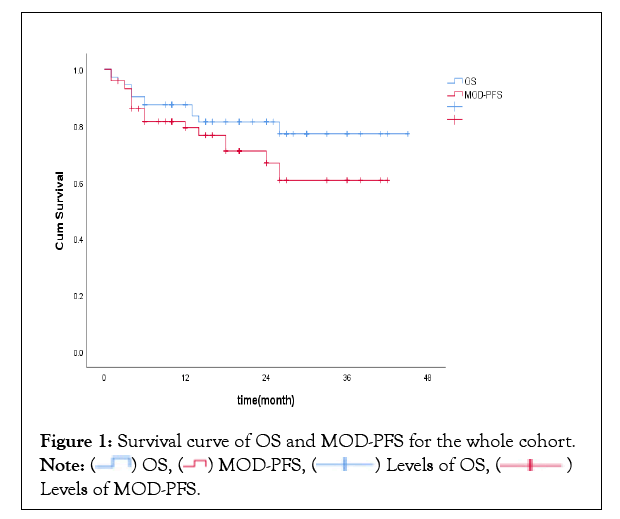
Figure 1: Survival curve of OS and MOD-PFS for the whole cohort.  Levels of MOD-PFS.
Levels of MOD-PFS.
Comparison between prognosis in Bortezomib and Thalidomide based regimen
Baseline characteristics were not statistically different between the 2 groups (Table 1). The median follow-up time was 18 months in bortezomib and 28 months in thalidomide. Patients under bortezomib-based regimen received median 4 cycles of treatment. Among those who received thalidomide-based therapy, 60% of patients under CTD treatment, others received TD regimen for at least 6 months. 3 patients treated with TD regimen then switched to VD or VCD as a second-line regimen due to no hematologic response or organ dysfunction progression. There were 6 patients in VCD regimen who received second-line therapy,of which 2 patients switched to CTD regimen, 2 patients received Lenalidomide, Dexamethasone (RD) or Pomalidomide, Dexamethasone (PD) regimen, and 2 patients under autologous stem cell transplantation after achieving VGPR.
| Bortezomib based regimen (n=52) | Thalidomide based regimen (n=20) | P-value | |
|---|---|---|---|
| Age | 59.7 ± 10.3 | 62.8 ± 6.9 | 0.523 |
| Male, n (%) | 30 (57.7%) | 14(70.0%) | 0.087 |
| Mayo stage III, | 19 (36.5%) | 9(45.0%) | 0.569 |
| Cardiac involved %, n (%) | 24 (46.2%) | 11(55.0%) | 0.448 |
| Involved organ >2, n (%) | 6 (12.2%) | 4(15.0%) | 0.882 |
| NT-pro BNP, pg/mL | 445 (145-2012) | 1296(165-4519) | 0.274 |
| cTnI, ug/L | 0.019 (0.010-0.045) | 0.028(0.010-0.082) | 0.444 |
| IVS,mm | 11 (10-12) | 12(8-15) | 0.985 |
| LVEF (%) | 66.5 (63.0-70.0) | 69.5(68.0-71.0) | 0.531 |
| Proteinuria, g/24 h | 4.4 (2.9-5.5) | 3.2(2.7-5.5) | 0.999 |
| Serum albumin, g/L | 23.7 ± 6.6 | 23.9 ±5.5 | 0.589 |
| Serum creatinine, umol/L | 55 (45-78) | 69(61-99) | 0.281 |
| eGFR,ml/min/1.73 m2 | 90.4 ± 37.2 | 80.1 ± 30.8 | 0.648 |
| dFLC (mg/L) | 73.1 (35.8-260.3) | 145.4(62.7-342.6) | 0.626 |
| M protein (g/L) | 7.3 (4.4-10.2) | 10.4(2.0-20.8) | 0.207 |
| Positive on IFE,n (%) | 20 (38.5%) | 10 (50.0%) | 0.547 |
| K type, n (%) | 7 (13.5%) | 2(10.0%) | 0.729 |
Table 1: Baseline characteristics of Bortezomib and Thalidomide-based regimen.
By intention-to-treat analysis,the overall hematologic response rate was 67.4% in bortezomib-based therapy, which was not statistically different compared to thalidomide-based regimen but complete responserate (27.9%) and overall responses at 3 months (51.2%) or 6 months (65.1%) were higher in patients treated with bortezomib-based regimen (Figure 2 and Table 2).
| Bortezomib-based regimen(n=52) | Thalidomide-based regimen(n=20) | P-value | |
|---|---|---|---|
| Follow up time(m) | 18 | 28 | 0.011 |
| Second-line therapy, n (%) | 6 (11.5%) | 3 (15.0%) | 1 |
| CR, n (%) | 12 (27.9%) | 0 | 0.015 |
| Overall response, n (%) | 29 (67.4%) | 7 (43.8%) | 0.061 |
| Overall response at 3 m, n (%) | 22 (51.2%) | 2 (11.8%) | 0.006 |
| Overall response at 6 m, n (%) | 28 (65.1%) | 5 (29.4%) | 0.013 |
| Time to response (m) | 3.0 (1.0-6.0) | 6.0 (4.3-15.0) | 0.134 |
| Renal response at 6 m, n (%) | 8 (15.4%) | 1 (5.0%) | 0.426 |
| Renal response at 12 m, n (%) | 15 (28.8%) | 3 (15.0%) | 0.224 |
| Progression to ESRD, n (%) | 3 (5.7%) | 4 (20.0%) | - |
| 1 year renal survival | 93.70% | 89.50% | 0.111 |
| Cardiac response, n (%) | 5 (20.8%) | 0 | 0.91 |
| MOD-PFS, n (%) | 9 (17.3%) | 10 (50.0%) | - |
| Median MOD-PFS (m) | NR | 18 | - |
| 1 year MOD-PFS | 86.20% | 64.20% | 0.026 |
| Death, n (%) | 6 (8.1%) | 7 (28.6%) | - |
| Median OS(m) | NR | NR | - |
| 1 year OS | 90.40% | 80.00% | 0.127 |
Note: 60 patients were available for hematologic response with dFLC>50 mg/L (43 in bortezomib based regimen and 17 in Thalidomide based regimen).
Table 2: Responses and prognosis in different groups.
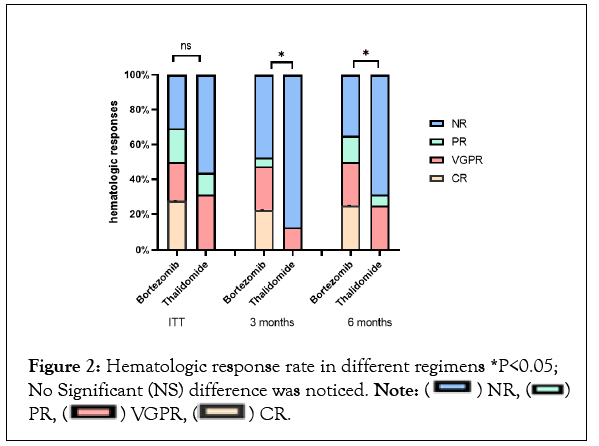
Figure 2: Hematologic response rate in different regimens *P<0.05; No Significant (NS) difference was noticed. Note:
Median OS was not reached in the bortezomib-based regimen and 1 year survival rate was 90.4%, with no difference compared to thalidomide-based regimen. However, MOD-PFS was significantly improved in the bortezomib-based regimen (1-year MOD-PFS was 86.2% and 64.2% respectively, P=0.026). The survival curve was shown in Figures 3a and 3b.
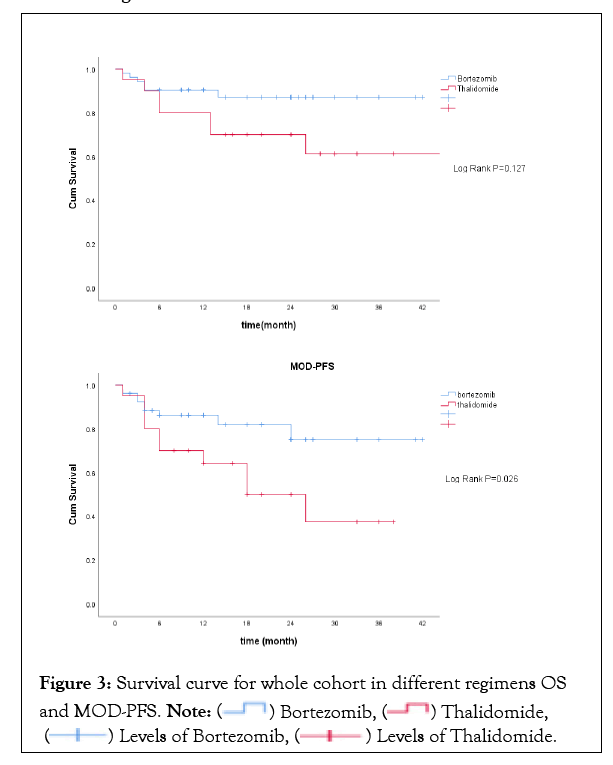
Figure 3: Survival curve for whole cohort in different regimens OS and MOD-PFS 
Patients receiving bortezomib therapy tended to achieve higher renal responses at 6 months and 12 months (15.4% and 28.8% respectively), but no statistical difference was found compared to thalidomide therapy (Figure 4). There was no significant difference between the two groups regarding the incidence of ESRD progression. By the end of follow-up, a total of 7 patients progressed to dialysis-dependent,and 1-yearrenal survival was 93.7% and 89.5% respectively in the bortezomib group and thalidomide group (P>0.05). The survival curve for renal survival was shown in Figure 5.
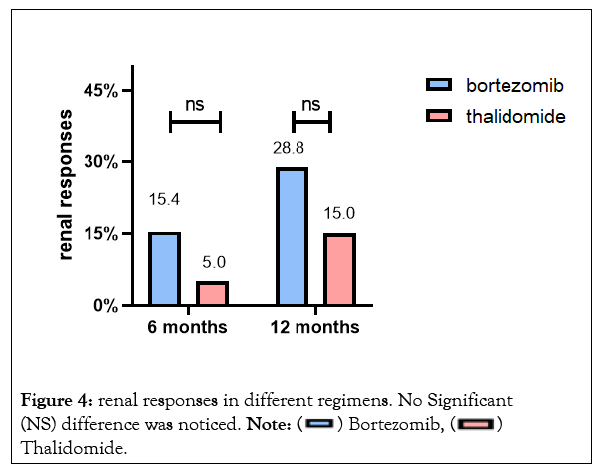
Figure 4: renal responses in different regimens. No Significant (NS) difference was noticed. Note: Thalidomide.
Thalidomide.
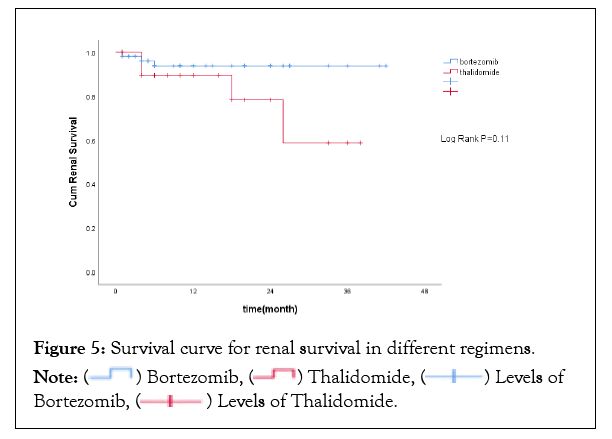
Figure 5: Survival curve for renal survival in different regimens. 
Comparison between prognosis in CVD and VD regimen
VD regimen was given in 36 patients and VCD in 16 patients. Patients were not matched for baseline characters but there were no statistical differences in baseline cardiac and renal function. No difference was noticed regarding to the numberof treatment cycles or bortezomib doses between VD and VCD group. Overall response rate was 83.3% and 61.3% respectively and higher VGPR rate was revealed in VCD regimen(83.3% vs. 38.7%, P=0.022). Theaddition of pulsed cyclophosphamide to VD regimen was not associated with longer OS or MOD-PFS (Supplementary Table 1).
Prognosis in Mayo stage III patients
Among 28 patients with Mayo stage III, 19 patients were treated with VD regimen and the other 9 patients were treated with TD or CTD regimen. No difference was noticed in overall response rates, but renal and cardiac response rates were higher in patients treated with bortezomib-based regimen (Supplementary Table 2). Median OS for all Mayo stage III patients was not reached with 1 year OS rate of 68.8%. 3 patients died within the first 3 months from initiation of therapy. Landmark analysis at 3 months revealed that Mayo stage III patientswho received VD regimen had improved overall survival, with 1-year survival rate being 86.7% compared with 62.5% inthe thalidomide-based regimen(P=0.048). Kaplan Meier survival curve for Mayo stage III patients who survived more than 3 months was shown in Figure 6.
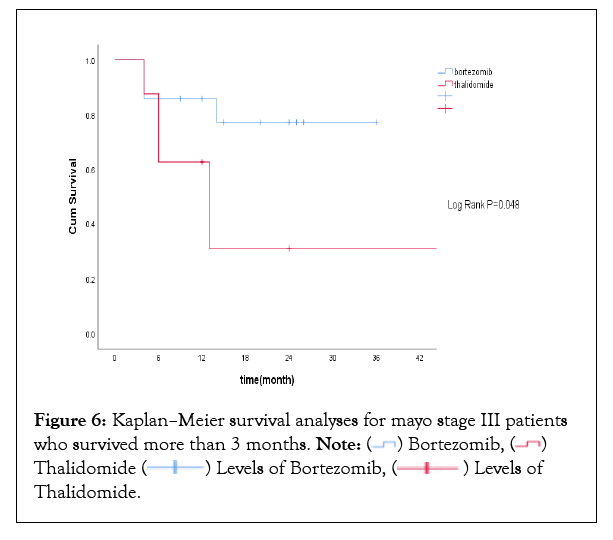
Figure 6: Kaplan–Meier survival analyses for mayo stage III patients who survived more than 3 months. Note:
 Thalidomide.
Thalidomide.
Adverse event
Incidence of all-grade adverse events for the whole cohort was 45.8%, of which 29.2% were >3 grade. Common adverse events include fluid retention,infection,and weakness. The incidence of hematological toxicity was low in both treatments.And higher proportion (30.0%) of patients in the Thalidomide-based regimen endured fluid retention compared to bortezomib (P<0.05), of which 5 patients needed CRRT to reduce volume overload (Supplementary Table 3).
Risk factors for overall survival
ROC curves were used to determine the cut-off values for the continuous variables, and the best predicted values were 1100 ng/L for NT-proBNP (area under the curve: 0.807, P=0.001), 0.0625 ng/ml for serum troponin T (area under the curve: 0.854, P<0.001), and 50 mg/L for serum dFLC (area under the curve: 0.612, P=0.021),ROC curves for different variables are shown in Supplementary Figure 1.
13 (18.1%) patients died during follow up. Risk factors for inferior OS by uni-variate analysis included involved organs ≥ 3,NT proBNP>1100 ng/L, troponin T>0.0625 ng/ml, IVS ≥ 13 mm, cardiac dysfunction progression, eGFR<50 ml/min/1.73 m2, dFLC>50 mg/l and Mayo stage III (compared to Mayo stage I),while achievement of VGPR was associated with reduced risk of death. Detailed results are shown in Table 3.
| Variables | Uni-variate | |
|---|---|---|
| HR(95% CI) | P-value | |
| age>70 | 1.32(0.30-5.81) | 0.713 |
| Involved organ ≥ 3 | 3.84(1.37-10.77) | 0.011 |
| dFLC>50 mg/l | 9.81(1.23-78.43) | 0.031 |
| NT-proBNP>1100 ng/L | 10.69(3.44-33.28) | <0.001 |
| cTnT>0.0625 ng/ml | 9.07(2.95-27.87) | <0.001 |
| IVS ≥ 13 mm | 3.57(1.06-12.03) | 0.04 |
| LVEF<50% | 4.87(0.98-24.18) | 0.053 |
| eGFR<50 ml/min/1.73 m2 | 3.08(1.14-8.35) | 0.018 |
| Urinary protein>5 g/24 h | 1.41(0.53-3.76) | 0.492 |
| VGPR response* | 0.23(0.07-0.70) | 0.01 |
| NT-proBNP increase>30%* | 3.13(1.12-8.71) | 0.029 |
| eGFR decrease>30%* | 1.50(0.43-5.18) | 0.524 |
| Urinary protein decrease>30* | 0.03(0.00-2.36) | 0.116 |
| Mayo stage I | Reference | |
| Mayo stage II | 1.43(0.13-16.00) | 0.765 |
| Mayo stage III | 10.40(2.32-46.48) | 0.002 |
Abbreviations: IVS: Interventricular Septum; LVEF: Left Ventricular Ejection Fraction; eGFR: Estimated Glomerular Filtration Rate; Mayo stage I: NT proBNP<332 ng/L and cTnT<0.035 μg/LcTnI<0.01 g/L; Mayo stage II: either NT proBNP>332 ng/L or cTnT>0.035 μg/L, cTnI>0.01 g/L; Mayo stage III: both NT proBNP>332 ng/L and cTnT>0.035 μg/L, cTnI>0.01 g/L.
Table 3: Uni-variate analysis of factors associated with OS.
Risk factors for renal survival
7 patients (9.7%) progressed to dialysis during follow-up. Progression to renal replacement therapy was used as the endpoint to analyze the risk factors for renal prognosis. By uni-variate analysis, baseline eGFR<50 ml/min/1.73 m2 ,eGFR decrease>30% ,and renal stage 2 or 3 (compared to renal stage 1) were associated with risk of renal failure.However, there was no difference in the risk of renal failure between bortezomib and thalidomide treatment (HR=0.33, 95%CI:0.06-1.65,P=0.175). Detailed results were shown in Table 4.
| Variables | Uni-variate | |
|---|---|---|
| HR(95%CI) | P-value | |
| age>70 | 1.59(0.33-7.67) | 0.562 |
| dFLC>50 mg/l | 2.87(0.59-13.85) | 0.189 |
| NT-proBNP>1100 ng/L | 3.04(0.63-14.65) | 0.165 |
| Urinary protein>5 g/24 h | 1.33(0.33-5.41) | 0.695 |
| Serum albumin<30 g/L | 2.28(0.58-8.89) | 0.235 |
| eGFR<50 ml/min/1.73 m2 | 6.46(1.86-22.50) | 0.003 |
| Renal response* | 0.29(0.00-9.72) | 0.233 |
| VGPR response* | 0.35(0.09-1.34) | 0.124 |
| eGFR decrease>30%* | 11.79(3.15-44.14) | <0.001 |
| Bortezomib regimen | 0.31(0.07-1.42) | 0.134 |
| Renal stage 1 | Reference | |
| Renal stage 2 | 6.81(1.25-37.17) | 0.027 |
| Renal stage 3 | 38.93(4.66-325.2) | 0.001 |
Abbreviations: VGPR: Very Good Partial Response; *Evaluated at 3 month. Renal stage 1: Urinary protein<5 g/24 h and eGFR>50 ml/min/1.73 m2 Renal stage 2: either urinary protein>5 g/24 h or eGFR<50 ml/min/1.73 m2 Renal stage 3: Urinary protein>5 g/24 h and eGFR<50 ml/min/1.73 m2.
Table 4: Uni-variate analysis of factors associated with renal survival.
Discussion
Our cohort proved the high efficacy of bortezomib in promoting rapid response and reducing the risk of cardiac and renal failure in AL amyloidosis. Furthermore, we revealed that VD regimen improved the survival of Mayo stage III patients who survived over 3 months. The innovation of this study was that as a nephrology department we focused on the kidney outcome among different regimens and our results indicated no improvements regarding to urine protein remission rates and the incidence of ESRD in bortezomib based therapy compared to thalidomide. In addition, we compared efficacy of doublet and triplet regimen in bortezomib based therapy and concluded that the addition of cyclophosphamide to VD regimen did not further improve OS or MOD-PFS in AL amyloidosis patients.
Daratumumab-CyBorD regimen was approved for first-line treatment for newly diagnosed AL amyloidosis with satisfactory results of ANDROMEDA trial, which presented high CR rate and long MOD-PFS. Due to the absence of Daratumumab in some region, VCD regimen became the alternative regimen in our department, which was also effective in reducing circulating free light chain concentrations and promoting organ responses. Previous studies showed an overall response rate of 60%-94% for VCD regimen, which was reduced in this study [2,7-9]. Possible explanations may be that lower doses of bortezomib and less treatment cycles were applied in some fragile patients. Another possible reason might be that we did not exclude patients with heart failure and renal insufficiency. Besides, we proved the safety of the bortezomib regimen with the incidence of grade 3 adverse events being 26.9%. Among all AEs, pulmonary and gastrointestinal infections were most common in VD or VCD regimen, followed by herpes zoster and volume retention, which differed from previous reports that peripheral neuropathy was common AE with bortezomib [10]. We speculatedthat the low incidence of peripheral neuropathy may be due to bortezomib being administered by subcutaneous injection and the low cumulative dose in our study.
According to previous research, overall responses in TD regimen were 48% and 69% in CTD regimen in AL amyloidosis patients, which was comparable to the Melphalan Dexamethasone (MD) regimen [11,12]. CP Venner compared the efficacy of CVD and CTD therapy in 138 newly diagnosed AL amyloidosis patients and revealed no statistical difference in overall remission rate (79.7% vs. 71.0%),but a higher complete remission rate (40.5% vs. 24.6%) and prolonged progression-free survival (28 months vs. 14 months) were found in VCD regimen. Similarly, in our cohort, no difference was noticed in the overall response rate but complete remission and early remission at 3 months and 6 months were significantly higher in bortezomib-based therapy [13]. Regarding to overall survival, our results showed that thalidomide-based therapy was as effective as bortezomib in improving OS. Furthermore, thalidomide-based regimen had the advantage of being less expensive and easy to administer as oral medication, making it one of the optional treatments for AL amyloidosis.
No agreement was settled on whether the addition of cyclophosphamide to VD regimen had superior efficacy. Our results were consistent to Kastritis,which revealed no significant differences in remission rate and overall survival between VD regimen and VCD regimen [14]. In our cohort, choices of treatment between VD or VCD regimen were based on physician’s decision, therefore the patients in VCD group were younger, had less severe organ dysfunction to tolerate cyclophosphamide treatment, which were also predictions for a better prognosis for VCD patients. However, we found no further improvement in OS and MOD-PFS on the condition of better performance at baseline with VCD regimen. This finding had practical implications for reducing costs and avoiding toxic effects associated with cyclophosphamidesuch as infections and hemorrhagic cystitis. Nonetheless, we admitted that the discrepancy in bortezomib doses and treatment cycles between VD and VCD regimen could be confounding factors for prognosis analysis. Prospective, randomized trials are needed to further confirm the differences in efficacy between VD and VCD regimens. Besides, addition of Melphalan to VD regimen could overcome the negative impact of thalidomide abnormalities on remission rate,which might further improve the prognosis of AL amyloidosis [15].
The overall response rate in Mayo stage III patients under bortezomib-based regimen was 52.9% in our cohort, lower than Jaccard’s research which revealed the overall response rate being 68% in Mayo stage III patients [16]. The fact that we did not exclude patients at mayo stage IIIb or those who received only one cycle of therapy because of poor tolerance may account for the low response rate. AL amyloidosis patients with severe cardiac involvement had high mortality rate. Basset, et al., included 249 patients at mayo stage IIIb and 84% of them died during 52 months follow-up, with median overall survival being 4.2 months [17]. General chemotherapy proved less effective in improving OS and quality of life in these high risk patients. Wechalekar enrolled 346 cases of AL amyloidosis patients at Mayo stage III who were mainly treated with melphalan and thalidomide, and showed that hematologic response was 40% with a 1-year survival rate being 46% [18]. Other options such as lenalidomide combined with Dexamethasone (RD) were also applied in high-risk patients with a 1-year survival rate being 40% [19]. On the other hand, studies revealed prolonged survival in mayo stage IIIb patients if they achieved rapid hematologic responses and cardiac responses in first 3 months [17,20]. Bortezomib based therapy had the potential to improve OS of AL amyloidosis patients with severe cardiac involvement Palladini G showed that patients with severe cardiac insufficiency treated with MVD regimen (Melphalan,Bortezomib, Dexamethasone) had longer median OS (20 months) compared to MD regimen [21,22,23]. Similarly, our cohort proved Bortezomib-based regimen significantly improve OS of mayo stage III patients who survived more than 3 months, with 1 year survival rate being 86.7% in VD regimen. However, a major limitation of low proportion of cardiac involvement and short follow up duration in our cohort may explain for the better prognosis. Additionally, our results did not observe the cardiac response in Mayo stage III patients treated with thalidomide, possibly due to the potential cardio-toxicity of thalidomide, similar to the previous study which showed poor tolerance to thalidomide treatment in cardiac AL amyloidosis [24].
Cardiac involvement is the most important risk factor for OS in AL amyloidosis patients.However, an increased level of NT-proBNP could also be seen in patients with renal insufficiency or treated with drugs such as thalidomide and lenalidomide [25]. It was suggested that Mayo stage was not directly applicable for patients in renal failure [26]. Due to a low proportion of patients with severe kidney dysfunction in this research, and a high threshold value for serum NT-proBNP in prognosis evaluation, we found serum NT-proBNP was still helpful to predict OS in our cohort. Besides, echocardiography is useful in evaluation of cardiac function such as Interventricular Septum (IVS) thickness, Left Ventricular Ejection Fraction (LVEF) and electrocardiographic parameters were reported to be associated with the extent of myocardial impairment and overall survival in patients with AL amyloidosis [27-29]. In line with previous findings, we found that patients who presented with reduced LVEF and increased IVS thickness had worse overall survival.
Kidney involvement in AL amyloidosis is common in Chinese population, and those who progressed to end-stage renal disease had inferior OS. Palladini established Renal stage to evaluate renal prognosis which combines baseline urine protein levels with glomerular filtration rate [30]. We validated the prognostic role of Renal stage in evaluation the risk of ESRD in our cohort. In addition, Rubinstein found that the semi-quantitative area of amyloid deposition in the kidney was positively associated with the risk of ESRD progression [31]. Therefore, mass amyloid deposition in kidney and chronic pathological lesions such as interstitial fibrosis could also be risk factors for renal failure. However, incomplete pathological data for quantity of amyloid deposition prevented us to make further analysis and further efforts are needed to refine the research. Quiet a few studies had focused on the renal prognosis with different treatments. Kastritis suggested that among patients with renal stage 2-3, bortezomib could reduce the risk of renal failure compared to lenalidomide [32]. However, our results did not reveal a reduced risk of ESRD with bortezomib based regimen compared to thalidomide, possibly due to lower doses in the bortezomib regimen and the limited samples or a relative shorter follow-up duration in this cohort. Application of standard treatment protocols and longer follow-up duration are needed to verify the advantage of bortezomib in improving renal prognosis.
Major limitation of this study was that baseline characters and bortezomib doses or regimen cycles in each group could not be fully matched due to the nature of retrospective studies, and randomized controlled trials are needed. Secondly, limited endpoints event such as death and dialysis was observed at the end of this cohort, preventing us to make strong conclusion for factors predicting OS and renal survival. Thirdly, selecting bias of patients inclusion could not be avoided, since this study was conducted at department of nephrology, resulting in a lower rates of cardiac involvement and better prognosis of whole cohort. Besides, we did not take into account kidney pathological changes into renal prognosis because of the incomplete pathological data, which was also important factors for kidney outcome.
Conclusion
Bortezomib-based regimen had superiority over thalidomide in promoting rapid hematologic responses and reducing the risk of cardiac and renal deterioration in AL amyloidosis. VD regimen could effectively improve the OS of high risk patients who were at mayo stage III and survived over 3 months. The addition of cyclophosphamide into VD regimen could not further improve the overallremission or survival of AL amyloidosis patients.
Declarations
Acknowledgement
Thanks for Jie Feng and Liyi Xie for providing writing ideas and suggestions on paper revision, thanks Ping Lan, Xiaoyang Yu, Jie Feng, Dapeng Hao at Department of Nephrology for providing pathological suggestions.
Ethical approval and consent to participate
All patients have given their written informed consent and that the study protocol was approved by ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University (No: XJTU1AF2021LSK-033). All methods were carried out in accordance with relevant guidelines and regulations..
Conflict of interest statement
The authors have no conflicts of interest to declare.
Funding sources
There was no funding in this study..
Author contributions
Research idea and study design: Yuemeng Sun, Jie Feng, Liyi Xie; data acquisition, data analysis/interpretation.
Statistical analysis: Yuemeng Sun, Xiaoyang Yu; Biopsy performance.
Pathology analysis: Ping Lan, Wanhong Lu, Jie Feng, Dapeng Hao; Jing Lv, Jiping Sun, Dan Niu, Yaning Hao.
Supervision or mentorship: Wanhong Lu, Liyi Xie.
Data availability statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.Further enquiries can be directed to the corresponding author.
References
- Huang X, Wang Q, Jiang S, Chen W, Zeng C, Liu Z. The clinical features and outcomes of systemic AL amyloidosis: A cohort of 231 Chinese patients. Clin Kidney J. 2015;8(1):120-126.
[Crossref] [Google Scholar] [Pubmed]
- Kastritis E, Palladini G, Minnema MC, ANDROMEDA Trial Investigators. Daratumumab-Based Treatment for Immunoglobulin Light-Chain Amyloidosis. N Engl J Med. 2021;385(1):46-58.
[Crossref] [Google Scholar] [Pubmed]
- Manwani R, Cohen O, Sharpley F, Mahmood S, Sachchithanantham S, Foard D, et al. A prospective observational study of 915 patients with systemic AL amyloidosis treated with upfront bortezomib. Blood. 2019;134(25):2271-2280.
[Crossref] [Google Scholar] [Pubmed]
- Gertz MA, Comenzo R, Falk RH, Fermand JP, Hazenberg BP, Hawkins PN, et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): A consensus opinion from the 10th international symposium on amyloid and amyloidosis, tours, France, 18–22 April 2004. Am J Hematol. 2005;79:319–328.
[Crossref] [Google Scholar] [Pubmed]
- Palladini G, Dispenzieri A, Gertz MA. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: Impact on survival outcomes. J Clin Oncol. 2012;30(36):4541-4549.
[Crossref] [Google Scholar] [Pubmed]
- The clinical features and outcomes of systemic AL amyloidosis: A cohort of 231 Chinese patients
- Kastritis E, Anagnostopoulos A, Roussou M, Toumanidis S, Pamboukas C, Migkou M, et al. Treatment of light chain (AL) amyloidosis with the combination of bortezomib and dexamethasone. Haematologica. 2007;92(10):1351-1358.
[Crossref] [Google Scholar] [Pubmed]
- Palladini G, Sachchithanantham S, Milani P, Gillmore J, Foli A, Lachmann H, et al. A European collaborative study of cyclophosphamide, bortezomib, and dexamethasone in upfront treatment of systemic AL amyloidosis. Blood. 2015;126(5):612-615.
[Crossref] [Google Scholar] [Pubmed]
- Venner CP, Lane T, Foard D, Rannigan L, Gibbs SD, Pinney JH, et al. Cyclophosphamide, bortezomib, and dexamethasone therapy in AL amyloidosis is associated with high clonal response rates and prolonged progression-free survival. Blood. 2012;119(19):4387-4390.
[Crossref] [Google Scholar] [Pubmed]
- Reece DE, Hegenbart U, Sanchorawala V, Merlini G, Palladini G, Bladé J, et al. Efficacy and safety of once-weekly and twice-weekly bortezomib in patients with relapsed systemic AL amyloidosis: results of a phase 1/2 study. Blood. 2011;118(4):865-873.
[Crossref] [Google Scholar] [Pubmed]
- Palladini G, Perfetti V, Perlini S, Obici L, Lavatelli F, Caccialanza R, et al. The combination of thalidomide and intermediate-dose dexamethasone is an effective but toxic treatment for patients with primary amyloidosis (AL). Blood. 2005;105(7):2949-2951.
[Crossref] [Google Scholar] [Pubmed]
- Liu B, Wang Y, Bai M, Wang D, Zhao J, Zhang M, et al. Cyclophosphamide + thalidomide + dexamethasone versus melphalan + dexamethasone for the treatment of amyloid light-chain amyloidosis with kidney involvement: A retrospective study in chinese patients. Clin Ther. 2019;41(6):1186-1198.
[Crossref] [Google Scholar] [Pubmed]
- Venner CP, Gillmore JD, Sachchithanantham S, Mahmood S, Lane T, Foard D, et al. A matched comparison of cyclophosphamide, bortezomib and dexamethasone (CVD) versus risk-adapted cyclophosphamide, thalidomide and dexamethasone (CTD) in AL amyloidosis. Leukemia. 2014;28(12):2304-2310.
[Crossref] [Google Scholar] [Pubmed]
- Kastritis E, Gavriatopoulou M, Roussou M. Addition of cyclophosphamide and higher doses of dexamethasone do not improve outcomes of patients with AL amyloidosis treated with bortezomib. Blood Cancer J. 2017;7(6):e570.
[Crossref] [Google Scholar] [Pubmed]
- Kastritis E, Leleu X, Arnulf B, Zamagni E, Cibeira MT, Kwok F, et al. Bortezomib, Melphalan, and Dexamethasone for light-chain amyloidosis. J Clin Oncol. 2020;38(28):3252-3260.
[Crossref] [Google Scholar] [Pubmed]
- Jaccard A, Comenzo RL, Hari P, Hawkins PN, Roussel M, Morel P, et al. Efficacy of bortezomib, cyclophosphamide and dexamethasone in treatment-naïve patients with high-risk cardiac AL amyloidosis (Mayo Clinic stage III). Haematologica. 2014;99(9):1479-1485.
[Crossref] [Google Scholar] [Pubmed]
- Basset M, Milani P, Foli A, Nuvolone M, Benvenuti P, Nanci M, et al. Early cardiac response is possible in stage IIIb cardiac AL amyloidosis and is associated with prolonged survival. Blood. 2022;140(18):1964-1971.
[Crossref] [Google Scholar] [Pubmed]
- Wechalekar AD, Schonland SO, Kastritis E, Gillmore JD, Dimopoulos MA, Lane T, et al. A European collaborative study of treatment outcomes in 346 patients with cardiac stage III AL amyloidosis. Blood. 2013;121(17):3420-3427.
[Crossref] [Google Scholar] [Pubmed]
- Kumar SK, Hayman SR, Buadi FK, Roy V, Lacy MQ, Gertz MA, et al. Lenalidomide, cyclophosphamide, and dexamethasone (CRd) for light-chain amyloidosis: long-term results from a phase 2 trial. Blood. 2012;119(21):4860-4867.
[Crossref] [Google Scholar] [Pubmed]
- Manwani R, Foard D, Mahmood S. Rapid hematologic responses improve outcomes in patients with very advanced (stage IIIb) cardiac immunoglobulin light chain amyloidosis. Haematologica. 2018;103(4):e165-e168.
[Crossref] [Google Scholar] [Pubmed]
- Feng J, Zhang C, Shen K, Sun J, Fang Q, Zhang L, et al. Outcome of cardiac light-chain amyloidosis in the era of novel therapy: A single-center cohort study of 227 patients. Circ J. 2019;83(4):775-782.
[Crossref] [Google Scholar] [Pubmed]
- Shen KN, Zhang CL, Tian Z, Feng J, Wang YN, Sun J, et al. Bortezomib-based chemotherapy reduces early mortality and improves outcomes in patients with ultra-high-risk light-chain amyloidosis: a retrospective case control study. Amyloid. 2019;26(2):66-73.
[Crossref] [Google Scholar] [Pubmed]
- Palladini G, Milani P, Foli A, Vidus Rosin M, Basset M, Lavatelli F, et al. Melphalan and dexamethasone with or without bortezomib in newly diagnosed AL amyloidosis: A matched case-control study on 174 patients. Leukemia. 2014;28(12):2311-16.
[Crossref] [Google Scholar] [Pubmed]
- Palladini G, Russo P, Lavatelli F, Nuvolone M, Albertini R, Bosoni T, et al. Treatment of patients with advanced cardiac AL amyloidosis with oral melphalan, dexamethasone, and thalidomide. Ann Hematol. 2009;88(4):347-350.
[Crossref] [Google Scholar] [Pubmed]
- Tapan U, Seldin DC, Finn KT, Fennessey S, Shelton A, Zeldis JB, et al. Increases in B-type natriuretic peptide (BNP) during treatment with lenalidomide in AL amyloidosis. Blood. 2010;116(23):5071-5072.
[Crossref] [Google Scholar] [Pubmed]
- Merlini G, Wechalekar AD, Palladini G. Systemic light chain amyloidosis: an update for treating physicians. Blood. 2013;121(26):5124-5130.
[Crossref] [Google Scholar] [Pubmed]
- Agrawal T, Nagueh SF. Echocardiographic assessment of cardiac amyloidosis. Heart Fail Rev. 2021.
[Crossref] [Google Scholar] [Pubmed]
- Boldrini M, Cappelli F, Chacko L. Multiparametric echocardiography scores for the diagnosis of cardiac amyloidosis. JACC Cardiovasc Imaging. 2020;13(4):909-920.
[Crossref] [Google Scholar] [Pubmed]
- Li H, Wang Y, Lan P, Xie L, Zhao Y, Lu W, et al. Electrocardiographic parameters and prognosis of renal light chain amyloidosis. Clin Cardiol. 2020;43(10):1160-1166.
[Crossref] [Google Scholar] [Pubmed]
- Palladini G, Hegenbart U, Milani P, Kimmich C, Foli A, Ho AD, et al. A staging system for renal outcome and early markers of renal response to chemotherapy in AL amyloidosis. Blood. 2014;124(15):2325-2332.
[Crossref] [Google Scholar] [Pubmed]
- Rubinstein S, Cornell RF, Du L, Concepcion B, Goodman S, Harrell S, et al. Novel pathologic scoring tools predict end-stage kidney disease in light chain (AL) amyloidosis. Amyloid. 2017;24(3):205-211.
[Crossref] [Google Scholar] [Pubmed]
- Kastritis E, Gavriatopoulou M, Roussou M, Migkou M, Fotiou D, Ziogas DC, et al. Renal outcomes in patients with AL amyloidosis: Prognostic factors, renal response and the impact of therapy. Am J Hematol. 2017;92(7):632-639.
[Crossref] [Google Scholar] [Pubmed]
Citation: Sun Y, Yu X, Lan P, Lu W, Sun J, Niu D, et al (2023) Long-Term Prognosis of AL Amyloidosis in Patients Treated with Bortezomib or Thalidomide. J Vasc Surg. 11:508.
Copyright: © 2023 Sun Y, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

