Indexed In
- Open J Gate
- Academic Keys
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- Google Scholar
- SHERPA ROMEO
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 0, Issue 0
Is there a Link between Sex and the Ablation Outcome of Atrial Fibrillation?: An Updated Systematic Review and Meta-Analysis
Tian Zheng2, Qianwei Huang1, Xiao Huang1, Qianghui Huang1, Jianxin Hu1, Xiaoshu Cheng1, Bo Zhu1 and Biming Zhan1*2Department of Radiology, The Second Affiliated Hospital of Nanchang University, Nanchang, China
Received: 25-Jan-2023, Manuscript No. JVMS-23-19662; Editor assigned: 27-Jan-2023, Pre QC No. JVMS-23-19662 (PQ); Reviewed: 10-Feb-2023, QC No. JVMS-23-19662; Revised: 17-Feb-2023, Manuscript No. JVMS-23-19662 (R); Published: 27-Feb-2023, DOI: 10.35248/2329-6925.23.S14.501
Abstract
Aim: While females are at a higher risk of Atrial Fibrillation (AF), it is unclear whether gender differences are associated with AF recurrences after catheter ablation for AF. The goal of this study was to conduct a systematic review of the medical literature to evaluate the clinical outcomes of catheter ablation for AF in men and women.
Methods: A systematic review of databases (PubMed, World of Science, and Embase) was conducted to identify studies published since 2010 that reported AFCA by sex. The primary endpoints were freedom from recurrence of AF/Atrial Tachycardia (AT), and the procedure complications of interest were (1) vascular/groin complications; (2) pericardial effusion/tamponade; (3) stroke/TIA; (4) permanent phrenic nerve injury; and (5) procedural mortality. When the heterogeneity between studies was 50% (freedom from AF/atrial tachycardia), random effects models were used for meta-analysis, and fixed effects models were used for all other endpoints.
Results: 22 studies met the inclusion criteria, with 281872 patients undergoing AFCA, 34% of whom were women. Women were older (63.54.13 vs. 60.254.00 years), more likely to be hypertensive (46.2% vs. 44.7%), and more likely to be diabetic (18.6% vs. 16.7%) (P=0.0001 for all comparisons). Our analyses revealed that the rate of freedom from AF/AT recurrence was lower in women than men at the long year follow-up (Odds Ratio (OR): 0.67, 95% Confidence Interval (CI): 0.57-0.79; P=0.00001), but there were no statistically significant differences in all-cause mortality between men and women (OR=1.07, 95% CI 0.88-1.30, P=0.49). Other complications (pericardial effusion/tamponade, stroke/TIA, vascular complication, and hematoma) were significantly higher in women.
Conclusion: Women who had AF catheter ablation may have less efficacy and a higher risk of stroke/TIA and major complications than men. More in-depth research is required to better define the mechanisms of increased risk in women and to identify strategies for closing the gender gap.
Keywords
Ablation; Atrial fibrillation; Complications; Female; Gender
Introduction
The most common type of sustained arrhythmia in the world is Atrial Fibrillation (AF) [1]. This escalating epidemic is costly and complicates the treatment of other medical conditions. In addition to an increased risk of stroke, heart failure, and cognitive impairment, atrial fibrillation is associated with a lower quality of life [2]. Several studies have been conducted to investigate the role of gender in the etiology, presentation, and prognosis of AF [3]. Women with AF are more likely than men to have a stroke and to die. The Framingham cohort study found that AF is associated with an odds ratio for death of 1.5 in men and 1.9 in women [4]. As a result, women may benefit more from maintaining sinus rhythm.
Over the last decade, catheter ablation has become a more common option for rhythm control in patients who have failed medical therapy [5]. Women are less likely than men to be referred for catheter ablation, despite the well-established role of Pulmonary Vein (PV) isolation in the treatment of symptomatic AF [6]. Several clinical scoring systems include female sex as a predictor of procedural failure or procedure-related complications, and vascular injury or cardiac tamponade after catheter ablation appear to occur AFCAmore frequently in women [7]. However, no gender difference in outcome or complications after ablation was found in several other long-term observational studies [8]. As a result of this, we conducted an updated systematic review and meta-analysis to investigate the clinical outcomes associated with AF catheter ablation.
Methodology
Data sources and searches
We conducted a thorough online search of the literature (up to December 2022) in the Medline and EMBASE databases to identify all published clinical studies that compared the outcomes of catheter ablation of AF in men and women. Relevant keywords and medical subject heading terms such as ‘atrial fibrillation,’ ‘gender, “sex, “men,’ ‘women, “male,’ ‘female,’ ‘pulmonary vein isolation,’ and ‘ablation’ were used both individually and in combination in the retrieval strategy. The bibliographies of the reviewed manuscripts were manually retrieved to avoid missing relevant data.
Studies were considered if they reported primary endpoints of interest in patients undergoing catheter ablation of AF and provided specific information on women vs. men within the main article or subgroup. Catheter ablation was used to describe an endocardial ablation procedure (radiofrequency or cryoablation). To determine which studies would be included, the following criteria were used: (i) Studies had to provide accurate information on at least one of the primary endpoints; (ii) All studies had to include at least 500 participants to reduce the risk of small-study effects; and (iii) Endpoints had to be reported as numerical events rather than just odds rates. If relevant data were not provided in the published papers, we made an effort to contact the corresponding authors to ask for more information.
Outcomes and definitions
The primary goals were to determine the incidence rates of freedom from AF/Atrial Tachycardia (AT) recurrence. Serious complications including mortality from all causes, stroke/ Transient Ischemic Attack (TIA), pericardial effusion/tamponade and severe hemorrhage requiring transfusion were secondary outcomes. Without antiarrhythmic medication, no episode of AF, flutter, or tachycardia lasting longer than 30 seconds after a 1-or3- month break was regarded as being free from recurrence of AF/AT (AADs). All-cause mortality and the first incidence of a stroke or TIA were both referred to as these terms, as well as any deaths that happened during hospitalization or follow-up.
Data extraction and quality assessment
Data was gathered and reported in line with the preferred reporting items for systematic reviews and meta-analyses in order to compile this article. Using a specified, standardized process and a data gathering tool, three writers abstracted data independently. From the main paper, the following details about the study, the patient, and the operation were taken. Any discrepancies were discussed and settled among the authors. The Newcastle-Ottawa Quality Assessment Scale (NOS) for observational trials was used to rate the effectiveness of the included research [9,10]. The NOS scale has a maximum score of nine and eight questions. We assessed the data based on the populations we selected, how comparable the groups were, and how a star system represented the exposure/outcome of interest [11-15].
Data synthesis and analysis
Dichotomous data were analyzed using the Odds Ratio (OR) with 95% Confidence Intervals (CIs), whilst continuous variables were analyzed using weighted mean differences. The Cochran Q test was used to determine whether there was study heterogeneity, and the Higgins I 2 tests was used to determine how much heterogeneity was present. These tests show that there is 0-25%, 25-50%, and 50-75% heterogeneity, respectively. The pooled effects of our metaanalyses were estimated using a random-effects model due to the inherent heterogeneity in research design and demographics [16-20].
In order to demonstrate that our results were not significantly influenced by any particular study, pre-specified sensitivity analyses for the primary endpoints were carried out by systematically deleting one study at a time. To further assess the stability of our metaanalysis, numerous subgroup analyses (based on research design, study quality, and patient age) were carried out. We used a randomeffects meta-regression analysis to examine how preselected factors affected the total effect. All statistical analyses were done with STATA 12.0 and the Rev Man software suite (Review Manager, Version 5.3). Two-tailed P-values were used, and a P-value of 0.05 or above was regarded as statistically significant [21-25].
Results
22 studies met the inclusion criteria and were included in the analysis out of a total of 1316 citations that were generated by the database searches (Figure 1). A total of 281872 patients who underwent AFCA were included in these trials; 33.6% of these patients were women (94930 women) [26-31]. Table 1 lists baseline characteristics broken down by sex. Women receiving ablation had considerably higher rates of diabetes (18.6% vs. 16.7%, p=0.0001) and hypertension (46.2% vs. 44.7%), as well as significantly older ages (63.5 ± 4.13 vs. 60.25 ± 4.00 years). As a result, women had significantly higher CHADS2-VASc ratings in the studies that reported them (n=12, respectively). Left Ventricle Ejection Fraction (LVEF) was normal in the great majority of patients, regardless of gender, when reported (n=9 studies), while women had statistically higher ejection fractions (60.9 ± 4.7% vs. 58.4 ± 4.8%, P<0.0001) and more often had a paroxysmal AF pattern at enrollment. Men were more likely to have coronary artery disease, to have enlarged LA diameter, increased body mass index and less paroxysmal AF.
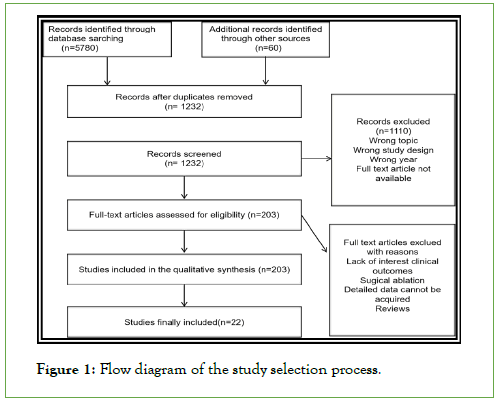
Figure 1: Flow diagram of the study selection process.
| Study | Age | BMI | Hypertension (%) | Diabetes (%) | LA area | CHA2DS2-VASc | Ejection fraction (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | |
| Alexis Hermida 2022, [10] | 63 ± 9 | 60 ± 10 | 29 ± 6 | 29 ± 5 | 84 (46%) | 254 (46%) | 19 (10%) | 66 (12%) | 23 ± 5 | 25 ± 5 | 2.5 ± 1.2 | 1.4 ± 1.3 | 58 ± 10 | 56 ± 11 |
| Andrea M. Russo 2021, [11] | 69.4 (65.3, 74.0) | 66.6 (59.8, 71.2) | 30.0 (26.1, 34.9) | 30.0 (26.8, 33.9) | 338/413(81.8%) | 538/695 (77.4%) | NG | NG | NG | NG | 3.0 (3.0, 4.0) | 2.0 (1.0, 3.0) | NG | NG |
| Daniel W. Kaiser 2016, [12] | 61.9 ± 11.4 | 58.1 ± 10.5 | NG | NG | 4,061 (66.2%) | 9,246 (61.8%) | 1,400 (22.8%) | 3,097 (20.7%) | NG | NG | 2.9 ± 1.5 | 1.6 ± 1.4 | NG | NG |
| Fahd N. Yunus 2021, [13] | 66.8 ± 9.6 | 63.4 ± 10.6 | 31.3 ± 7.7 | 30.8±6.0 | 1423 (72.3%) | 2449 (72.3%) | 360 (18.3%) | 707 (20.9%) | NG | NG | 3.4 ± 1.5 | 2.4 ± 1.6 | 57.8 ± 10.0 | 54.0 ± 11.5 |
| Moon-hyun Kim 2021, [14] | 60.8 ± 10.2 | 56.0 ± 10.5 | NG | NG | 1797 (81.5%) | 5320 (76.3%) | 972 (13.9%) | 315 (14.3%) | NG | NG | 3.5 ± 1.7 | 2.0 ± 1.6 | NG | NG |
| Jim W. Cheung 2019, [15] | 62.2 ± 0.16 | 67.9 ± 0.16 | NG | NG | 12 459 (60.4%) | 19 245 (56.6%) | 4223 (20.5%) | 6854 (20.2%) | NG | NG | NG | NG | NG | NG |
| Claude S. Elayi 2018, [16] | 65.1 ± 10.9 | 59.5 ± 10.9 | NG | NG | 16,521 (59.4%) | 31,433 (54%) | 4705 (16.9%) | 8303 (14.3%) | NG | NG | 3 (2) | 1 (2) | NG | NG |
| Jonathan P. Piccini 2012, [17] | NG | NG | NG | NG | NG | NG | NG | NG | NG | NG | NG | NG | NG | NG |
| Hee Tae Yu 2018, [18] | 50.8 ± 8.6 | 49.5 ± 7.4 | 23.8 ± 3.4 | 25.5 ± 3.1 | 58 (26.0%) | 301 (36.0%) | 11 (4.9%) | 84 (10.0%) | 39.5 ± 7.5 | 41.0 ± 6.2 | 1.6 ± 0.8 | 0.8 ± 1.0 | 63.8 ± 7.3 | 62.1 ± 8.5 |
| Karl-Heinz Kuck 2018, [19] | 64 ± 8 | 57 ± 10 | 28 ± 5 | 28 ± 4 | 183 (62%) | 253 (55%) | 21 (7%) | 39 (9%) | 39.9 ± 6.5 | 41.2 ± 5.8 | NG | NG | 64 ± 6 | 62 ± 7 |
| Dimpi Patel 2010, [20] | 59 ± 13 | 56 ± 19 | 28 ± 8 | 27.3 ± 9 | 286 (55.2%) | 1100 (40%) | 57 (11%) | 414 (15%) | NG | NG | NG | NG | 56 ± 8 | 49 ± 5 |
| XIAO-DONG ZHANG 2013, [21] | 62.7 ± 10.6 | 61.1 ± 10.4 | 24.2 ± 2.6 | 24.1 ± 2.5 | 31 (42.5%) | 59 (40.1%) | 6 (8.2%) | 22 (15.0%) | 45.9 ± 4.6 | 45.5 ± 5.7 | NG | NG | 59.6 ± 4.2 | 58.3 ± 6.2 |
| Marielle Kloostermn 2020, [22] | 66 (60–72) | 63 (57–69) | 28.0 (24.9–32.7) | 28.4 (25.4–31.2) | 186 (89.0%) | 385 (90.8%) | 22 (10.5%) | 54 (12.7%) | NG | NG | 3.2 ± 1.1 | 2.1 ± 1.1 | NG | NG |
| Danilo Ricciardi 2019, [23] | 62.4 ± 9.9 | 58.8 ± 10.6 | 27.1 ± 5.2 | 27.1 ± 3.6 | 298 (50.7%) | 764 (49.6%) | 29 (4.8%) | 86 (5.6%) | 40.8 ± 12.2 | 42.6 ± 5.9 | NG | NG | NG | NG |
| Laura Ueberham 2020, [24] | 69.0 ± 8.2 | 62.7 ± 10.1 | NG | NG | NG | NG | 160 | 233 | NG | NG | 3.9 ± 1.2 | 2.6 ± 1.2 | NG | NG |
| Masateru Takigawa 2013, [25] | 63.2 ± 9.1 | 60.0 ± 10.5 | 23.2 ± 3.2 | 23.7 ± 2.8 | 121 (46.5%) | 372 (43.1%) | 30 (11.5%) | 90 (10.4%) | 37.2 ± 5.0 | 38.0 ± 5.1 | 0.9 ± 1.0 | 0.8 ± 0.9 | 68.6 ± 6.2 | 65.6 ± 7.0 |
| Maura M. Zylla 2016, [26] | 63.6 ± 9.6 | 59.1 ± 10.5 | NG | NG | 139 (64.4%) | 247 (60.2%) | 102 (8.5%) | 186 (7.6%) | NG | NG | NG | NG | NG | NG |
| Seung-Young Roh 2018, [27] | 59 ± 10 | 59 ± 10 | 25 ± 4 | 25 ± 3 | 133 (36%) | 124 (34%) | 29 (8%) | 35 (10%) | 41 ± 6 | 41 ± 6 | NG | NG | 55 ± 6 | 56 ± 6 |
| Winkle RA 2011, [28] | 65.7 ± 9.8 | 60.1 ± 10.1 | 28.7 ± 6.4 | 29.6 ± 4.8 | 117 | 245 | 17 | 47 | NG | NG | NG | NG | NG | NG |
| Mihaela Grecu 2020, [29] | NG | NG | NG | NG | 709 (62.1%) | 1245 (51.1%) | NG | NG | NG | NG | NG | NG | NG | NG |
| Reed Mszar 2022, [8] | 67.5 (9.7) | 64.2 (10.4) | 31.5 (9.4) | 31.4 (16.0) | 1887 (9.3%) | 3294 (8.6%) | 3851 (18.9%) | 7912 (20.6%) | NG | NG | 3.4 (1.5) | 2.2 (1.6) | NG | NG |
| Nobuaki Tanaka 2020, [30] | 67.9 ± 8.8 | 63.0 ± 10.6 | 23.2 ± 3.8 | 24.3 ± 3.4 | 753 (55.0%) | 1881 (51.7%) | 158 (11.5%) | 561 (15.4%) | 38.9 ± 6.8 | 40.4 ± 6.6 | 2.96 | 1.72 | 65.8 ± 8.4 | 62.5 ± 9.7 |
Note: Data are presented as mean ± standard deviation or %. Abbreviations: CHADS2:Congestive Heart Failure, Hypertension, Age > 75, Diabetes, Stroke (2 points); CHA2DS2‐VASc, Congestive Heart Failure, Hypertension, Age > 75 (2 points), Diabetes, Stroke (2 points), Vascular Disease, Age 65–74, female sex.
Table 1: Baseline characteristics of the included studies.
Table 2 describes the characteristics of the included studies and the definitions applied to each of the procedural complication endpoints. The majority of studies that documented the ablation energy source (n=17 studies) used Radio-Frequency (RF) ablation in their AFCA procedures. Only 4 studies reported using Cryoballoons (Cryo). Fifteen studies were evaluated as good quality (NOS ≥ 6), indicating a low risk of bias.
| Study | Study characteristics | Ablation energy source | Follow-up months | Reported primary outcomes | NOS points |
|---|---|---|---|---|---|
| Alexis Hermida 2022, [10] | retrospective cohort | Cryoballoon ablations | 26 ± 21months | recurrence of ATa | 5 |
| Andrea M. Russo 2021, [11] | prospective randomized | catheter ablation | 12months | death, disabling stroke, serious bleeding, or cardiac arrest | 7 |
| Daniel W. Kaiser 2016, [12] | retrospective cohort | catheter ablation | 12months | vascular complications, hematoma or hemorrhage, perforation or tamponade, all-cause hospitalization, stroke or TIA | 7 |
| Fahd N. Yunus 2021, [13] | retrospective cohort | catheter ablation | NG | hemopericardium, phrenic nerve injury, perforation/tamponade requiring surgery, pulmonary vein stenosis, stroke/transient ischemic attack) | 6 |
| Moon-hyun Kim 2021, [14] | retrospective cohort | catheter ablation | 12months | - | 7 |
| Jim W. Cheung 2019, [15] | Observational Retrospective | RF | 30days | 30-day all-cause readmission | 6 |
| Claude S. Elayi 2018, [16] | Observational Retrospectivestudy | RF | NG | occurrence of at least one major complication during the index hospitalization for the ablation procedure | 6 |
| Jonathan P. Piccini 2012, [17] | Observational Retrospective | RF | 12months | diagnosis of atrial fibrillation or atrial flutter | 5 |
| Hee Tae Yu 2018, [18] | Observational Retrospective | RF | 24.5 ± 18.9months | AF recurrence | 6 |
| Karl-Heinz Kuck 2018, [19] | RCT secondary analysis | catheter ablation+Cryoballoon ablations | 12months | recurrence of atrial arrhythmia (AF >30 seconds, atrial flutter, or atrial tachycardia), (2) prescription of an antiarrhythmic drug, or (3) repeat catheter ablation. | 7 |
| Dimpi Patel 2010, [20] | Observational Prospective data collection | RF | 24 ± 16months | freedom from AF/atrial tachycardia | 6 |
| XIAO-DONG ZHANG 2013, [21] | Observational Retrospective | RF | 19 ± 5.0months | freedom of AF/AT recurrences | 5 |
| Marielle Kloostermn 2020, [22] | RCT secondary analysis | RF+cryoballon ablations | 3months | composite all-cause death, stroke, or major bleeding, Cardiac tamponade | 7 |
| Danilo Ricciardi 2019, [23] | Observational Prospective data collection | Cryoballoon ablations | 16.8 ± 13.9months | Freedom from atrial fibrillation (AF) recurrence. | 7 |
| Laura Ueberham 2019, [24] | Observational Retrospective | catheter ablation | NG | Vascular access complications (%) | 7 |
| Masateru Takigawa 2013, [25] | Observational Retrospective | catheter ablation | 1/2/3year | AF-recurrence | 6 |
| Maura M. Zylla 2016, [26] | Observational Prospective | catheter ablation+Cryoballoon ablations | 1year | AF-recurrence | 5 |
| Seung-Young Roh 2018, [27] | Matched cohort Retrospective | catheter ablation | 55 ± 38months | recurrence of atrial arrhythmia | 6 |
| Winkle RA 2011, [28] | observational cohort study | catheter ablation | 1/2/3yeas | AF-free rates | 6 |
| Mihaela Grecu2020, [29] | Observational Prospective | catheter ablation+Cryoballoon ablations | 12months | AF recurrence | 7 |
| Reed Mszar 2022, [8] | observational cohort study, prospective cohort study | catheter ablation+Cryoballoon ablations | 10years | A-V fistula requiring intervention | 6 |
| Nobuaki Tanaka 2020, [30] | observational study | catheter ablation | 1year/3year | Atrial fibrillation recurrence | 6 |
Table 2: Baseline demographics in the included studies for the different endpoints.
Freedom from atrial fibrillation/at recurrence
Eighteen studies were included in the quantitative synthesis of freedom from AF/AT recurrence in women vs. men. Some degree of heterogeneity was present as ascertained through the heterogeneity test with I2>50. A random effects model was selected for the analysis. The sex-related difference in freedom from AF/ AT recurrence was independent of follow-up time (at 1 year, OR: 0.98, 95% CI: 0.82-1.16; P=0.79; at 1-3 years, OR: 0.67, 95% CI: 0.57-0.79; P<0.00001; at ≥ 3 years, OR=0.78, 95% CI: 0.66–0.92; P=0.003 (Figure 2).
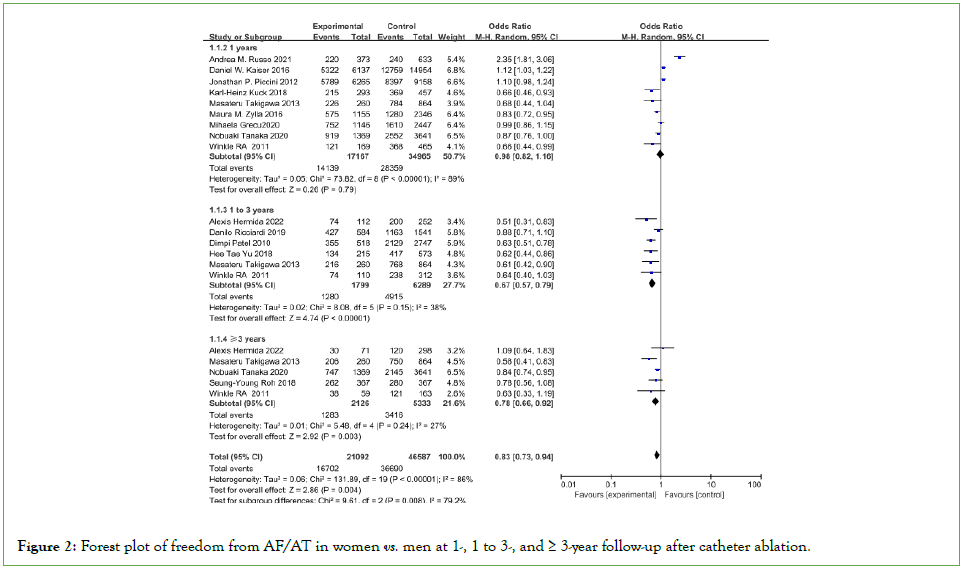
Figure 2: Forest plot of freedom from AF/AT in women vs. men at 1-, 1 to 3-, and ≥ 3-year follow-up after catheter ablation.
Although the need for intervention was not specified in all studies, efforts were made to include only pericardial effusions that required intervention (Table 2). The overall incidence of AFCAmore related pericardial effusion/tamponade was 2.4%, with women having a significantly higher incidence (2.6% vs. 2.2%, OR=1.24, 95% CI: 1.18–1.31) See Figure 3.
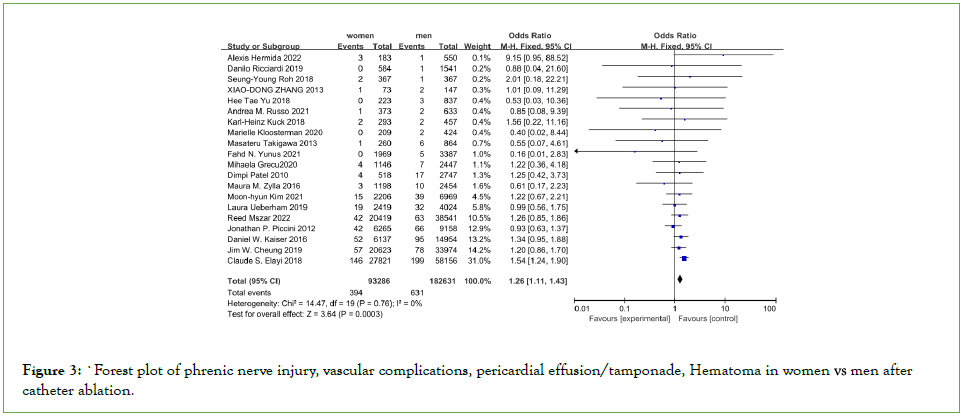
Figure 3: Forest plot of phrenic nerve injury, vascular complications, pericardial effusion/tamponade, Hematoma in women vs. men after
catheter ablation.
Stroke/TIA
In 24 studies, 1031 periprocedural stroke/TIA events were reported in 276420 ablations (overall incidence 0.37%). Women had 397 strokes/TIAs out of 93454 AFCA procedures (0.42%), while men had 1031 events out of 182966 procedures (0.34%), resulting in a 27% higher relative risk of stroke/TIA in women (OR: 1.26, 95% CI: 1.11-1.43), P=0.0003) (Figure 4).

Figure 4: Forest plot of stroke/TIA in women vs. men in-hospital after catheter ablation.
Other complications
10 studies reported phrenic injury, three of which included outcomes with both RF and Cryo, and most studies all included only RF (Table 2). Studies that included both energy modalities did not specify whether phrenic complications occurred with RF or Cryo. Overall, the incidence of phrenic injury was very low in both sexes, and there was no different between both groups (0.11% vs. 0.09%, OR: 1.44, 95% CI: 0.91-2.26, P=0.12) (Figure 3). Our analysis indicated that women undergoing catheter ablation of AF had an increased risk of vascular compliation (OR: 1.67, 95% CI: 1.56–1.78; P<0.0001), Hematoma (OR: 1.52, 95% CI: 1.43–1.62; P<0.0001).
Procedural mortality
Mortality was reported in 12 studies, the overall mortality rate associated with AFCA was very low at 0.17% (443 deaths out of 257008 patients undergoing AFCA in 12 studies). And there was no significant difference in the risk of in hospital mortality between the two groups (OR=1.07, 95%CI 0.88-1.30, P=0.49) (Figure 5).
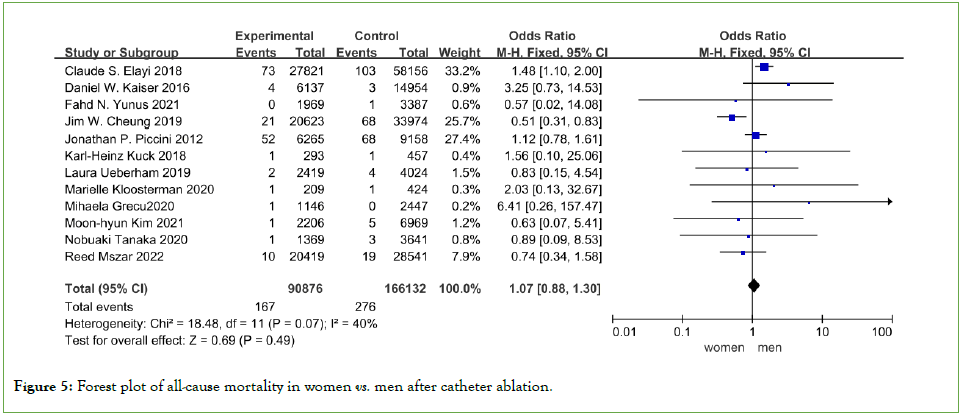
Figure 5: Forest plot of all-cause mortality in women vs. men after catheter ablation.
Discussion
To the best of our knowledge this is the largest study evaluating sex difference in the characteristics and outcomes between females and males following catheter ablation of AF. (i) Females AF patients experienced higher AF recurrence rates after the initial RFCA procedures; and (ii) Females experienced more frequent incidence of procedure-related complications.
It is still difficult to identify people who are at risk for arrhythmia recurrence after AF ablation. There have been reports of several prediction models based on clinical, anatomical, imaging, and serological traits [32]. A residual non-PV arrhythmogenic substrate after the PVI and the restoration of isolated PV potentials were the two main mechanisms of recurrence following AF ablation [33]. The PVI techniques were not well defined in the past, and PV re-conduction was common. The impact of patient variables, particularly gender, may have been muted in earlier research since PV re-conduction was such a significant factor influencing recurrence [34]. Today, PVI is more durable and the influence of patient features on the success of the ablation may be revealed advancements in the PVI technique like 3D mapping systems, irrigation catheters, contact force catheters, consequently, emphasis is gradually being directed to sexy differences [35].
Numerous inflammatory reactions, including oxidative stress, calcium overload, and myofibroblast activation, are brought on by prolonged atrial stimulation [36]. These inflammatory responses, which may be more severe in women than in males, may result in deformation of the extracellular matrix and of the electrophysiological characteristics of the atria [37]. Inflammatory markers like high-sensitivity C-reactive protein, soluble intercellular adhesion molecule-1, and fibrinogen have been linked to incident AF in women even when established risk factors have been taken into account, according to the women’s health study [38]. Higher frequency of non-pulmonary triggered activity, longer action potential duration in female atria, and more pronounced AF-associated fibrotic remodeling result in higher AF recurrence in females than in males [39]. Females in this study who were scheduled for ablation were older, had less paroxysmal AF, and had more comorbid conditions; these factors were linked to more advance structural remodeling and resulted in poorer success rates.
Women undergoing AFCA have a higher risk of pericardial effusion tamponade [40]. According to previous research, tamponade is more common in women than in men. Our research backs up this finding. Pericardial effusion is a risk with both transseptal puncture and catheter manipulation, with left atrial perforation being the most common complication [41]. Despite the fact that routine use of ICE is associated with a significantly lower rate of pericardial complications, cost considerations limit its routine use [42]. Patients receiving continuous oral anticoagulation may be at increased risk of refractory bleeding despite pericardiocentesis and protamine administration [43]. Andexanet alfam idarucizumab can be used to reverse dabigatran, rivaroxaban, or apixaban in such cases, and to lower the danger of pericardial effusion/tamponade, numerous innovative catheter ablation techniques have been deployed, including ultra-low temperature cryoablation catheters and extremely high-power short-duration catheter ablation [44-46].
Vascular surgeries were relatively infrequent in this study’s reported vascular access problems [47]. A higher percentage of obese women, a known risk factor for more difficult vascular access, smaller vascular diameter in women than in men, and the close anatomic relationship between the femoral artery and its branches, which makes veins in women more difficult to puncture and increases the risk of unintentional arterial puncture [48,49]. This gap could be filled by using vascular ultrasonography for femoral venous access on a regular basis.
Even after controlling for other risk factors, females with AF have an increased risk of stroke compared to males. In line with previous findings, our study reported 0.37% in-hospital stroke/TIA. Our findings are consistent with a previous meta-analysis of AFCA outcomes stratified by gender, which found that women had significantly higher rates of stroke/TIA and mortality after AFCA [50]. However, the precise mechanism of gender and procedural stroke is unknown, and there are several possible explanations. First, it is assumed that procedural strokes/TIAs are primarily caused by thrombus formation, though non-thrombotic mechanisms may also contribute to neurologic events associated with AFCA [51]. Second, differences in heparin pharmacokinetics between men and women may contribute to the higher rate of stroke/TIA observed in women. More research is needed to identify optimal peri and intra-procedural anticoagulation strategies in women that balance not only thrombotic risk but also the increased risk of bleeding complications [52]. Third, differences in genetic, vascular biology, hormonal between men and women may increase the risk of stroke [52]. Physicians must discuss the ongoing risks and benefits of anticoagulant therapy, especially when combined with a high CHA2DS2-VASc score and a high likelihood of AF recurrence.
The risk of in-hospital mortality in the current study was extremely low (0.14%). These findings matched the 0.06-0.60% estimated death risk for AF catheter ablation. Our research looked at over 257008 patients and discovered that there was no difference in in-hospital mortality between male and female patients. Campbell found that women had significantly higher rates of mortality after AFCA [52]. However, one significant difference between our analysis and Campbell’s is that we included five newly published studies totaling 75230 patients. Patients were included in a larger sample size, resulting in a different conclusion.
Our analysis included 22 studies from various institutions and countries. It is the largest study aimed at providing real-world information about sex-specific catheter ablation outcomes to physicians and their patients. This meta-analysis, however, has several limitations. First, the majority of the included studies are retrospective in nature, potentially biasing our findings. Despite our efforts to overcome this limitation by conducting multiple sensitivity and subgroup analyses, as well as meta-regression analyses, selection bias could not be ruled out. Second, most studies did not provide detailed information on AF type, ablation strategy, and perioperative medication use (AAD, b-blocker, platelet inhibitors, and anticoagulant). As a result, unmeasured confounders could exist in our findings. Finally, the study data did not allow for a separate analysis of cardiac and non-cardiac death, haemorrhagic and non-haemorrhagic stroke.
Conclusion
In conclusion, updated analysis of our study’s data revealed that women may have a lower percentage of patients who experience no AF/AT recurrence following catheter ablation of AF. Furthermore, female sex was linked to a higher risk of stroke/TIA. Major problems also occurred more frequently in the female sex. Further research is required to determine the causes of these sex-related variations in catheter ablation of AF and more ways to reduce the recurrence rate of atrial fibrillation caused by gender differences.
Funding
The project was supported by funding from the following: The National Natural Science Foundation of China (82060075).
References
- Fabritz L, Obergassel J. Artificial intelligence for early atrial fibrillation detection. Lancet. 2022;400(10359):1173-1175.
[Crossref] [Google Scholar] [PubMed]
- Davidson KW, Mangione C, Ogedegbe G. US preventive services task force recommendation statement on screening for atrial fibrillation-reply. JAMA. 2022;327(20):2022.
[Crossref] [Google Scholar] [PubMed]
- Compagnucci P, Guerra F, Capucci A. Early or delayed cardioversion in recent-onset atrial fibrillation. N Engl J Med. 2019;381(4):386.
- Vinter N, Huang Q, Fenger-Grøn M, Frost L, Benjamin EJ, Trinquart L. Trends in excess mortality associated with atrial fibrillation over 45 years (framingham heart study): Community based cohort study. BMJ. 2020;370:m2724.
[Crossref] [Google Scholar] [PubMed]
- Kistler PM, Chieng D, Sugumar H, Ling LH, Segan L, Azzopardi S, et al. Effect of catheter ablation using pulmonary vein isolation with vs without posterior left atrial wall isolation on atrial arrhythmia recurrence in patients with persistent atrial fibrillation: The CAPLA randomized clinical trial. JAMA. 2023;329(2):127-135.
[Crossref] [Google Scholar] [PubMed]
- Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JE, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: The CABANA randomized clinical trial. JAMA. 2019;321(13):1261-1274.
[Crossref] [Google Scholar] [PubMed]
- Bollmann A, Ueberham L, Schuler E, Wiedemann M, Reithmann C, Sause A, et al. Cardiac tamponade in catheter ablation of atrial fibrillation: German-wide analysis of 21 141 procedures in the helios atrial fibrillation ablation registry (SAFER). Europace. 2018;20(12):1944-1951.
[Crossref] [Google Scholar] [PubMed]
- Mszar R, Friedman DJ, Ong E, Du C, Wang Y, Zeitler EP, et al. Sex-based differences in atrial fibrillation ablation adverse events. Heart. 2022.
[Crossref] [Google Scholar] [PubMed]
- Oremus M, Oremus C, Hall GB, McKinnon MC, ECT & Cognition Systematic Review Team. Inter-rater and test-retest reliability of quality assessments by novice student raters using the Jadad and Newcastle-Ottawa Scales. BMJ open. 2012;2(4):e001368.
[Crossref] [Google Scholar] [PubMed]
- Hermida A, Burtin J, Kubala M, Fay F, Lallemand PM, Buiciuc O, et al. Sex differences in the outcomes of cryoablation for atrial fibrillation. Front Cardiovasc Med. 2022;9:893553.
[Crossref] [Google Scholar] [PubMed]
- Russo AM, Zeitler EP, Giczewska A, Silverstein AP, Al-Khalidi HR, Cha YM, et al. Association between sex and treatment outcomes of atrial fibrillation ablation versus drug therapy: Results from the CABANA trial. Circulation. 2021;143(7):661-672.
[Crossref] [Google Scholar] [PubMed]
- Kaiser DW, Fan J, Schmitt S, Than CT, Ullal AJ, Piccini JP, et al. Gender differences in clinical outcomes after catheter ablation of atrial fibrillation. JACC Clin Electrophysiol. 2016;2(6):703-710.
[Crossref] [Google Scholar] [PubMed]
- Yunus FN, Perino AC, Holmes DN, Matsouaka RA, Curtis AB, Ellenbogen KA, et al. Sex differences in ablation strategy, lesion sets, and complications of catheter ablation for atrial fibrillation: An analysis from the GWTG-AFIB Registry. Circ Arrhythm Electrophysiol. 2021;14(11):e009790.
[Crossref] [Google Scholar] [PubMed]
- Kim MH, You SC, Sung JH, Jang E, Yu HT, Kim TH, et al. Safety and long-term outcomes of catheter ablation according to sex in patients with atrial fibrillation: A nationwide cohort study. Int J Cardiol. 2021;338:95-101.
[Crossref] [Google Scholar] [PubMed]
- Cheung JW, Cheng EP, Wu X, Yeo I, Christos PJ, Kamel H, et al. Sex-based differences in outcomes, 30-day readmissions, and costs following catheter ablation of atrial fibrillation: The united states nationwide readmissions database 2010-2014. Eur Heart J. 2019;40(36):3035-3043.
[Crossref] [Google Scholar] [PubMed]
- Elayi CS, Darrat Y, Suffredini JM, Misumida N, Shah J, Morales G, et al. Sex differences in complications of catheter ablation for atrial fibrillation: Results on 85,977 patients. J Interv Card Electrophysiol. 2018;53:333-339.
[Crossref] [Google Scholar] [PubMed]
- Piccini JP, Sinner MF, Greiner MA, Hammill BG, Fontes JD, Daubert JP, et al. Outcomes of medicare beneficiaries undergoing catheter ablation for atrial fibrillation. Circulation. 2012;126(18):2200-2207.
[Crossref] [Google Scholar] [PubMed]
- Yu HT, Yang PS, Kim TH, Uhm JS, Kim JY, Joung B, et al. Poor rhythm outcome of catheter ablation for early-onset atrial fibrillation in women-mechanistic insight. Circ J. 2018;82(9):2259-2268.
[Crossref] [Google Scholar] [PubMed]
- Kuck KH, Brugada J, Fürnkranz A, Chun KJ, Metzner A, Ouyang F, et al. Impact of female sex on clinical outcomes in the fire and ice trial of catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol. 2018;11(5):e006204.
[Crossref] [Google Scholar] [PubMed]
- Patel D, Mohanty P, Di Biase L, Sanchez JE, Shaheen MH, Burkhardt JD, et al. Outcomes and complications of catheter ablation for atrial fibrillation in females. Heart Rhythm. 2010;7(2):167-172.
[Crossref] [Google Scholar] [PubMed]
- Zhang XD, Tan HW, Gu J, Jiang WF, Zhao L, Wang YL, et al. Efficacy and safety of catheter ablation for long‐standing persistent atrial fibrillation in women. Pacing Clin Electrophysiol. 2013;36(10):1236-1244.
[Crossref] [Google Scholar] [PubMed]
- Kloosterman M, Chua W, Fabritz L, Al-Khalidi HR, Schotten U, Nielsen JC, et al. Sex differences in catheter ablation of atrial fibrillation: Results from AXAFA-AFNET 5. Europace. 2020;22(7):1026-1035.
[Crossref] [Google Scholar] [PubMed]
- Ricciardi D, Arena G, Verlato R, Iacopino S, Pieragnoli P, Molon G, et al. Sex effect on efficacy of pulmonary vein cryoablation in patients with atrial fibrillation: Data from the multicenter real-world 1STOP project. J Interv Card Electrophysiol. 2019;56:9-18.
[Crossref] [Google Scholar] [PubMed]
- Ueberham L, König S, Hohenstein S, Mueller-Roething R, Wiedemann M, Schade A, et al. Sex differences of resource utilization and outcomes in patients with atrial arrhythmias and heart failure. Heart. 2020;106(7):527-533.
[Crossref] [Google Scholar] [PubMed]
- Takigawa M, Kuwahara T, Takahashi A, Watari Y, Okubo K, Takahashi Y, et al. Differences in catheter ablation of paroxysmal atrial fibrillation between males and females. Int J Cardiol. 2013;168(3):1984-1991.
[Crossref] [Google Scholar] [PubMed]
- Zylla MM, Brachmann J, Lewalter T, Hoffmann E, Kuck KH, Andresen D, et al. Sex-related outcome of atrial fibrillation ablation: Insights from the german ablation registry. Heart Rhythm. 2016;13(9):1837-1844.
[Crossref] [Google Scholar] [PubMed]
- Roh SY, Shim J, Lee KN, Ahn J, Kim DH, Lee DI, et al. Gender-related difference in clinical outcome of the patient with atrial fibrillation after radiofrequency catheter ablation. Korean Circ J. 2018;48(7):605-618.
[Crossref] [Google Scholar] [PubMed]
- Winkle RA, Mead RH, Engel G, Patrawala RA. Long-term results of atrial fibrillation ablation: The importance of all initial ablation failures undergoing a repeat ablation. Am Heart J. 2011;162(1):193-200.
[Crossref] [Google Scholar] [PubMed]
- Grecu M, Blomström-Lundqvist C, Kautzner J, Laroche C, Van Gelder IC, Jordaens L, et al. In-hospital and 12-month follow-up outcome from the ESC-EORP EHRA atrial fibrillation ablation long-term registry: Sex differences. Europace. 2020;22(1):66-73.
[Crossref] [Google Scholar] [PubMed]
- Tanaka N, Inoue K, Kobori A, Kaitani K, Morimoto T, Kurotobi T, et al. Sex differences in atrial fibrillation ablation outcomes: Insights from a large-scale multicenter registry. Europace. 2020;22(9):1345-1357.
[Crossref] [Google Scholar] [PubMed]
- Schmidt M, Dorwarth U, Straube F, Daccarett M, Rieber J, Wankerl M, et al. Cryoballoon in AF ablation: Impact of PV ovality on AF recurrence. Int J Cardiol. 2013;167(1):114-120.
[Crossref] [Google Scholar] [PubMed]
- Özlem K, Selçuk K. High ABSI values and association with AF recurrence after AF ablation: A prospective single-center study. Eur Rev Med Pharmacol Sci. 2022;26(19):7151-7160.
[Crossref] [Google Scholar] [PubMed]
- Jian B, Li Z, Wang J, Zhang C. Correlation analysis between heart rate variability, epicardial fat thickness, visfatin and AF recurrence post radiofrequency ablation. BMC Cardiovasc Disord. 2022;22(1):65.
[Crossref] [Google Scholar] [PubMed]
- Huang Z, Liang X, Wang W, Mao Z, Lin Y, Zhang L, et al. Relationship between plasma Cancer Antigen (CA)-125 level and one-year recurrence of atrial fibrillation after catheter ablation. Clin Chim Acta. 2020;502:201-206.
[Crossref] [Google Scholar] [PubMed]
- Gomez SE, Parizo J, Ermakov S, Larson J, Wallace R, Assimes T, et al. Evaluation of the association between circulating IL-1β and other inflammatory cytokines and incident atrial fibrillation in a cohort of postmenopausal women. Am Heart J. 2023.
[Crossref] [Google Scholar] [PubMed]
- Zhou Y, Song X, Ma J, Wang X, Fu H. Association of inflammation indices with left atrial thrombus in patients with valvular atrial fibrillation. BMC Cardiovasc Disord. 2023;23(1):9.
[Crossref] [Google Scholar] [PubMed]
- Cong X, Tian B, Zhu X, Zhang X, Gu W, Zhao H, et al. Interleukin-11 is elevated in patients with atrial fibrillation, correlates with serum fibrosis markers, and represents a therapeutic target for atrial fibrosis. Cerebrovasc Dis. 2023;1-12.
[Crossref] [Google Scholar] [PubMed]
- Zhang T, Wang Y, Liang Z, Zhao H, Han Z, Wang Y, et al. Effect of combined pulmonary vein and superior vena cava isolation on the outcome of second catheter ablation for paroxysmal atrial fibrillation. Am J Cardiol. 2020;125(12):1845-1850.
[Crossref] [Google Scholar] [PubMed]
- Zghaib T, Allison JD, Barrett C, Arkles J, D'Souza B, Luebbert J, et al. Multicenter experience with andexanet alfa for refractory pericardial bleeding during catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2023.
[Crossref] [Google Scholar] [PubMed]
- Mo BF, Wu CQ, Wang QS, Li YG. Case report: Intrapericardial thrombus aspiration in early stage of pericardial thrombosis for cardiac tamponade complicating percutaneous left atrial appendage closure. Front Cardiovasc Med. 2022:924570.
[Crossref] [Google Scholar] [PubMed]
- Tsai J, Chishinga N, Mannil SV, Schaffer R, Kuchciak A, Gomez SI, et al. Acute cardiac tamponade as a complication of pulmonary vein isolation ablation. Cureus. 2021;13(11): e19572 .
[Crossref] [Google Scholar] [PubMed]
- Wu C, Li X, Lv Z, Chen Q, Lou Y, Mao W, et al. Second-generation cryoballoon versus contact force radiofrequency ablation for atrial fibrillation: An updated meta-analysis of evidence from randomized controlled trials. Sci Rep. 2021;11(1): 17907.
[Crossref] [Google Scholar] [PubMed]
- Kurdziel M, Hudzik B, Kazik A, Piegza J, Szkodzinski J, Gąsior M. Idarucizumab for dabigatran reversal in cardiac tamponade complicating percutaneous intervention in ST elevation myocardial infarction. Advances in Interventional Cardiology/Postępy w Kardiologii Interwencyjnej. 2021;17(1):129-130.
[Crossref] [Google Scholar] [PubMed]
- Tohoku S, Schmidt B, Bordignon S, et al. Initial clinical experience of pulmonary vein isolation using the ultra-low temperature cryoablation catheter for patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2022;33(7):1371-1379.
[Crossref] [Google Scholar] [PubMed]
- Popa MA, Bourier F, Lengauer S, Krafft H, Bahlke F, Förschner LV, et al. Safety profile and long-term efficacy of very high-power short-duration (60–70 W) catheter ablation for atrial fibrillation: Results of a large comparative analysis. Europace. 2022.
[Crossref] [Google Scholar] [PubMed]
- Martínez-Montesinos L, Rivera-Caravaca JM, Agewall S, Soler E, Lip GY, Marín F, et al. Polypharmacy and adverse events in atrial fibrillation: Main cause or reflection of multimorbidity? Biomed Pharmacother. 2023;158:114064.
[Crossref] [Google Scholar] [PubMed]
- Szegedi N, Széplaki G, Herczeg S, Tahin T, Salló Z, Nagy VK, et al. Repeat procedure is a new independent predictor of complications of atrial fibrillation ablation. Europace. 2019;21(5):732-737.
[Crossref] [Google Scholar] [PubMed]
- Behrendt CA, Thomalla G, Rimmele DL, Petersen EL, Twerenbold R, Debus ES, et al. Prevalence of peripheral arterial disease, abdominal aortic aneurysm, and risk factors in the Hamburg City Health Study: A Cross-Sectional Analysis. Eur J Vasc Endovasc Surg. 2023.
[Crossref] [Google Scholar] [PubMed]
- Xue S, Qiu X, Wei M, Kong Q, Dong J, Wang Q, et al. Changing trends and factors influencing anticoagulant use in patients with acute ischemic stroke and NVAF at discharge in the NOACs era. J Stroke Cerebrovasc Dis. 2023;32(2):106905.
[Crossref] [Google Scholar] [PubMed]
- Cheng X, Hu Q, Gao L, Liu J, Qin S, Zhang D. Sex-related differences in catheter ablation of atrial fibrillation: A systematic review and meta-analysis. Europace. 2019;21(10):1509-1518.
[Crossref] [Google Scholar] [PubMed]
- Liu H, Lin M, Han W, Ge J, Maduray K, Zhong J. The risk factors of thrombus formation and the effect of catheter ablation on repetitive thrombus formation in patients with atrial fibrillation: A single center retrospective study in China. BMC Cardiovasc Disord. 2023;23(1):1-2.
[Crossref] [Google Scholar] [PubMed]
- Huo S, Wang Q, Jiang Y, Shi W, Luo P, Guo J, et al. Efficiency and safety of high-power ablation guided by Lesion size index: An ex vivo porcine heart study. Pacing Clin Electrophysiol. 2023.
[Crossref] [Google Scholar] [PubMed]
Citation: Zheng T, Huang Q, Huang X, Huang Q, Hu J, Cheng X, et al., (2023) Is there a Link between Sex and the Ablation Outcome of Atrial Fibrillation?: An Updated Systematic Review and Meta-Analysis. J Vasc Surg. S14:501.
Copyright: © 2023 Zheng T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

