Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 14, Issue 6
Long Term Immunity Duration against Peste des Petits Ruminants Mediated by an Inactivated Vaccine in Sheep and Goats
Zahra Bamouh1, Fatima-Zohra Fakri1, Soufiane Elmejdoub1, Amal Elarkam1, Lamya Rafi1, Khalid Omari Tadlaoui1, Douglas M Watts2* and Mehdi Elharrak12Department of Biological Sciences, University of Texas at El Paso, El Paso, Texas 79968, USA
Received: 29-May-2023, Manuscript No. JVV-23-21516; Editor assigned: 31-May-2023, Pre QC No. JVV-23-21516 (PQ); Reviewed: 14-Jun-2023, QC No. JVV-23-21516; Revised: 22-Jun-2023, Manuscript No. JVV-23-21516 (R); Published: 30-Jun-2023, DOI: 10.35248/2157-7560.23.14.535
Abstract
Small Ruminant Morbillivirus (SRMV) or Peste des Petits Ruminants (PPR) is caused by Peste des Petits Ruminants Virus (PPRV) of the Paramyxoviridae family. As one of the most contagious transboundary disease that affects sheep, goats and wild ruminants, the disease has a devastating impact on the livelihood of small farmers in enzootic countries. Efforts to control and prevent Peste des Petits Ruminants based on inactivated vaccines have rarely been investigated. In this study, we developed an inactivated oily adjuvanted vaccine, using a virulent field isolate, and evaluated the safety and efficacy in goats and sheep by challenge of goats and serological monitoring of the antibody response in sheep during 2 years. The vaccine was completely safe and induced a significant neutralizing antibody response starting on day 7 post vaccination, and reached 100% seroconversion at day 14 with a marked booster effect. The immunological response lasted for at least 12 months in sheep while goats showed full protection against challenge. The inactivated vaccine may represent a valuable tool for the prevention of PPR that avoids the safety and thermos-sensitivity associated with live vaccines.
Keywords
Peste des Petits Ruminants Virus (PPRV); Small Ruminant Morbillivirus (SMRV); Inactivated vaccine; Immunity duration
Introduction
Small Ruminant Morbillivirus (SRMV) or Peste des Petits Ruminants Virus (PPRV) is one of the most contagious transboundary viral disease affecting sheep, goats and wild ruminants [1-3]. SRMV causes a devastating impact on small farmers’ livelihood in endemic countries [4-7]. The disease is caused by Peste des Petits Ruminants Virus (PPRV) that belongs to the Morbillivirus genus of the Paramyxoviridae family [8-10]. Clinically, the disease is characterized by high fever, catarrhal inflammation of ocular and nasal mucous that progresses to an oculo-nasal discharge and mucopurulent, dyspnea and profuse diarrhea at the terminal stage. The infection leads to up-to 100% morbidity and 90% mortality rates in non-immunized sheep and goat populations [11,12]. Since the first outbreak in Ivory Coast in 1942, PPR distribution has expanded and the disease is currently enzootic in large regions of Africa, Middle East, Central, South and East Asia [13].
The control of PPR relies on vaccination, which has been based on live attenuated vaccines for decades [14,15]. However, live vaccines present several limitations due to thermo-sensitivity, risk of extraneous agent transmission, potential reversion to virulence and virus shedding. Inactivated vaccines may represent a good alternative, through their safety and stability in the warm tropical environments and in disease free or at risk countries to prevent the introduction and spread of the PPRV. In this study, we developed an inactivated vaccine using a local PPRV isolate and evaluated its safety and efficacy in sheep and goats by challenge studies and monitoring of the serological response for up to 2 years Post Vaccination (PV).
Materials and Methods
Cells and virus production
Vero cells (African green monkey kidney cells, ATCC No.CCL-81) were used for vaccine production and testing. Cells were grown on Dulbecco’s-modified Eagle medium (DMEM) supplemented with 5% fetal calf serum, 2 mM l-glutamine and 1 mM sodium pyruvate.
The virulent PPRV strain used was isolated from tissues of an infected lamb that had clinical manifestation of PPR disease during an outbreak in 2015 in Morocco. The isolate belongs to lineage IV (KY197740) and was recovered from infected tissues of the lobar pneumonia lungs and enlarged mesenteric lymph nodes using qPCR a threshold cycle (Ct) value of 20 [16]. Inoculation of Vero cells was carried out using a multiplicity of infection of 0.01, in DMEM with 1% fetal calf serum. Virus titration to determine the titer was then performed according the method described in the OIE Terrestrial Manual [13].
Virus inactivation
The virus inactivation was performed using Binary Ethyleneimine (BEI) with an optimal concentration of 1 mM. The inactivation rate was determined from the infectious titers by calculating the regression coefficient, which allow to fix the inactivation end point. An appropriate volume of BEI was added to the live virus suspension, aliquots were homogenized at 37°C during 3 hours, with regular sampling at 30 minutes intervals to determine the inactivation kinetic. Sodium thiosulphate was added at a final concentration of 2% (v/v) to neutralize the BEI. The total inactivation was confirmed by 3 blind passages of the viral suspension on Vero cells.
Vaccine preparation
The inactivated PPRV antigen was mixed with an oily adjuvant (MontanideTM, Seppic) to prepare an Oil-in-Water (O/W) emulsion. Two formulations were prepared targeting two vaccine doses: 105.0 and 106.0 TCID50 infectious titre before inactivation. The final vaccine was tested for emulsion stability, sterility and was stored at 5°C until use.
Animals
Animal experiments were carried out in accordance with the international guidelines for care and handling of experimental animals. The study protocol was approved by the Internal Laboratory Ethic Committee of MCI Santé Animale (Protocol number RD1V1531). The care and use of experimental animals complied with animal welfare laws, guidelines and policies.
Twenty local Sardi sheep and six Alpine goats were used in this study, both breeds are known to be susceptible to PPRV infection [17,18]. Animals were 6 months old and tested negative for PPRV antibody by cELISA. Animals were keept in experimental farm for two weeks to acclimatize and were monitored daily for body temperature and general health conditions prior to use in the experiments. Animals were divided into 5 groups (G) to test safety, immunogenicity and efficacy by challenge test of the inactivated PPR vaccine as described in Table 1.
| Groups | Species | Test | Categories | Number of animals | Vaccine dose | Vaccination program |
|---|---|---|---|---|---|---|
| 1 | Sheep | Immunity duration | Vaccinated | 8 | 105.0 TCID50 | D0, D28 and 12 months |
| 2 | 8 | 106.0 TCID50 | ||||
| 3 | Unvaccinated | 4 | - | - | ||
| 4 | Goats | Challenge | Vaccinated | 4 | 105.0 TCID50 | D0 and D28 |
| 5 | Unvaccinated | 2 | - | - |
Table 1: The groups of animals used in this study.
Immunogenicity study
Groups 1 and 2, including eight sheep each that were vaccinated with the PPR inactivated vaccine at a dose of 105.0 and 106.0 TCID50, respectively. Vaccination consisted of two administrations of 1 ml by subcutaneous route behind the elbow at 4 weeks interval (at D0 and D28). Both groups received an annual booster at 12 months, same dose and administration route. Group 3 of four sheep was inoculated with Phosphate- Buffered Saline (PBS) for use as controls and housed in the same pen as the vaccinated animals. Sheep were monitored for general health, clinical symptoms, hyperthermia and local inflammation at the inoculation site during 14 days following each vaccination.
Sera were collected weekly until day 42 post vaccination and monthly for twenty-four month PV to determine the duration of the antibody response. Antibody response was monitored by Serum virus Neutralization (SNT) and Enzyme-Linked Immunosorbent Assay (ELISA). SNT was performed in 96- microwell plates. The test is based on a serial 1/4 dilutions of heat inactivated sera mixed with the PPR infectious virus (100 TCID50), plates were incubated for 7 days at 37°C under 5% CO2. The neutralizing antibody titre was calculated following the Reed and Muench method [19]. ELISA test was performed using cELISA kit based on N protein (ID-vet, PPRC-4P).
Vaccine potency in goats
The experiment was performed under biosafety level 3 containment. Group 4 of four goats was vaccinated with the PPR inactivated vaccine at a dose of 105.0 TCID50, with a volume of 1 ml by subcutaneous route behind the elbow at day 0 and boosted at D28. Group 5 of two goats was kept unvaccinated as control. Animals were monitored for clinical signs, hyperthermia and local inflammation at the inoculation site during 14 days following each vaccination.
The efficacy of the PPR inactivated vaccine was evaluated by challenge of goats with a virulent strain of PPRV. Two weeks following the second vaccination (at D42 pv), vaccinated and unvaccinated goats of groups 4 and 5, were challenged with the virulent PPRV strain (PPRV/MOR/2015). This PPRV strain was confirmed to induce typical symptoms of the PPR disease. Goats were challenged by Intravenous (IV) injection (1 ml) and Intra-Nasal (IN) spray (1 ml) of the virus as reported by El Harrak et al. [20]. Monitoring was based on a daily observation of hyperthermia and clinical signs from 0 to 14 days post infection (dpi). Clinical score was used to assess the severity of the infection and to allow comparison between animals and groups [21]. Clinical scoring system was followed with a ranking from 0 to 4 based on the severity of: general behavior, hyperthermia, alimentation, diarrhea, salivation, and respiratory symptoms including nasal discharge, dyspnea, coughing and sneezing. A total cumulative score of the assessed signs per animal per day was the calculated. Animals with evident of clinical signs of the disease and a clinical score of 15 to 18 were euthanized. All surviving animals at the end of the observation period were euthanized by overdose anesthesia through intravenous xylazine and intramuscular ketamine injections followed by exsanguination. Lacrimal, nasal and buccal swabs, as well as blood samples were collected from goats every 3 dpi and analyzed by quantitative real time reverse transcriptasepolymerase chain reaction (RT-qPCR) to monitor the viral load. Specific Post Mortem (PM) samples were taken from lung, mesenteric nodes, pulmonary nodes, trachea, spleen and liver for virus assay by RT-qPCR [22]. RNA extraction was accomplished using a RNA kit (Bioline BIO-52075, isolate II RNA Mini kit). Amplification was carried out with the Invitrogen Superscript III Platinum R one step qRT-PCR system (Cat. no. 11745-100).
Statistical analysis
Significant differences among groups regarding hyperthermia, clinical scoring and serological response were determined using Student’s t-test. A p-value of ≤ 0.05 was considered statistically significant.
Results
Inactivation and vaccine preparation
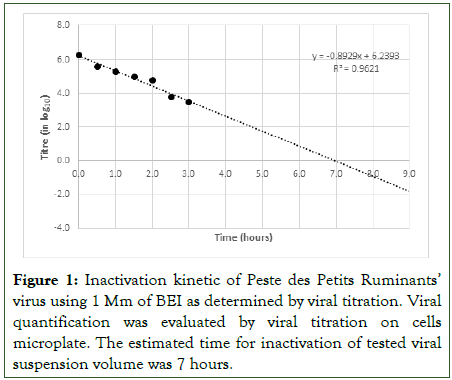
The PPRV suspension used to prepare the PPR vaccine was harvested after five days of incubation of infected Vero cells at 37°C when the Cytopathic Effect (CPE) was 100% with 30% lysis of the cells. The infectious virus harvest titer was 7.2 log TCID50/ml. The inactivation of viruses with BEI was a linear reaction. The regression line equation from the inactivation kinetic was y=-0,8929x+6,2393 with a R2 coefficient of 0,9621 (Figure 1).
Figure 1: Inactivation kinetic of Peste des Petits Ruminants’ virus using 1 Mm of BEI as determined by viral titration. Viral quantification was evaluated by viral titration on cells microplate. The estimated time for inactivation of tested viral suspension volume was 7 hours.
The estimated time for complete inactivation of the viral suspension was 7 hours. Total inactivation was confirmed by 3 blind passages of the suspension in Vero cells that did not show any evidence of infection based on observations for CPE.
Vaccine safety
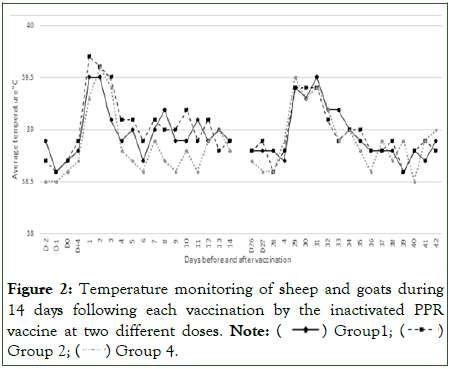
All vaccinated sheep and goats remained healthy with no clinical signs or adverse effect on animal’s behavior or feed uptake following the first and second primary vaccination with the inactivated PPR vaccine. A transient hyperthermia (0.5 above the normal temperature) was observed from day 1 to day 3 pv (Figure 2).
Figure 2: Temperature monitoring of sheep and goats during 14 days following each vaccination by the inactivated PPR vaccine at two different doses.
Note: ( ) Group1; (
) Group1; ( ) Group 2; (
) Group 2; ( ) Group 4.
) Group 4.
Almost all the animals developed a local swelling (1-2 cm of diameter) at the injection site between day 4 and day 12 pv. There was no significant difference (p<0.05) between the three vaccinated groups 1, 2 and 4. Temperature of unvaccinated controls sheep and goat remains within normal values.
Serological response in sheep
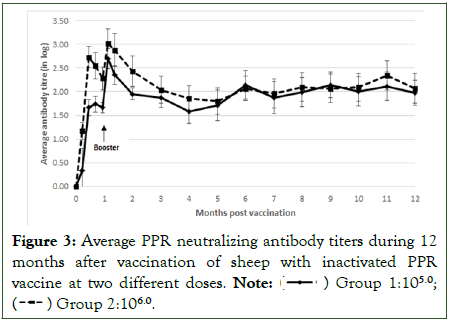
In the group 1 sheep vaccinated with a dose of 105.0 TCID50, neutralizing antibodies were detected in 3 animals at 7 dpv, then in all animals at day 14 pv. Whereas in the group 2 sheep, vaccinated with a dose of 106.0 TCID50, all animals developed neutralizing antibodies from 7 dpv. Animals of group 1 had a mean antibody titre of 1.7 (equivalent to 1/60) at day 28 (day of the second injection), which increased to a mean value of 2.7 (equivalent to 1/500) 7 days after the second injection. Animals of group 2 had a mean antibody titre of 2.3 (equivalent to 1/200) at day 28 pv which increased to mean value of 3.0 (equivalent to 1/1000) 7 days following the second injection. All vaccinated sheep showed a significant increase of antibody response following the second injection (p<0.05). Unvaccinated animals did not develop any detectable anti PPRV antibody response. The serological monitoring for 12 months showed persistence of neutralizing antibodies, with a mean value of 2.0 (equivalent to 1/100) with no significant difference between the two groups (Figure 3).
Figure 3: Average PPR neutralizing antibody titers during 12 months after vaccination of sheep with inactivated PPR vaccine at two different doses.
Note: ( ) Group 1:105.0; (
) Group 1:105.0; ( ) Group 2:106.0.
) Group 2:106.0.
After the annual booster, sheep in both groups showed a significant increase (p<0.05) in antibody titers from 2.0 to 3.1, maintained above 2.0 during 12 months after. The antibody response in group 2 was significantly higher (p<0.05).
Serological response and protection against challenge in goats
Serological response show that there is a response to the first vaccination and all vaccinated goats had developed PPRV neutralizing antibody at 7 dpv with average titer of 1.03. Antibody titres reach a maximum four week after the first vaccination with a mean titre of 2.35 at 28 dpv, which increased to a mean value of 2.65, one week after the second vaccination. All unvaccinated controls remained negative indicating an absence of spread of the vaccine virus between the groups of vaccinated and unvaccinated animals.
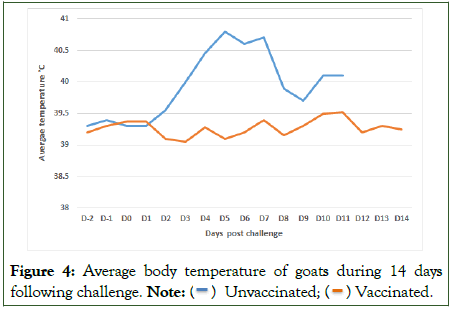
Following the virulent challenge, the two unvaccinated goats showed hyperthermia from D2 post challenge and during the observation period. A temperature peak of 40°C was observed at day 5 pi (Figure 4).
Figure 4: Average body temperature of goats during 14 days following challenge.
Note: ( ) Unvaccinated; (
) Unvaccinated; ( ) Vaccinated.
) Vaccinated.
Goats developed specific PPR disease clinical signs, starting by nasal and ocular discharges at D4 pi, followed by decreased appetite, dyspnoea, cough and diarrhea at the last stage. Both goats were euthanized at D9 and D11 pi, when the clinical score reached the endpoint. The virus was detected in all collected swabs at 6 dpi with average Ct values between 18 and 20. Viremia was detected in both infected goats with Ct between 28 and 35. Seroconversion was detected in both animals starting at 6 dpi, with an antibody neutralization titer of 1.22 log10 in goat 1 at 9 dpi and 1.5 log10 in animal 2 at 11 dpi. At PM, lesions were observed in lungs (congestion and consolidation), with swelling in mesenteric and pulmonary lymph nodes and congestion in the small intestine and colon. The lung and lymph nodes were the tissue with the highest Ct value (between 15 and 17), followed by trachea, spleen and liver, with average Ct value of 18, 20 and 29 respectively.
In-group of vaccinated goats, body temperatures remained normal, and no clinical signs were reported after challenge during the 14 days observation period and clinical scoring was nil (Figure 4). No lesions were observed at necropsy and no viral genome detection by qPCR in swabs or tissues.
Discussion
Small Ruminant Morbillivirus, one of the most economically important diseases of small ruminants, continues to cause the death of millions of sheep and goats annually in many countries of Africa, Middle East, and Asia. Given its social and economic impact, PPR is presently being targeted by international organizations for global eradication by 2030 [23,24]. Vaccination is the most effective way to control the disease in endemic regions [25]. Vaccines that are currently used are live-attenuated vaccines, based commonly on PPRV/Nigeria/75 or PPRV/ India/Sungri/96 strains [26]. They are highly effective to control the disease and provide a strong long-lasting immunity [27-29]. However, they are thermo-sensitive and need to be properly stored and distributed within an efficient cold-chain, they present risk of adventitious agent transmission or virus shed and are not DIVA compliant.
Inactivated vaccines present various advantages in safety, stability and can be used in non-endemic regions which are under threat, when live vaccines are not permitted by veterinary authorities. Within this scope, we developed and tested safety and potency of a new inactivated PPR vaccine using a Morocco isolate that was adapted to growth on Vero cell with an interesting infectious titer.
Only two studies have tested PPR inactivated vaccines with successful results in goats. Ronchi et al. reported an adjuvanted inactivated PPR vaccine that induced 100% seroconversion rate in goats at day 9 and remained positive up to 4 months [30]. This study has several limitations; the vaccine was tested in a limited number of five goats with serological follow-up note exceeding 4 months and the vaccine potency by challenge was not tested. Cosseddu et al. also developed an adjuvanted inactivated PPR vaccine based on field isolate that induced 100% seroconversion rate in vaccinated goats which resisted to a virulent challenge [31]. The serological monitoring in this study was conducted during 4 months without specifying the vaccine dose.
In our study, we developed an oily adjuvanted vaccine using an Oil-in-Water (O/W) emulsion, known to be safe and enhance humoral immunity [32,33]. We formulate the vaccine accordingly, as several authors reported that oily adjuvanted vaccines induced long lasting protection against viruses compared to aqueous preparation [34,35].
Two different doses were tested by serology monitoring on sheep. After one injection, the vaccine induced an early antibody response, at day 7 pv, with a 100% seroconversion rate at day 14 pv. Serological response showed significant difference between the two tested doses during the first 4 months, and then antibody titers become similar. Goats are known to be more sensitive to PPR disease compared to sheep, with severe clinical signs and high mortality rate [36-38]. For this reason, the low dose (105.0 TCID50) was tested in goats through a virulent PPRV challenge test. Vaccination induced full protection in goats as demonstrated by absence of hyperthermia, virus shedding, viremia and clinical signs after challenge of vaccinated animals. Control goats showed typical clinical signs of PPR disease in accordance with several previous studies. The efficacy of the low vaccine dose to protect goats supports evidence of the vaccine compatibility with large industrial scale production at low cost. The serology monitoring confirmed that a booster injection, one year after the primary vaccination, allowed a significant increase of antibody response that stabilized above 2.0 for at least 12 months. To our knowledge, this is the first study on immunity duration of PPR inactivated vaccine up to 24 months. Nevertheless, the need of a booster injection four weeks following the first administration could be a constraint compared to live attenuated vaccine that only requires one shot to elicit long-term immunity. It will thus be interesting to conduct further study to assess the protection and immunity duration following one vaccine injection.
Combinations of several inactivated antigens are commonly used as vaccines and confer protection against a wide range of viral and bacterial pathogens through a single injection [39-43]. This advantage can allow PPR vaccine to be one component of multivalent vaccines used routinely in small ruminants in several countries of Africa and Middle East. This inclusion may contribute efficiently to control PPR without specific vaccination campaigns organized by the authority. This combination need however to be tested by experimental studies and field trials.
Conclusion
In conclusion, this study brings evidence that the developed inactivated PPR vaccine induced high level of neutralizing antibodies against PPRV and conferred a complete protection against a virulent PPRV challenge. The vaccine conferred immunity of at least 12 months and the annual booster is required. Moreover, the vaccine is safe in small ruminants with no risk to virulence, shedding or transmission of extraneous agents. The vaccine is a promising candidate to prevent introduction of the PPRV in free at risk countries and may be an interesting tool to be included in the global PPR eradication program, using combined vaccines.
References
- Aguilar XF, Fine AE, Pruvot M, Njeumi F, Walzer C, Kock R, et al. PPR virus threatens wildlife conservation. Science. 2018;362(6411):165-166.
[Crossref] [Google Scholar] [Pubmed]
- Mahapatra M, Sayalel K, Muniraju M, Eblate E, Fyumagwa R, Shilinde S, et al. Spillover of peste des petits ruminants virus from domestic to wild ruminants in the Serengeti ecosystem, Tanzania. Emerg Infect Dis. 2015;21(12):2230.
[Crossref] [Google Scholar] [Pubmed]
- Pruvot M, Fine AE, Hollinger C, Strindberg S, Damdinjav B, Buuveibaatar B, et al. Outbreak of peste des petits ruminants among critically endangered Mongolian saiga and other wild ungulates, Mongolia, 2016–2017. Emerg Infect Dis. 2020;26(1):51-62.
[Crossref] [Google Scholar] [Pubmed]
- Albina E, Kwiatek O, Minet C, Lancelot R, de Almeida RS, Libeau G. Peste des petits ruminants, the next eradicated animal disease? Vet Microbiol. 2013;165(1-2):38-44.
[Crossref] [Google Scholar] [Pubmed]
- Buczkowski H, Muniraju M, Parida S, Banyard AC. Morbillivirus vaccines: Recent successes and future hopes. Vaccine. 2014;32(26):3155-3161.
[Crossref] [Google Scholar] [Pubmed]
- Parida S, Muniraju M, Altan E, Baazizi R, Raj GD, Mahapatra M. Emergence of PPR and its threat to Europe. Small Rumin Res. 2016;142:16-21.
[Crossref] [Google Scholar] [Pubmed]
- Zhao H, Njeumi F, Parida S, Benfield CT. Progress towards eradication of peste des petits ruminants through vaccination. Viruses. 2021;13(1):59.
[Crossref] [Google Scholar] [Pubmed]
- Amarasinghe GK, Ayllón MA, Bào Y, Basler CF, Bavari S, Blasdell KR, et al. Taxonomy of the order Mononegavirales: update 2019. Arch Virol. 2019;164(7):1967-1980.
[Crossref] [Google Scholar] [Pubmed]
- Gibbs PJ, Taylor WP, Lawman MJ, Bryant J. Classification of peste des petits ruminants virus as the fourth member of the genus Morbillivirus. Intervirology. 1979;11(5):268-274.
[Crossref] [Google Scholar] [Pubmed]
- ICTV. Implementation of taxon-wide non-Latinized binomial species names in the family Paramyxoviridae. 2016
[Crossref]
- Kumar N, Maherchandani S, Kashyap SK, Singh SV, Sharma S, Chaubey KK, et al. Peste des petits ruminants virus infection of small ruminants: a comprehensive review. Viruses. 2014;6(6):2287-327.
[Crossref] [Google Scholar] [Pubmed]
- Singh RK, Balamurugan V, Bhanuprakash V, Sen A, Saravanan P, Yadav MP. Possible control and eradication of peste des petits ruminants from India: technical aspects. Vet Ital. 2009;45(3):449-462.
[Google Scholar] [Pubmed]
- OIE Terrestrial Manual. Peste des petits ruminants (infection with small ruminant morbillivirus). 2022
- Kumar N, Barua S, Riyesh T, Tripathi BN. Advances in peste des petits ruminants vaccines. Vet Microbiol. 2017;206:91-101.
[Crossref] [Google Scholar] [Pubmed]
- Mariner JC, Gachanja J, Tindih SH, Toye P. A thermostable presentation of the live, attenuated peste des petits ruminants vaccine in use in Africa and Asia. Vaccine. 2017;35(30):3773-3779.
[Crossref] [Google Scholar] [Pubmed]
- Sen A, Saravanan P, Balamurugan V, Rajak KK, Sudhakar SB, Bhanuprakash V, et al. Vaccines against peste des petits ruminants virus. Expert Rev Vaccines. 2010;9(7):785-796.
[Crossref] [Google Scholar] [Pubmed]
- Fakri F, Embarki T, Parida S, Bamouh Z, Jazouli M, Mahapatra M, et al. Re-emergence of Peste des Petits Ruminants virus in 2015 in Morocco: Molecular characterization and experimental infection in Alpine goats. Vet Microbiol. 2016;197:137-141.
[Crossref] [Google Scholar] [Pubmed]
- Fakri FZ, Elhajjam A, Bamouh Z, Jazouli M, Boumart Z, Tadlaoui K, et al. Susceptibility of Moroccan sheep and goat breeds to peste des petits ruminants virus. Acta Vet Scand. 2017;59(1):56.
[Crossref] [Google Scholar] [Pubmed]
- Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Epidemiol. 1938;27(3):493-497.
- Harrak M, Touil N, Loutfi C, Hammouchi M, Parida S, Sebbar G, et al. A reliable and reproducible experimental challenge model for peste des petits ruminants virus. J Clin Microbiol. 2012;50(11):3738-3740.
[Crossref] [Google Scholar] [Pubmed]
- Bamouh Z, Fakri F, Jazouli M, Safini N, Omari Tadlaoui K, Elharrak M. Peste des petits ruminants pathogenesis on experimental infected goats by the Moroccan 2015 isolate. BMC Vet Res. 2019;15(1):1-8.
[Crossref] [Google Scholar] [Pubmed]
- Hammouchi M, Loutfi C, Sebbar G, Touil N, Chaffai N, Batten C, et al. Experimental infection of alpine goats with a Moroccan strain of peste des petits ruminants virus. Vet Microbiol. 2012;160(1-2):240-244.
[Crossref] [Google Scholar] [Pubmed]
- Batten CA, Banyard AC, King DP, Henstock MR, Edwards L, Sanders A, et al. A real time RT-PCR assay for the specific detection of Peste des petits ruminants virus. J Virol Methods. 2011;171(2):401-404.
[Crossref] [Google Scholar] [Pubmed]
- Dundon WG, Diallo A, Cattoli G. Peste des petits ruminants in Africa: a review of currently available molecular epidemiological data, 2020. Arch Virol. 2020;165(10):2147-2163.
[Crossref] [Google Scholar] [Pubmed]
- FAO O. Global Strategy for the Control and Eradication of PPR. FAO and OIE. 2015.
- Jia XX, Wang H, Liu Y, Meng DM, Fan ZC. Development of vaccines for prevention of peste-des-petits-ruminants virus infection. Microb Pathog. 2020;142:104045.
[Crossref] [Google Scholar] [Pubmed]
- Diallo A, Taylor WP, Lefèvre PC, Provost A. Atténuation d’une souche de virus de la peste des petits ruminants: candidat pour unvaccin homologue vivant. Rev Elev Med Vet Pays Trop. 1989;42(3):311–319.
[Google Scholar] [Pubmed]
- Hodgson S, Moffat K, Hill H, Flannery JT, Graham SP, Baron MD, et al. Comparison of the immunogenicities and cross-lineage efficacies of live attenuated peste des petits ruminants virus vaccines PPRV/Nigeria/75/1 and PPRV/Sungri/96. J Virol. 2018;92(24):e01471-18.
[Crossref] [Google Scholar] [Pubmed]
- Saravanan P, Sen A, Balamurugan V, Rajak KK, Bhanuprakash V, Palaniswami KS, et al. Comparative efficacy of peste des petits ruminants vaccines. Biologicals. 2010;38(4):479-485.
[Crossref] [Google Scholar] [Pubmed]
- Ronchi GF, Monaco F, Harrak ME, Chafiqa L, Capista S, Bortone G, et al. Preliminary results on innocuity and immunogenicity of an inactivated vaccine against Peste des petits ruminants. Vet Ital. 2016;52(2):101-109.
[Crossref] [Google Scholar] [Pubmed]
- Cosseddu GM, Polci A, Pinoni C, Capobianco DA, Iapaolo F, Orsini G, et al. Evaluation of humoral response and protective efficacy of an inactivated vaccine against peste des petits ruminants virus in goats. Transbound Emerg Diseas. 2016;63(5):e447-452.
[Crossref] [Google Scholar] [Pubmed]
- Schijns VE, Strioga M, Ascarateil S. Oil‐based emulsion vaccine adjuvants. Curr Protoc Immunol. 2014;106(1):2-18.
[Crossref] [Google Scholar] [Pubmed]
- Tahara Y, Mizuno R, Nishimura T, Mukai SA, Wakabayashi R, Kamiya N, et al. A solid-in-oil-in-water emulsion: An adjuvant-based immune-carrier enhances vaccine effect. Biomaterials. 2022;282:121385.
[Crossref] [Google Scholar] [Pubmed]
- Es-Sadeqy Y, Bamouh Z, Ennahli A, Safini N, El Mejdoub S, Tadlaoui KO, et al. Development of an inactivated combined vaccine for protection of cattle against lumpy skin disease and bluetongue viruses. Vet Microbiol. 2021;256:109046.
[Crossref] [Google Scholar] [Pubmed]
- Hamdi J, Boumart Z, Daouam S, El Arkam A, Bamouh Z, Jazouli M, et al. Development and evaluation of an inactivated lumpy skin disease vaccine for cattle. Vet Microbiol. 2020;245:108689.
[Crossref] [Google Scholar] [Pubmed]
- Couacy-Hymann E, Bodjo C, Danho T, Libeau G, Diallo A. Evaluation of the virulence of some strains of peste-des-petits-ruminants virus in experimentally infected West African dwarf goats. Vet J. 2007;173(1):178-183.
[Crossref] [Google Scholar] [Pubmed]
- Nanda YP, Chatterjee A, Purohit AK, Diallo A, Innui K, Sharma RN, et al. The isolation of peste des petits ruminants virus from northern India. Vet Microbiol. 1996;51(3-4):207-216.
[Crossref] [Google Scholar] [Pubmed]
- Shaila MS, Purushothaman V, Bhavasar D, Venugopal K, Venkatesan RA. Peste des petits ruminants of sheep in India. The Veterinary Record. 1989;125(24):602.
[Google Scholar] [Pubmed]
- Jorge S, Dellagostin OA. The development of veterinary vaccines: A review of traditional methods and modern biotechnology approaches. Biotechnol Res Innov. 2017;1(1):6-13.
- Meeusen EN, Walker J, Peters A, Pastoret PP, Jungersen G. Current status of veterinary vaccines. Clin Microbiol Revi. 2007;20(3):489-510.
[Crossref] [Google Scholar] [Pubmed]
- Minke J, Audonnet JC, Fischer L. Equine viral vaccines: the past, present and future. Vet Res. 2004;35(4):425-443.
[Crossref] [Google Scholar] [Pubmed]
- Fernandez AX, Mahapatra M, Begovoeva M, Kalema-Zikusoka G, Driciru M, Ayebazibwe C, et al. Peste des petits ruminants at the wildlife–livestock interface in the Northern Albertine Rift and Nile Basin, East Africa. Viruses. 2020;12(3):293.
[Crossref] [Google Scholar] [Pubmed]
- Shabbir MZ, Munir M. Peste des petits ruminants virus. InMononegaviruses of veterinary importance. 2016;2:71-80.
Citation: Bamouh Z, Fakri FZ, Elmejdoub S, Elarkam A, Rafi L, Tadlaoui KO, et al. (2023) Long Term Immunity Duration against Peste des Petits Ruminants Mediated by an Inactivated Vaccine in Sheep and Goats. J Vaccines Vaccin. 14:535.
Copyright: © 2023 Bamouh Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited