Indexed In
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Proquest Summons
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Case Report - (2023) Volume 14, Issue 1
Isolated Factor VII Deficiency of Coagulation: A Case Diagnosed in the Departement of Onco-hematology of National Hospital of Niamey
Djibrilla Almoustapha A1,2*, Abdou-Moussa H1, Abba-Ousmane F1, Abdoulaye-Soumana O1, Malam-Abdou B1, Maman Brah M3, Chefou M4 and Diori A52Department of Health Science, Abdou Moumouni University of Niamey, Niamey, West Africa
3Department of Health Science, Andre Salifou University of Zinder, Zinder, West Africa
4Department of Health Science, Reference Hospital of Maradi, Maradi, West Africa
5Department of Ophthalmology, Hospital Center of Lamorde, Niamey, West Africa
Received: 17-Jul-2022, Manuscript No. JBDT-22-17494; Editor assigned: 19-Jul-2022, Pre QC No. JBDT-22-17494 (PQ); Reviewed: 03-Aug-2022, QC No. JBDT-22-17494; Revised: 16-Sep-2022, Manuscript No. JBDT-22-17494 (R); Published: 23-Sep-2022, DOI: 10.4172/2155-9864.22.14.541
Abstract
Factor VII deficiency is a rare coagulopathy that may be congenital or acquiered, the transmission of which is autosomal and recessive, due to a decrease or absence of factor VII in coagulation without impact on other factors. The prevalence is estimated at 1-2/1,000,000 inhabitants in France. Its clinical expression is polymorphic. We report the observation of a case of isolated factor VII deficiency in 10 years old child diagnosed with onco-hematologie department at the national hospital of Niamey.
Keywords
Factor VII deficiency; Onco-hematology; National hospital of Niamey; Coagulopathy
INTRODUCTION
Factor VII deficiency (proconvertin) or Alexander disease is a congenital or acquired coagulopathy, characterized in the majority of cases by a hemorrhagic syndrome associated with a biological decrease in proconvertin [1]. It is a condition that is very rare, it is estimated at 1-2/1,000,000 inhabitants in France [2]. It is characterized biologically by a decrease in the Prothrombin rate (PT) without disturbance of the TCA. The disease can be asymptomatic or can be manifested by sudden hemorrhages provoked. Its management consists of the administration of the missing factor. We report a case of isolated factor VII deficiency revealed by chronic intracranial hemorrhage.
Case Presentation
It is a subject of male gender, black race, 10 years old, of normal morphotype, of a 38 years old mother who died of an undocumented pathology. He is from a consanguineous marriage, 3rd of a family of 7 children, 2 of whom died (two girls) of unspecified causes. There is a history of epistaxis and prolonged bleeding at the age of 4 years during a scrotal hernia repair and at the age of 6 years during circumcision. No notable family history. Following a head trauma, the patient presented with a chronic distilling hemorrhage that caused a parietal hematoma objectified on the CT scan (Figure 1). On admission to the emergency room, he was conscious and presented with marked mucocutaneous pallor. Weight: 27 kg, height: 1.34 m, Blood Pressure (PA) at 100/50 mmHg, pulse at 112 bts/min and Respiratory Rate (RR) at 32 cycles/min. Complete Blood Count (NFS) showed anemia (Hb: 6.6 g/dl), microcytic (VGM: 78.8 fl), hypochromic (CCMH: 28.6 g/dl), normal white blood cell count (WBC: 8500/mm3) and a normal platelet count (Plq: 253,000/mm3). A preoperative assessment was carried out, namely a Rhesus blood group (B positive).
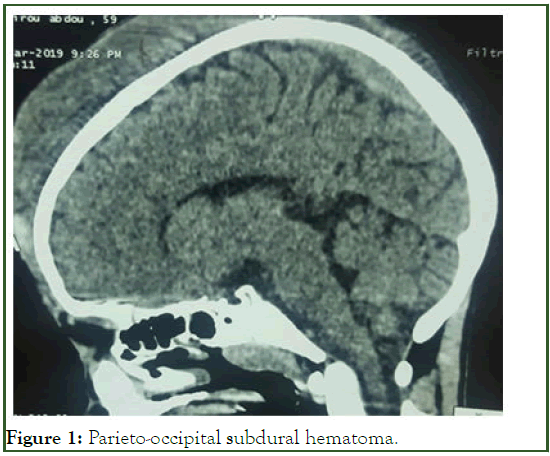
Figure 1: Parieto-occipital subdural hematoma.
A craniotomy for resorption of the hematoma was performed urgently after transfusion of three bags of whole blood. In view of the persistence of the hemorrhage, he was transferred to the Onco-hematology service for exploration.
On admission to the service, the patient was hemodynamically and respiratory stable and presented with moderate mucocutaneous pallor. There was proptosis with bilateral blindness and 2 frontal craniotomy points. Assessment made: TP at 36% and normal TCA, factorial dosage by chronometric method in factor VII at 57% and an increase in factors VIII and IX respectively at 265% (N: 53%-130%) and 201 (N: 60-120), fibrin at 6.26 g/l (N: 2-4 g/l).
Taking into account the clinical data, the haemostasis assessment, the factorial dosage of factor VII, the diagnosis is that of an isolated factor deficiency.
Given the unavailability of recombinant factor VII, the patient was treated with 6 transfusions of Fresh Frozen Plasma (FFP). The evolution was characterized by the disappearance of the hematoma and the healing of the craniotomy points. The blindness remained permanent, linked to a corneal exposure ulcer (Figure 2). Family screening was not carried out.
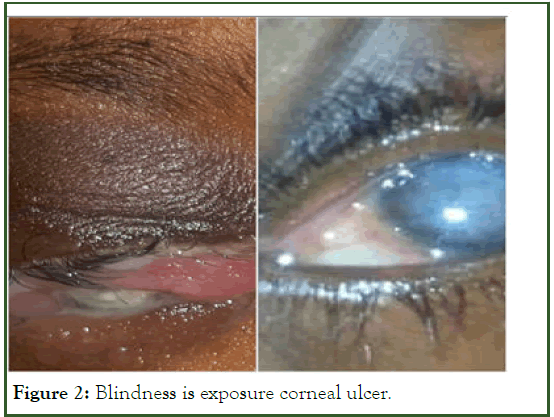
Figure 2: Blindness is exposure corneal ulcer.
Discussion
Factor VII or proconvertin is a plasma glycoprotein synthesized by the liver in the presence of vitamin K1, it belongs to the factors of the prothrombin complex (II, V, VII, X), it is involved in the exogenous pathway of coagulation [3]. Its plasma concentration varies from 0.35 to 0.60 mg/l, i.e., 10 times less than that of the other vitamin K dependent factors and its half life is very short: 4 to 6 hours.
Factor VII deficiency is a rare coagulopathy. Its incidence is probably underestimated because often in this deficit, the clinical expression is rough or absent, estimated between 1/500,000 to 1/1,000,000 [4]. On the other hand, one in 500 people may carry the defective gene. This rarity is not documented in Niger. According to data from the literature, the frequency of this condition is higher in subjects from consanguineous marriages, this is the case of our patient who is from a second degree consanguineous marriage. The age of presentation can vary widely from birth to adulthood. It can be hereditary (autosomal recessive disorder) [5]. The gene responsible is located on chromosome 13(3) or acquired (secondary to hepatocellular insufficiency, treatment with vitamin K, vitamin K deficiency or malabsorption, a state of fibrinolysis or an autoimmune origin). In the symptomatic forms, the disease can be revealed in the neonatal period by haemorrhage at the fall of the cord, in early childhood by haemorrhage at the fall of the teeth, at puberty by menorrhagia. The signs may remain crude and only appear in adulthood following trauma or surgery [6]. According to Kadda A [7], the hemorrhagic syndrome was the most frequent mode of revelation (66%). Our patient presented with a haemorrhagic syndrome such as recurrent epistaxis and post-surgical haemorrhage and currently with head trauma complicated by fronto parietal hematoma. The diagnosis was suspected in front of a drop in TP to 36% and a normal TCA. The dosage of the factor by chronometric method confirmed the isolated deficiency of Factor VII. Normal values vary from 70% to 140% but vary depending on the thromboplastin used [8]. The correlation between the severity of bleeding and the size of the deficit has not been proven [9].
However, very many patients are totally asymptomatic with a factor VII activity level visibly lower than 5% or even in some cases lower than 1%. There are even reports of cases of surgery without replacement therapy that did not result in any hemorrhagic manifestation [10]. Like all genetic diseases, there is no cure for congenital factor VII deficiency. Treatment is only possible in two circumstances; symptomatic treatment during bleeding and preventive treatment. The aim was to provide a sufficient quantity of factor VII to stop the bleeding and ensure haemostasis. Several therapeutic approaches are possible, namely PFC infusion, factor VII concentrate and recombinant factor VII concentrate [11]. The efficacy and safety of rFVIIa is clearly established in patients with a constitutional hemostasis disorder such as hemophilia, thrombopathies and severe FVII deficiency [12]. The action of rFVIIa is rapid (2 to 3 h) but brief. Its halflife is estimated at about 2-7 hours in adults and is shorter in children. The mechanisms of action explain that the effectiveness of rFVIIa requires a circulating platelet count of at least 50,000/mm3 and a minimal plasma fibrinogen level (0.5 to 1 g/L) [12]. PFC is an alternative to rFVIIa which remains of great benefit in our context despite the risks associated with transfusion. Our patient had received a series of transfusions of fresh frozen plasma due to the unavailability of rFVIIa.
Conclusion
Factor VII deficiency or Alexander disease, is a rare, congenital or acquired coagulopathy that is often asymptomatic or mild and can have consequences in cases of neglected trauma. It must be evoked in front of a disturbed PT and a normal TCA associated with hemorrhagic signs. Its treatment remains based on PFC transfusion in the absence of the recommended factor.
References
- Imane Z, Mdaghri-Alaoui A, Hamdani S, el Harim-Lamdouar L, Lamdouar-Bouazzaoui N. Congenital factor VII deficiency: About a case. J de Pediatrie et de Pueric. 2004;17(3):139-142.
[Crossref] [Google Scholar] [Indexed]
- Ariffin H, Lin HP. Neonatal intracranial hemorrhage secondary to congenital factor VII deficiency: Two case reports. Am J Hematol. 1997;54(3):263.
[Crossref] [Google Scholar] [Indexed]
- Levy G. Concept of physiology and exploration of hemostasis. Encycl Med Chir (Elsevier SAS Paris) Anesthésie-Réanimation. 1995:1-536-657-10.
- Benajiba N, Ayyad A, Aabdi C, Amrani R, Rkain M, Benajiba M. Congenital coagulation factor VII deficiency: About two family cases. Pan Afr Med J. 2018; 31(1):242-246.
[Crossref] [Google Scholar] [Indexed]
- Zied TE. The science of health: Factor VII (FVII) deficiency. Health Sci. 2016.
- Mansour LS, Thabet A, Aloulou H, Turki H, Chabchoub I, Mhiri F, et al. Congenital coagulation factor VII deficiency revealed by cerebral hemorrhage. Arch Pédiatr. 2009;16(7):1024-1027.
[Crossref] [Google Scholar] [Indexed]
- Kada A. Factor VII deficiency in children. DSpace at Univ. Mohammed-V Rabat (Thesis). 2017.
- Samama MM, Elalamy I, Conard J, Achkar A, Horellou MH. Hemorrhage and thrombosis: from diagnosis to treatment. Collection “Les abreges” Edition Masson, Paris, 2004.
- Ranchère JY, Ménait C, Lienhart A, Attali O. Factor VII deficiency and surgery. Ann Fr Anesth Reanim 1999;18(7):772-775
[Crossref] [Google Scholar] [Indexed]
- Peyvandi F, Mannucci PM, Asti D, Abdoullahi M, Di Rocco N, Sharifian R. Clinical manifestations in 28 Italian and Iranian patients with severe factor VII deficiency. Haemophilia. 1997;3(4):242-246.
[Crossref] [Google Scholar] [Indexed]
- Federation Mondiale de I’Hemophilie (FMH), Committee in charge of Von Will brand's disease and rare coagulopathies, coagulation disorders, rare deficiency of coagulation factors, factor VII deficiency.
- Monpoux F, Chambost H, Haouy S, Benadiba J, Sirvent N. Recombinant activated factor VII in paediatrics. Universal hemostat? Archives de Pédiatrie. 2010;17(8):1210-1219.
[Crossref] [Google Scholar] [Indexed]
Citation: Almoustapha DA, Abdou-Moussa H, Chefou M, Brah MM, Diori A, Abba-Ousmane F, et al. (2022) Isolated Facteur VII Deficiency of Coagulation: A Case Diagnosed in the Departement of Onco-Hématology of National Hospital of Niamey. J Blood Disord Transfus. 14:541.
Copyright: © 2022 Almoustapha DA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.

