Indexed In
- Open J Gate
- Academic Keys
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- Google Scholar
- SHERPA ROMEO
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Case Report - (2019) Volume 7, Issue 1
Life Threatening Aortoesophageal Fistula Following Modified Hemiarch Repair and Aortic Valve Replacement in Acute Type A Aortic Dissection
Yue LI1, Kingsfield Ong1, Md Faizud Sazzad2 and Giap Swee Kang1*2Ibrahim Cardiac Hospital and Research Institute, Dhaka, Bangladesh
Received: 10-Jan-2019 Published: 22-Feb-2019, DOI: 10.35248/2329-6925.19.7.378
Abstract
Acute type A aortic dissection mandates emergent surgical intervention to prevent life-threatening complications and sudden death. Despite the advances in healthcare and medical technology, surgical repair of the dissection is high-risk and associated with significant morbidity and mortality. Aortoesophageal fistula (AEF) is an extremely rare but severe complication of aortic dissection and survivors of this sequalae are sparsely documented in previous literature. We report the successful management of a case with catastrophic perioperative complication of AEF after repair of acute Stanford type A aortic dissection.
Keywords
Thoracic endovascular aortic repair; Aortoesophageal fistula (AEF); Aortic dissection
Introduction
Acute type A aortic dissection remains a disastrous condition mandating emergency surgical intervention to prevent life-threatening complications and sudden death. Despite advances in medical technology and surgical techniques, the International Registry of Acute Aortic Dissection (IRAD) revealed an overall surgical mortality of 18-25% [1]. The 5-year survival rates reported from various studies ranged between 50-80% [2]. Amongst the numerous post-operative complications, aortoesophageal fistula (AEF) is an extremely rare but severe complication and survivors of this sequalae are sparely reported in the literature. We report an aggressive surgical management of a patient with catastrophic perioperative complication of AEF after successful repair of acute Stanford type A aortic dissection.
Case Presentation
A 66-year-old man presented to the emergency department with an acute onset of pulling sensation in the lower jaw, unsteady gait and severe right buttock pain. He had a past medical history of duodenal ulcer and colonic polyps. His vitals were stable, with blood pressure of 104/56, heart rate 60 beat per min and saturating 97% on room air. Physical examination was unremarkable but chest X-ray revealed widened mediastinum. Emergent computed tomography of the whole aorta demonstrated extensive Stanford type A aortic dissection beginning 1.5 cm distal to the aortic root, involving the ascending, arch and descending aorta, and extending into the right common iliac artery. The right brachiocephalic trunk and right common carotid arose from the true lumen but were involved by the dissection. The diameters of the ascending, arch and descending aorta were 4.8 cm, 3.6 cm and 3.4 cm respectively. Transthoracic echocardiogram showed ejection fraction of 65%, dissection flap in the ascending aorta with moderate aortic regurgitation and poor coaptation of the valve leaflets (Figure 1A-B). The measurements of the annulus, sinus and sinotubular junction were 22 mm, 39 mm and 25 mm respectively.
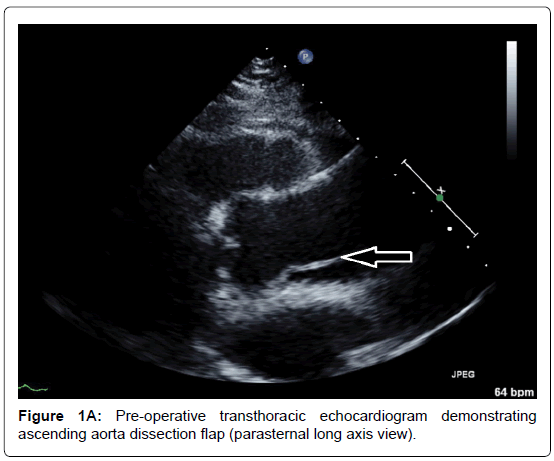
Figure 1A. Pre-operative transthoracic echocardiogram demonstrating ascending aorta dissection flap (parasternal long axis view).
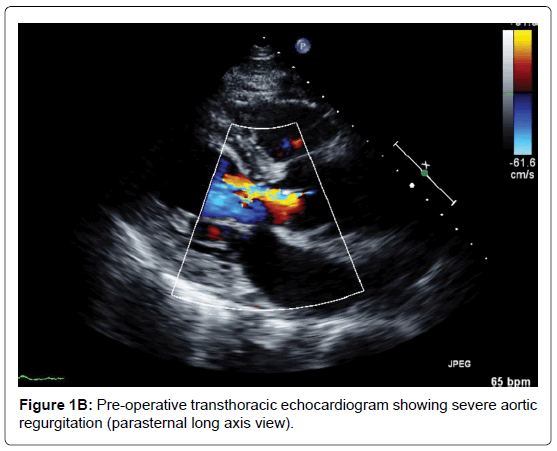
Figure 1B. Pre-operative transthoracic echocardiogram showing severe aortic regurgitation (parasternal long axis view).
The patient underwent emergent replacement of the ascending aorta & proximal arch (Gelweave 24 x 8 mm) with debranching of brachiocephalic artery (Uni-graft 10 mm) and aortic valve replacement (Epic Tissue Valve 23 mm). Median sternotomy and left femoral artery dissection performed simultaneously. Left femoral artery and right atrial two-stage cannula inserted, cardiopulmonary bypass commenced and cooled to 28 degrees Celsius. Heart fibrillated with fibrillator and aortic cross-clamp applied. Direct antegrade cardioplegia administered followed by retrograde cardioplegia every 20 minutes. On careful inspection, the entry tear was found 2 cm above the mid-point of the left and non-coronary cusps. In addition, the brachiocephalic and right common carotid arteries were dissected. As the right coronary cusp remained prolapsed with significant aortic regurgitation after commissure re-suspension, aortic valve replacement was performed. Distal dissection and transection of the aorta between brachiocephalic and left common carotid artery was made to facilitate percutaneous stenting in the future. Debranching of the brachiocephalic artery and replacement of the ascending aorta was performed under deep hypothermic circulatory arrest and selective antegrade cerebral perfusion of the brachiocephalic and left common carotid arteries. The patient made an uneventful recovery and was discharged on postoperative day 9.
Two week later, the patient returned to the emergency department with an acute onset of haematemesis of 3 episodes, approximately 1L in total. He also claimed to have passes large amounts of black stool. On examination he was pale, cold, clammy and hypotensive (BP 94/54). Per-rectal examination revealed fresh melena but his abdomen was soft, non-tender with normal bowel sounds. His haemoglobin level was 6.1 g/dL. As the patient was in hypovolaemic shock, he was aggressively resuscitated with a total of 8 units packed red blood cells, 6 units of platelets and 500 ml fresh frozen plasma. Emergency CT whole aortogram showed satisfactory repair of the ascending aorta and debranching of the brachiocephalic artery, with no active contrast extravasation. Subsequently, oesophago-gastroduodenalscopy (OGD) revealed active bleeding at the mid-oesophagus (nipple sign) with underlying pulsating mass without visible ulcer (Figure 2). Also, his stomach was filled with fresh blood and clots and there were no ulcers seen. These findings were highly suggestive of AEF. The patient underwent emergency thoracic endovascular aortic repair (TEVAR) via femoral access. No visible AEF visualised during the procedure but the false lumen was large. A total of 3 stent grafts were deployed, starting distal to the left common carotid artery up to just above the celiac artery. Post-procedure, the true lumen increased considerably in size, with minimal communicating to the false lumen, while the left subclavian artery was completely obliterated. Re-check OGD confirmed no active bleeding or altered blood in the oesophagus and the previous pulsatile mass was no longer visible. The patient recovered well and was discharged 7 days later. Repeat CT aortogram in clinic revealed satisfactory repair of the aorta, near obliteration of the proximal left subclavian artery but with normal opacification distally (Figure 3A-B). At the 4th-year review in clinic, he remained well and active.
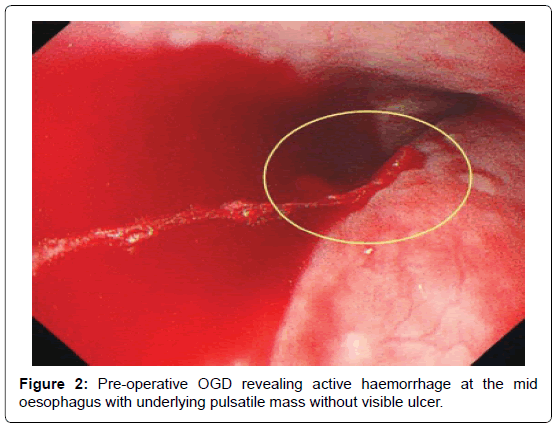
Figure 2. Pre-operative OGD revealing active haemorrhage at the mid oesophagus with underlying pulsatile mass without visible ulcer.
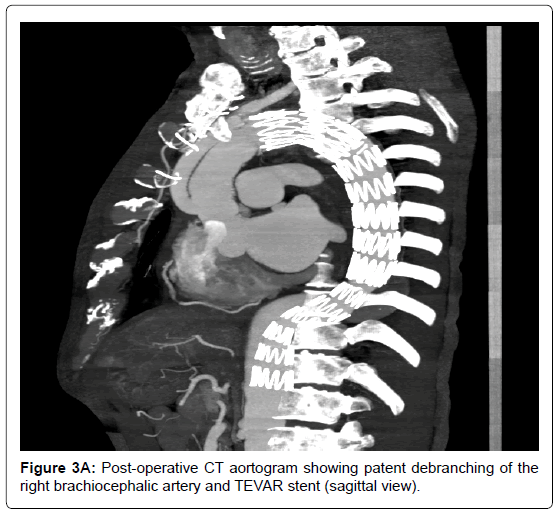
Figure 3A. Post-operative CT aortogram showing patent debranching of the right brachiocephalic artery and TEVAR stent (sagittal view).
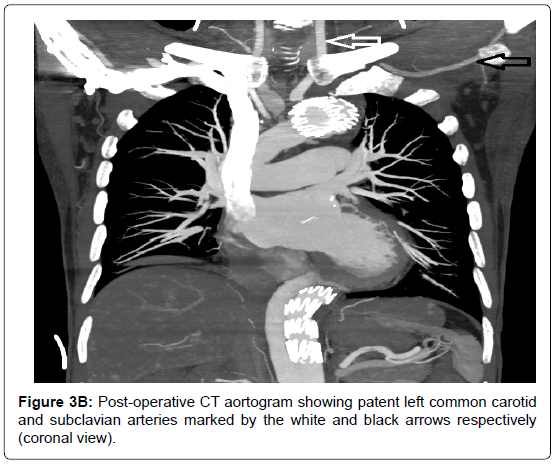
Figure 3B. Post-operative CT aortogram showing patent left common carotid and subclavian arteries marked by the white and black arrows respectively (coronal view).
Discussion
Almost a century ago in 1914, Chiari first described the cardinal features mid-thoracic pain, fatal haemorrhage and sentinel artery bleeding as “aorto-oesophageal syndrome”, which is currently termed AEF [3]. It is a rare condition and often life threatening. The common causes includes oesophageal malignancy, ingestion of foreign body and rupture of aortic aneurysm [4,5]. The exact mechanism of AEF is still unknown [4-6]. It is hypothesized that the pathogenesis of AEF could be either linked with oesophageal ischaemia secondary to changes in intrinsic or extrinsic pressure or primary local infection and inflammation [4-6]. The management of this condition is predominantly surgical as conservative medical management yields no long-term survival [4]. Previously, the 6-month mortality has been estimated to be >50% [4]. Open surgical therapies mainly involve primary closure of the oesophageal end of the fistula via oesophagectomy [7]. Nevertheless, advances in endovascular repair have evolved the treatment strategy of AEF [7,8]. TEVAR has shown to be effective in achieving control of the haematemesis and improving haemodynamic status in the acute setting [8,9]. Thereafter, most investigators recommend definitive open repair as soon as permitted, ideally within a week to prevent the development of any infective process on the TEVAR graft [9,10]. Studies have also shown better outcome with this strategy as compared to TEVAR alone or direct attempt at open repair [9]. A multi-centre analysis by Czerny and colleagues portrayed grim prognosis of zero survival at 1 year in patients with AEF who underwent TEVAR without oesophagectomy [6].
In this case, the patient underwent emergency TEVAR alone rather than open surgery or TEVAR followed by open repair as his perioperative mortality risk was too high in view of the recent type A aortic dissection and surgical repair. It is fortunate that he remained well at 4 years interval. There were a number of factors that were in his favour. First is the rapid access to treatment at a specialized tertiary centre in his country of origin. The small geographical size and established healthcare systems facilitated early delivery of high quality medical care and eliminated unnecessary time wastage which is critical during active bleeding. During his previous admission, discharge advice was given and hence he could recognize and present early to hospital. Second, the source of bleeding was through the false lumen and flow in that passage had been markedly reduced after the repair of the type A aortic dissection. Third, debranching of the brachiocephalic artery in the initially surgery provided a landing zone for the subsequent life-saving emergency TEVAR and reduced his stroke risk. As his left common carotid and subclavian arteries were not grossly dissected, he could avoid the serious complications of long circulatory arrest associated with the traditional total arch repair [11].
Essentially, this is a case of a two-stage hybrid modified repair of the type A aortic dissection, where the TEVAR was emergent for the AEF rather than elective repair of the residual type B aortic dissection. The less invasive hybrid approach for type A aortic dissection has been well recognised with satisfactory outcomes [12], which was proved likewise in this case. Debranching procedures in combination with TEVAR have also shown to reduce 30-day mortality to 11.9% with a stroke rate of 7.6% [13]. Early single debranching of the brachiocephalic artery is less complex surgically and effectively primes the patient for possible additional emergent hybrid aortic procedures.
Conclusion
In summary, this is a rare case of AEF after repair of Stanford type A repair that was successfully addressed by TEVAR. Although the durability and effectiveness of this strategy is potentially questionable, emergency TEVAR was clearly vital for this case and appeared to confer late survival benefit.
Consent
Written consent has been obtained from the patient for publication.
REFERENCES
- Pape LA, Awais M, Woznicki EM, Suzuki T, Trimarchi S, Evangelista A, et al. Presentation, Diagnosis, and Outcomes of Acute Aortic Dissection: 17-Year Trends from the International Registry of Acute Aortic Dissection. J Am Coll Cardiol. 2015;66(4):350-58.
- Chiappini B, Schepens M, Tan E, Amore AD, Morshuis W, Dossche K, et al. Early and late outcomes of acute type A aortic dissection: analysis of risk factors in 487 consecutive patients. Eur Heart J. 2004;26:180-6.
- Chiari H. About foreign coronary injury of the esophagus with aortic perforation. Ber Klin Wochenschr 1914;51:7-9.
- Hollander JE, Quick G. Aortoesophageal fistula: a comprehensive review of the literature. Am J Med. 1991;91(3):279-87.
- da Silva ES, Tozzi FL, Otochi JP, de Tolosa EM, Neves CR, Fortes F. Aortoesophageal fistula caused by aneurysm of the thoracic aorta: successful surgical treatment, case report, and literature review. J Vasc Surg. 1999;30(6):1150-7.
- Czerny M, Eggebrecht H, Sodeck G, Weigang E, Livi U, Verzini F. New insights regarding the incidence, presentation and treatment options of aorto-oesophageal fistulation after thoracic endovascular aortic repair: the European Registry of Endovascular Aortic Repair Complications. Eur J Cardiothorac Surg. 2014;45(3):452–7.
- Baril DT, Carroccio A, Ellozy SH, Palchik E, Sachdev U, Jacobs TS. Evolving strategies for the treatment of aortoenteric fistulas. J Vasc Surg. 2006;44(2):250–7.
- Kubota S, Shiiya N, Shingu N, Wakasa S, Ooka T, Tachibana T, et al. Surgical strategy for aortoesophageal fistula in the endovascular era. Gen Thorac Cardiovasc Surg. 2013;61(10):560–4.
- Marone EM, Coppi G, Kahlberg A, Tshomba Y, Chiesa R. Combined endovascular and surgical treatment of primary aortoesophageal fistula. Tex Heart Inst J. 2010;37(6):722–4.
- Okita Y, Yamanaka K, Okada K, Matsumori M, Inoue T, Fukase K, et al. Strategies for the treatment of aorto-oesophageal fistula. Eur J Cardiothorac Surg. 2014;46(5):894-900.
- Poon SS, Theologou T, Harrington D. Hemiarch versus total aortic arch replacement in acute type A dissection: a systematic review and meta-analysis. Ann Cardiothorac Surg. 2016;5(3):156-73.
- Tanaka A, Sandhu HK, Estrera AL. Descending endografts for type A dissections: con. Ann Cardiothorac Surg. 2016;5(3): 227-32.
- Moulakakis KG, Mylonas SN, Markatis F, Kotsis T, Kakisis J, Liapis CD. A systematic review and meta-analysis of hybrid aortic arch replacement. Ann Cardiothorac Surg. 2013;2(3): 247-60.
Citation: LI Y, Ong K, Sazzad MF, Kang GS (2019) Life Threatening Aortoesophageal Fistula Following Modified Hemiarch Repair and Aortic Valve Replacement in Acute Type A Aortic Dissection. J Vasc Med Surg 7: 1. doi: 10.35248/2329-6925.19.7.378
Copyright: © 2019 Yue LI, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

