Indexed In
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2021) Volume 5, Issue 3
Is Lignocaine Preconditioning Effective to Treat Severe Pulmonary Vasoconstriction Induced by Protamine During Cardiac Surgery?
Sanjeev Singh1,2,3* and Anbarasu Annamalai3,42Department of Cardiac Anaesthesia, Naval Healthcare Information Management System, Bangalore, India
3Department of Cardiac Anaesthesia, National Heart Centre, Royal Hospital, Muscat, Oman
Received: 03-Mar-2021 Published: 26-Mar-2021, DOI: 10.35248/2684-1606.21.5.148
Abstract
Protamine is a low molecular weight protein fraction (5.5-13.0 kDa) that is rich in basic arginine (67%) and lysine amino acids. They are basic polypeptides that neutralize the strongly negatively charged heparin. This study was aimed to explore the effect of lignocaine preconditioning on protamine-induced pulmonary vasoconstriction, which is used to reverse the effect of heparin during cardiac surgery. This was a prospective, single-centre, double-blind and randomised study performed among eighty paediatric patients of either sex in the age group between 1 to 12 years with acyanotic congenital heart disease, scheduled for elective on-pump cardiac surgery under general anaesthesia. The study participants were divided into four groups: Group A non-pulmonary hypertension+lignocaine preconditioning, group B- non-pulmonary hypertension+normal saline preconditioning, group C-pulmonary hypertension+lignocaine preconditioning and group D-pulmonary hypertension +normal saline preconditioning. Haemodynamic parametres, pulmonary inflammatory compounds, and pulmonary function were assessed intraoperatively at 6, 2, and 3-time points, respectively. Routine perioperative data were collected and analysed. Shapiro-Wilk test was used to test if the data were normally distributed. Continuous variables are expressed as mean ± standard deviation (SD) and compared across groups using one-way Analysis of Variance (ANOVA). Categorical variables were expressed as the number of patients together with the corresponding percentage of the total group and analyzed using chi-square tests or Fisher’s Exact test. Spearman’s correlation analysis was performed to evaluate the relationship between pulmonary haemodynamic indicators and inflammatory factors. A P-value<0.05 was considered statistically significant. Group B exhibited increased pulmonary artery pressure (PAP), Mean airway pressure (Paw), Respiratory index (RI), and alveolar-arterial oxygen difference (A-aDO ). Group D exhibited increased Paw, RI, and A-aDO and decreased dynamic pulmonary compliance (Cydn) and oxygen index (OI) after protamine administration. These changes were not observed in groups A and C. Compared with groups A and C groups, plasma thromboxane B2 (TXB2) level in groups B and D were higher, but 6-keto-prostaglandin F1a (6-keto-PGF1a) in groups B and D groups was lower. The incidence of protamine adverse reactions in groups A and C were lower than that in B and D groups respectively. The precondition of lignocaine before neutralization of heparin effectively reverses protamine-induced pulmonary vasoconstriction during cardiac surgery.
Keywords
Congenital heart disease; Lignocaine; Protamine; Pulmonary hypertension
Introduction
Protamines are simple alkaline proteins found in the sperm of clupeidae or salmonidae fishes. Protamine-HCl and protamine sulfate are polycationic agents that are used intravenously to reverse the effects of heparin prior to surgical procedures or for the treatment of heparin overdose. When administered intravenously protamine interacts with the polyanionic heparin to form a neutral charge 1:1 complex, which isolates heparin from antithrombin III, and leads to the loss of its anti-coagulating properties. Therefore, protamine has been often used in cardiac and other surgeries to confront anticoagulating heparin [1].
However, protamine causes adverse haemodynamic effects such as systemic hypotension, and catastrophic pulmonary vasoconstriction [2]. Also, it interacts with the peptides on the surfaces of the vasculature and blood cells. It triggers the release of a wide variety of vasoactive compounds and inflammatory mediators [3]. Even though the detailed mechanism of this interaction of protamine with immunoglobulins and the activation of the complement system remains unclear [4]. It causes a vast variety of adverse effects varying from 1.6% to 10.7%, including mild haemodynamic instability, severe pulmonary vasoconstriction, Pulmonary Artery Hypertension (PAH), hypoxaemia, life-threatening anaphylactic incident, fatal cardiovascular collapse, and death [5,6]. Regardless of the poor therapeutic index, with risk factors for life-threatening events, protamine remains the mainstay drug for heparin neutralisation in cardiac surgery.
The study by Guan et al. reported an incidence rate of 1 per 832 cases of severe pulmonary vasoconstriction from 2012 to 2016 at their centre in 2018 [7]. Dilating the pulmonary artery and decreasing the Pulmonary Artery Pressure (PAP) is a critical treatment of this complication. A few case reports described the use of prostaglandin E1 [8] and nitric oxide [9] to decrease PAP in this condition. There is a paucity of literature on this topic, a systemic research needs to be carried out to disclose the evidence- based knowledge concerning pulmonary vasoconstriction induced by protamine.
Lignocaine is the most commonly used local anesthetic in clinical practice. In addition to blocking the inhibitory effects of the nerve signal, lignocaine has a regulatory function in the immune system, which has been proven to be beneficial in multi-links of inflammatory response. Due to its significantly anti-inflammatory characteristics, lignocaine can alleviate acute lung injuries caused by various reasons [10,11]
To the best of our knowledge, no systemic research has been published to discuss the value of lidocaine in the treatment of severe pulmonary vasoconstriction induced by protamine during the repair of congenital heart disease. To address this relative lack of information, we performed this prospective clinical study to provide a theoretical basis for clinic drug use.
Materials and Methods
This study was undertaken after obtaining institutional ethical approval by the Committee on Human Research Publications and Ethics (CHRPE). The nature of the study was explained to all the parents/guardians of paediatric patients included in the study and written informed valid consent was taken from the parents/guardian of the patient on the separate consent form. The study population consisted of ASA physical status I and II, paediatric patients of either sex in the age between 1 to 12 years with acyanotic congenital heart disease scheduled for elective cardiac surgery requiring CPB. All of them received protamine to reverse heparin anticoagulation.
Study design
This study was a single-centre, prospective, randomised, and double-blinded clinical comparison study. The Sample size for the study was eighty generated using a sample size calculator.
The study participants were randomly divided into four groups by a computer-generated randomisation table, if fulfill the study criteria. Person A involved in the randomisation process, Person B prepared the study drug, whilst Person C was responsible for the patient’s intraoperative and Intensive Care Unit (ICU) records. Person A, C, and the patient were kept unaware of the study drugs to enable double-blinding.
Inclusion criteria
The study was conducted in paediatric patients of both genders, age 1 to 12 years, belonging to ASA grade I and II undergoing elective, acyanotic congenital heart disease surgery requiring a cardio-pulmonary bypass (CPB) under general anaesthesia.
Exclusion criteria
The exclusion criteria for the study included parents with cyanotic heart disease/guardian refusal on behalf of the patient, patients less than 1 year and more than 12 years, history of protamine allergy, preoperative respiratory disease, preoperative haemodynamic instability, emergency surgery, abnormalities found in the lung, liver, and kidney, and coagulation function, patients with infection, respiratory and circulatory accidents occurring during anaesthesia induction.
Preoperative preparation
The day prior to surgery all patients underwent a pre-anaesthetic evaluation with special consideration to elicit any new complication as well as review previous anaesthetic history and drug sensitivity. All preoperative routine investigations rechecked. All procedures were explained to the patients/guardians. Patients were fasted as per hospital protocol prior to surgery.
Anaesthetic protocol
After patient identification, a short preoperative history was taken, clinical examination, and routine investigations were rechecked in all patients. After arrival to the operating room, oxygen saturation in 4 limbs was checked without oxygen, Children were premedicated with injection (inj.) Midazolam 0.5 mg/kg and inj. Ketamine 5 mg/kg along with inj. Glycopyrrolate by oral route. The children over 5 years of age received inj. Midazolam 0.03 mg/kg and inj. Ketamine 0.5 mg/kg IV or inj. Ketamine 5 mg/kg IM, children were monitored by the electrocardiogram, pulse oximetry, and arterial pressures. The induction of anaesthesia was performed with opioids (inj. Fentanyl 10 ug/kg) and benzodiazepines (inj. Midazolam 0.1 mg/ kg). Inj. Pancuronium (0.1 mg/kg) was used as a muscle relaxant, intubation was performed after adequate muscle relaxation with Pancuronium. In all children, additional monitoring included central venous pressure, rectal and nasal temperatures. Anaesthesia was maintained with an infusion of inj Midazolam 0.02 mg/kg/hr and inj. Fentanyl 2 ug/kg/hr.
After a standard median sternotomy procedure and heparinisation with a bolus dose of 3 mg/kg heparin, the ascending aorta and vena cava was cannulated for arterial perfusion and venous drainage, respectively. The CPB technique was standardized. An initial dose of heparin (300 International Unit (IU)/kg)
was administered before CPB, and supplemental heparin was given into the CPB circuit for systemic anticoagulation effect, maintaining the activated clotting time (ACT) at above 480 seconds. The target CPB flow was 2 L/min/m , and the target pressure was >30 mmHg. Core cooling was used in all patients, monitored by rectal and oesophageal temperature.
Depending on whether pulmonary hypertension was directly measured in the pulmonary artery by a cardiac surgeon, and whether lignocaine (2 mg/kg in 5 ml syringe) was given before protamine neutralization, all patients were randomly divided into four groups using the random table method, with 20 patients in each group: A (non-pulmonary hypertension+lignocaine preconditioning), group B (non-pulmonary hypertension +normal saline preconditioning), group C (pulmonary hypertension+lignocaine preconditioning), and group D (pulmonary hypertension+normal saline preconditioning). The volume of preconditioning study drugs was kept 5 ml for all patients. According to the ratio of pulmonary/systemic circulatory pressure, ratio of ≤ 0.3 was considered normal, while the ratio >0.3 was considered as pulmonary hypertension [12].
Diagnostic criteria of protamine-induced adverse reactions
All study participants were successfully and easily weaned from CPB. After haemodynamic stability was established, following adequate volume loading with the pumping of blood, the A and C groups received lignocaine (2 mg/kg in 5 ml syringe) directly via a BD 22G intravenous indwelling needle placed into the pulmonary artery by a cardiac surgeon at one minute before heparin neutralization, and with the same manner, the B and D groups received normal saline (5 ml in 5 ml syringe). After CPB, the reversal of heparin was accomplished with protamine sulfate (1.3 mg/1 mg Heparin) was administered for five minutes through a central venous catheter with an infusion pump to neutralize the heparin. Adverse events potentially related to protamine mediated pulmonary vascular reaction were identified from the cohort when these occurred within 30 minutes of the initiation of protamine, and met one or more of the following criteria [2,5,7,9,13,14]: (A) an increase in pulmonary artery pressure of at least 25%, resulting in a decrease in systemic arterial pressure following protamine at ≥ 25% of the baseline, or a decrease of ≥ 10% requiring inotropic medications or reinstitution of CPB; (B) non-cardiogenic pulmonary edema, defined as any decrease in PO2 requiring an adjustment in ventilatory support; (C) bronchospasm (an elevation of peak inspiratory airway pressures of greater than 5 mm Hg). These events included those that were preceded by pulmonary hypertension, but clinically insignificant elevations of pulmonary pressures were not perceived as events. In all patients, if hemodynamic instability occurred, protamine administration was slowed or temporarily aborted.
Measurement of main outcomes
Pulmonary arterial Blood Pressure (PAP) was continuously measured via a BD 22G intravenous indwelling needle placed into the pulmonary artery by a cardiac surgeon at the following five intraoperative time points: (T0) baseline, one minute before CPB; (T1) one-minute pre-protamine start; (T2) one-minute post-
protamine start; (T3) three minutes post-protamine start; (T4) five minutes post-protamine start. The parameters of Paw, HR, MBP, and dynamic lung compliance (Cdyn) were recorded at the following six-time points: T1-T4, (T5) 10 minutes post-protamine end, and (T6) 20 minutes post-protamine end.
For the arterial blood gas analysis, the alveolar-arterial oxygen gradient (A-aDO ), respiratory index (RI) is the ratio of P (A- a) DO and PaO , and oxygenation index [(OI)=(mean airway pressure × FiO × 100)/PaO )] were documented at three-time points: T0, T1, and T6.
Sample collection and cryopreservation of radial artery blood and right ventricular blood were performed at the T1 and T6 time points, respectively. Thromboxane B2 (TXB2) and 6-keto- prostatin F1a (6-keto-F1a) in plasma were detected by Enzyme- Linked Immunosorbent Assay (ELISA). The data of adverse reactions of protamine was recorded.
Routine perioperative data were collected, including age, gender, weight, height, types of operation (ventricular septal defect or atrial septal defect), preoperative EF value, ACT value after protamine neutralization, operation time, CPB time, and aortic cross-clamp time.
Parameters and statistical analysis
Summary statistics of patient age, gender, and weight for all four groups were reported as means ± standard deviation (SD). Routine perioperative data were collected and analyzed. Shapiro-Wilk test was used for normally distributed data. Continuous variables are expressed as mean ± SD, and compared across groups using one- way Analysis of Variance (ANOVA). Categorical variables were expressed as the number and percentage of the total group and analyzed using chi-square tests or Fisher’s Exact test. Assessment of changes over time in haemodynamic indicators, pulmonary inflammatory factors, and pulmonary function indexes across groups were performed using repeated-measures ANOVA, with groups (A, B, C, and D) as the between-subject factor and time (with either 2, 3, or 6-time points, depending on the parameter) as the within-subject factor. Spearman’s correlation analysis was performed to evaluate the relationship between pulmonary hemodynamic indicators and inflammatory factors. A P-value <0.05 was considered statistically significant. The statistical package SPSS 14.0 was used.
Results
Twenty patients were randomly assigned to each group. All the demographic and clinical profiles in the groups’ A, B, C, and D were comparable (p>0.05) [Tables 1 and 2]. There was no case of pulmonary vasoconstriction in the group A. There was statistically no significant difference in the incidence of protamine adverse reactions between the four groups (P>0.05), but there was a statistically significant difference in the incidence of protamine adverse reactions between the lidocaine group (A and C) and normal saline group (B and D) (X =6.135; Fisher’s exact test, P=0.029).
| Variables | Group A (n=20) | Group B (n=20) | Group C (n=20) | Group D (n=20) | P-value |
|---|---|---|---|---|---|
| Age (yrs) | 4.25 ± 2.85 | 4.73 ± 2.31 | 4.38 ± 2.52 | 4.52 ± 2.83 | 0.847 |
| Weight (Kg) | 15.73 ± 6.41 | 15.96 ± 7.35 | 15.41 ± 8.51 | 15.67 ± 8.13 | 0.763 |
| Height (cm) | 101.25 ± 15.72 | 102.56 ± 17.19 | 100.25 ± 19.32 | 103.95 ± 18.47 | 0.642 |
| Sex M:F % | 1:1.85 (35%:65%) | 1:1.22 (45%:55%) | 1:2.33 (30%:70%) | 1:1.5 (40%:60%) | 0.325 |
Note: Data are presented as means ± standard deviation, ratio, and percentages. Group A: Non-pulmonary hypertension+lignocaine preconditioning; group B: Non-pulmonary hypertension+normal saline group; group C: Pulmonary hypertension+lignocaine preconditioning group; group D: Pulmonary hypertension+normal saline group. Yrs=Years, Kg=Kilogram, cm=centimetre, M=Male, F=Female, P is significant at <0.05
Table 1: Distribution of patient’s demographic profile.
| Variables | Group A (n=20) | Group B (n=20) | Group C (n=20) | Group D (n=20) | P-value |
|---|---|---|---|---|---|
| Types of surgery ASD:VSD | 6:14 | 8:12 | 7:13 | 8:12 | 0.465 |
| Preoperative EF (%) | 69.45 ± 6.89 | 71.53 ± 5.24 | 68 ± 6.31 | 69.38 ± 6.75 | 0.738 |
| CPB time (minutes) | 53.06 ± 21.35 | 46.32 ± 20.68 | 49.57 ± 19.06 | 48.10 ± 14.54 | 0.424 |
| Aortic cross-clamp time (minutes) | 33.32 ± 15.82 | 35.70 ± 16.53 | 29.55 ± 13.85 | 31.90 ± 17.35 | 0.561 |
| Post protamine ACT (seconds) | 125.35 ± 11.86 | 128.80 ± 17.63 | 123.35 ± 13.47 | 124.50 ± 16.39 | 0.743 |
Note: Data are presented as means ± standard deviation, ratio, and percentages. ASD: Atrial Septal Defect, VSD: Ventricular Septal Defect, EF: Ejection Fraction, CPB: Cardio Pulmonary Bypass, ACT: Activated Clotting Time.
Table 2: Distribution of patient’s clinical profile.
Nine cases of protamine-induced pulmonary vasoconstriction occurred from 80 cases in the three groups, with a total incidence of 11.25%. In group C, catastrophic pulmonary vasoconstriction responses occurred in the 3rd minute of protamine infusing in one patient, followed with an abrupt and significant increase in pulmonary artery pressure (PAPmax 88 mmHg), a slight elevation in airway pressure (Pawmax 17 mmHg), a sharp decrease in systemic BP (IBPmin 50/34 (41) mmHg), and the further development of right ventricular distension and bradycardia. With active treatment, the patient was immediately hyperventilated with 100% oxygen, multiple bolus doses of chloride for mild/moderate hypotension, or epinephrine for severe hypotension, and were immediately hyperventilated with 100% oxygen for hypoxia (Table 3).
| Number of cases suffered pulmonay vasoconstriction in each group | Manifestation PAP baseline → max (mmHg) |
Paw baselin → max (mmHg) | Cydn basely → min (ml/cmH2O) | BP baseline → min (mmHg) | HR baseline → min (beats/min) | Treatment |
|---|---|---|---|---|---|---|
| 3 cases group B | 17 → 30 | 14 → 25 | 16 → 5 | 105/56(83) → 80/40(56) | 116 → 94 | The symptoms relieved automatically after half a minute without intervention |
| 13 → 34 | 13 → 30 | 28 → 8 | 96/59(71) → 66/36(48) | 94 → 83 | Hyperventilated with 100% oxygen for hypoxia |
|
| 17 → 44 | 9 → 28 | 10 → 7 | 132/76(99) → 89/46(65) | 117 → 113 | Calcium chloride for mild/moderate hypotension; Epinephrine for severe hypotension | |
| 1 case in group C | 1 39 → 88 |
12 → 17 | 11 → 9 | 112/69(85) → 50/34(41) | 117 → 103 | Hyperventilated with 100% oxygen; Epinephrine: 1 mg, Dopamine: 3-8 ug/kg/min Milrinone: 0.5 ug/kg/min |
| 5 cases group D | 16 → 24 | 14 → 18 | 16 → 11 | 100/64(77) → 91/51(65) | 144 → 131 | The symptoms relieved automatically |
| 22 → 38 | 16 → 22 | 15 → 3 | 72/39(50) → 69/37(49) | 120 → 119 | after half a minute without intervention | |
| 23 → 35 | 13 → 17 | 19 → 11 | 78/48(58) → 47/34(40) | 112 → 87 | Hyperventilated with 100% oxygen for hypoxia |
|
| 35 → 48 | 17 → 26 | 13 → 4 | 82/53(68) → 66/40(51) | 138 → 135 | Calcium chloride for mild/moderate hypotension | |
| 33 → 58 | 17 → 32 | 10 → 5 | 125/86(107) → 66/43(54) | 131 → 122 | Epinephrine for severe hypotension |
Note: Group A: Non-pulmonary hypertension+lignocaine preconditioning; group B: Non-pulmonary hypertension+normal saline preconditioning; group C: Pulmonary hypertension+lignocaine preconditioning; group D: Pulmonary hypertension+normal saline preconditioning.
Table 3: Clinical manifestation of pulmonary vasoconstriction and treatments.
The intraoperative haemodynamic data during neutralization are presented in Figure 1 and Table 4. Compared with the group A, the PAP and Paw in the group B at the T3 time point were significantly higher, while the MAP in the group C at the T3 epinephrine were used to support the heart rate, and pumping with dopamine and milrinone was performed. A protamine- induced pulmonary vasoconstriction reaction occurred in the 3rd minute of protamine infusion in three patients in the group B, and five patients in the group D, and the symptoms were pulmonary hypertension, bronchospasm, hypotension, and bradycardia. One patient in the group B and two patients in the group D were relieved by themselves after half a minute without any interventions, while the rest of the patients received calcium time point was significantly lower. Compared with the group C, the Paw in the group D at the T3 time point was significantly higher, while the Cydn in the group D at the T3 time point was significantly lower. Compared with the T1 time point, the PAP and Paw in the B and D groups at the T3 time point significantly increased, while the Cdyn in the B and D groups at the T3 and T4 time points significantly decreased.
| T | T1 | T2 | T3 | T4 | T5 |
|---|---|---|---|---|---|
| MAP | (mmg) | ||||
| A | 73.65 ± 12.80 | 77.15 ± 12.85 | 81.00 ± 14.23 | 81.95 ± 13.19 | 75.20 ± 7.91 |
| B | 73.80 ± 14.15 | 74.90 ± 15.12 | 74.60 ± 17.17 | 80.70 ± 13.55 | 73.70 ± 10.28 |
| C | 73.70 ± 14.54 | 75.30 ± 13.63 | 75.75 ± 15.63 | 80.50 ± 16.22 | 74.00 ± 14.64 |
| D | 71.70 ± 16.59 | 74.10 ± 15.33 | 69.70 ± 15.99ӿ | 76.95 ± 15.48 | 71.30 ± 12.76 |
| HR | (beats/min) | ||||
| A | 112.60 ± 29.14 | 114.05 ± 15.72 | 112.70 ± 16.06 | 111.50 ± 15.73 | 114.60 ± 17.08 |
| B | 121.50 ± 14.65 | 119.75 ± 14.29 | 116.70 ± 15.60 | 117.40 ± 9.96 | 116.00 ± 11.83 |
| C | 115.05 ± 17.42 | 115.05 ± 15.67 | 112.10 ± 15.22 | 114.60 ± 17.82 | 114.35 ± 14.30 |
| D | 123.20 ± 15.87 | 120.30 ± 16.12 | 119.40 ± 16.32 | 117.15 ± 18.56 | 119.05 ± 12.72 |
| PAP | (mmHg) | ||||
| A | 17.30 ± 4.26 | 17.40 ± 4.42 | 20.60 ± 6.19 | 19.65 ± 4.38 | - |
| B | 19.40 ± 5.60 | 19.70 ± 5.62 | 24.40 ± 8.46 ӿ ¥ | 21.25 ± 5.87 | - |
| C | 27.75 ± 7.25 | 27.05 ± 6.95 | 31.40 ± 15.25 | 30.45 ± 9.85 | - |
| D | 27.40 ± 8.31 | 27.80 ± 7.98 | 32.35 ± 11.24 ¥ | 30.30 ± 9.22 | - |
| Paw | (mmHg) | ||||
| A | 13.80 ± 2.41 | 14.15 ± 4.87 | 15.95 ± 3.28 | 15.50 ± 2.43 | 13.75 ± 2.43 |
| B | 13.75 ± 3.18 | 14.00 ± 3.08 | 16.80 ± 7.67 | 18.25 ± 6.54 ӿ ¥ | 13.85 ± 3.82 |
| C | 14.40 ± 2.06 | 14.80 ± 2.73 | 15.95 ± 3.62 | 15.25 ± 3.09 | 14.35 ± 2.28 |
| D | 14.70 ± 2.89 | 14.70 ± 3.13 | 16.45 ± 5.48 | 18.75 ± 3.56 † | 14.40 ± 3.15 |
| Cdyn | (ml/cmH2O) | ||||
| A | 14.30 ± 4.16 | 14.35 ± 3.73 | 12.55 ± 4.68 | 12.05 ± 4.44 | 14.90 ± 4.24 |
| B | 13.65 ± 5.69 | 13.30 ± 5.55 | 11.45 ± 4.91¥ | 10.40 ± 4.86 ¥ | 14.50 ± 5.17 |
| C | 13.10 ± 4.94 | 13.05 ± 4.89 | 12.85 ± 4.79 | 12.05 ± 5.45 | 12.95 ± 4.05 |
| D | 13.60 ± 3.47 | 13.00 ± 3.51 | 11.20 ± 4.20 ӿ | 9.55 ± 4.07 † | 13.45 ± 5.92 |
Data are presented as mean ± SD. Significant differences are expressed as follows: Data compared with A group- ӿ, with C group- † with T1 time point- ¥ if p<0.05. MAP-mean arterial pressure, HR: Heart Rate, PAP: Pulmonary Artery Pressure, Paw: Airway Pressure, Cdyn: Dynamic Pulmonary Compliance, T: Time Point, T1: 1 min pre protamine start, T2: 1 min post protamine start, T3: 3 min post protamine start, T4: 5 min post protamine start, T5: 10 min post protamine end.
Table 4: Intraoperative haemodynamic variables compared with groups and time.
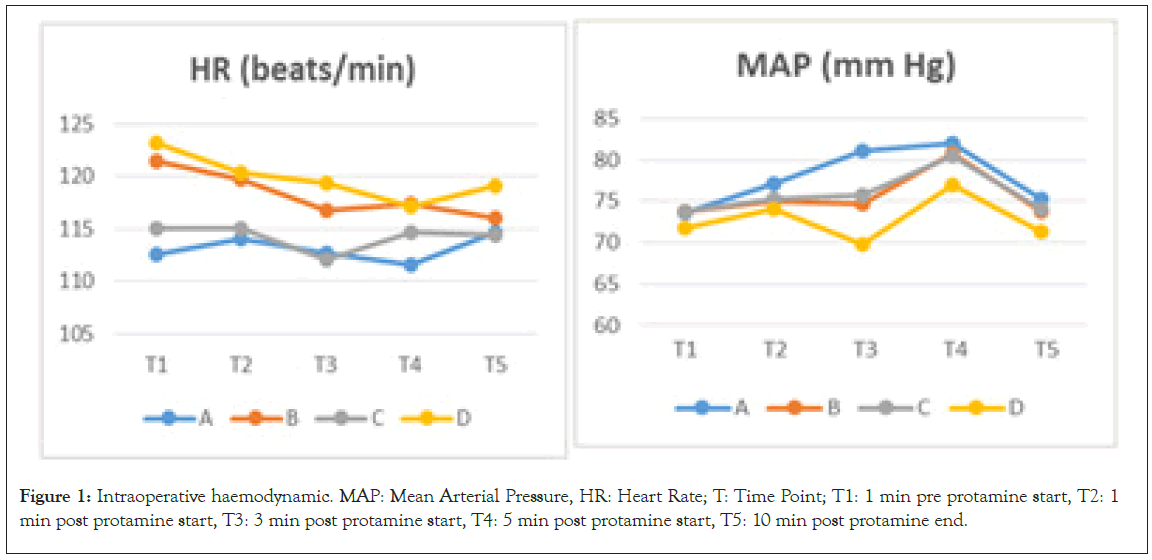
Figure 1: Intraoperative haemodynamic. MAP: Mean Arterial Pressure, HR: Heart Rate; T: Time Point; T1: 1 min pre protamine start, T2: 1 min post protamine start, T3: 3 min post protamine start, T4: 5 min post protamine start, T5: 10 min post protamine end.
The intraoperative pulmonary inflammatory data before neutralization and after neutralization are presented in Figure 2 and Table 5. Compared with the T1 time point, the plasma levels of TXB2 and 6-keto-PGF1a in the radial artery and right atrium in the four groups at the T6 time point decreased. At the T6 time point, the plasma TXB2 level in the A2 and B2 groups was higher than that in the A1 and B1 groups, respectively, while the level of 6-keto-PGF1a in the A2 and B2 groups was lower, respectively. Spearman’s correlation analysis was performed to evaluate the relationship between the plasma TXB2 level in the radial artery at the T6 time point and the PAP value at the T3 time point. The Paw value at the T4 time point revealed a (weak) correlation (correlation coefficient 0.44, P=0.000 and 0.25, P=0.027, respectfully). Furthermore, the plasma TXB2 level in the right atrium at the T6 time point, the PAP value in the T3 time point, and the Paw value in the T4 time point revealed a (weak) correlation (correlation coefficient 0.41, P=0.000 and 0.30, P=0.007, respectfully), while there was no correlation between the plasma 6-keto-PGF1a level in the radial artery or in the right atrium at the T6 time point, the PAP value at the T3 time point (correlation coefficient 0.09, P=0.413 and 0.2, P=0.85, respectively), and the Paw value at the T4 time point (correlation coefficient -0.20, P=0.079 and -0.14, P=0.216, respectively).
| Compounds | T1 Radial artery | T1 Right atrium | T6 Radial artery | T6 Right atrium |
|---|---|---|---|---|
| TXB2 | (pg/ml) | |||
| A | 4046.07 ± 1358.21 | 3800.72 ± 1367.60 | 2015.98 ± 1130.71 ¥ | 1806.45 ± 669.26¥ |
| B | 4062.27 ± 1297.22 | 3904.48 ± 1087.11 | 2835.11 ± 1536.47 ӿ ¥ | 2663.43 ± 1786.04 ӿ¥ |
| C | 4906.03 ± 1460.70 | 4563.12 ± 1235.46 | 3085.53 ± 2092.23¥ | 2754.69 ± 1400.07¥ |
| D | 4618.44 ± 618.00 | 4307.31 ± 1140.12 | 3532.93 ± 1535.05†¥ | 3149.82 ± 1446.41† ¥ |
| 6-keto- | PGF1 a (pg/ml) | |||
| A | 1402.50 ± 355.61 | 1326.95 ± 344.56 | 579.50 ± 142.28¥ | 568.95 ± 133.61¥ |
| B | 1383.90 ± 325.46 | 1327.80 ± 443.21 | 480.55 ± 148.62 ӿ ¥ | 473.30 ± 145.81 ӿ ¥ |
| C | 2042.55 ± 384.75 | 1828.35 ± 328.71 | 778.95 ± 165.02¥ | 667.35 ± 158.82¥ |
| D | 1882.45 ± 303.66 | 1777.95 ± 525.26 | 658.40 ± 155.89†¥ | 548.60 ± 143.73†¥ |
Note: Data are presented as mean ± standard deviation (SD). Data compared with A group- ӿ, with C group- † with T1 time point- ¥ and (p<0.05) significant differences are expressed. T1: 1 min pre protamine start, T6: 20 min post protamine end, TXB2: Thromboxane B2, 6-keto-PGF1a: 6-keto-prostaglandin F1a.
Table 5: Inflammatory compounds data compared with groups and time.
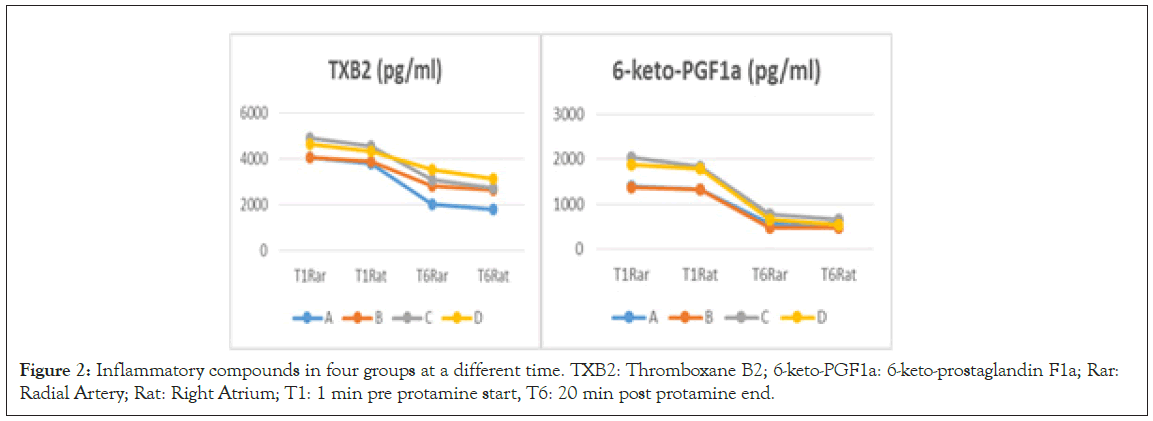
Figure 2: Inflammatory compounds in four groups at a different time. TXB2: Thromboxane B2; 6-keto-PGF1a: 6-keto-prostaglandin F1a; Rar: Radial Artery; Rat: Right Atrium; T1: 1 min pre protamine start, T6: 20 min post protamine end.
Intraoperative pulmonary function data before neutralisation and after neutralisation are presented in Table 6. Compared with the T0 time point, the OI in the group D at the T1 and T6 time points significantly decreased, while the levels of RI and A-aDO2 in the B, C, and D groups at the T1 time point were significantly higher. However, the levels of RI and A-aDO2 in the group C at the T6 time point recovered to that in the T0 time point.
| Group | T0 | T1 | T6 |
|---|---|---|---|
| OI (mmHg) | |||
| A | 423.85 ± 129.91 | 369.01 ± 115.22 | 403.60 ± 126.53 |
| B | 422.40 ± 109.69 | 359.95 ± 123.29 | 371.67 ± 128.45 |
| C | 426.93 ± 101.09 | 363.87 ± 148.86 | 378.61 ± 137.69 |
| D | 426.68 ± 115.65 | 349.85 ± 121.22 ¥ | 358.12 ± 139.08 ¥ |
| RI | |||
| A | 0.59 ± 0.47 | 0.94 ± 0.77 | 0.78 ± 0.70 |
| B | 0.40 ± 0.31 | 1.03 ± 0.92 ¥ | 0.84 ± 0.69 ¥ |
| C | 0.49 ± 0.44 | 1.05 ± 0.94 ¥ | 0.79 ± 0.57 |
| D | 0.53 ± 0.44 | 1.18 ± 1.12 ¥ | 1.08 ± 0.93¥ |
| A-aDO2 (mmHg) | |||
| A | 88.15 ± 48.10 | 143.10 ± 77.57 | 109.80 ± 62.49 |
| B | 74.40 ± 40.66 | 175.40 ± 95.38 ¥ | 131.10 ± 61.70 |
| C | 97.25 ± 92.04 | 183.65 ± 73.04 ¥ | 128.50 ± 35.31 |
| D | 102.15 ± 75.91 | 186.47 ± 112.13 ¥ | 145.43 ± 97.40 ¥ |
Note: Data are presented as mean ± standard deviation (SD). Data compared with T1 time point- ¥
Table 6: Intraoperative pulmonary function data compared at a different time.
Discussion
Protamine can induce severe pulmonary vasoconstriction in cardiac surgery. During the past decades, many cases were diagnosed as protamine-induced severe pulmonary vasoconstriction [7-9]. There are 2 mechanisms about pulmonary vasoconstriction induced by protamine infusion. The local excess of heparin-protamine complex, which is caused by rapid infusion of protamine, is thought to cause lift-threatened adverse haemodynamic reaction [2,3]. Besides, the animal and human experiments showed that contact with the oxygenator surface can activate the complement system through alternate pathways, leading to the generation of anaphylatoxins complements C3a and C5a [13,14]. This causes smooth muscle contraction, accumulation of platelets and leucocytes activation in the lungs, which subsequently induce the release of a large number of proteolytic enzymes to cause lung injury [13,15] As a result, pulmonary vasoconstriction, and potential lung injury would likely be mediated through complement-mediated C5a-induced TXA2 generation and might thereby result in the development of various cardiovascular adverse effects, such as pulmonary artery pressure elevation, right ventricular/right atrial pressures elevation, and systemic hypotension. Therefore, the regimen of treatment is to reduce pulmonary artery pressure and inhibit inflammatory responses, to reduce the generation of TXA2, which may be useful [3].
Our results indicate that precondition of lignocaine before the neutralisation of heparin effectively prevents protamine- induced pulmonary vascular reaction during CHD repair. The pulmonary circulation haemodynamic indexes (PAP, Paw, and Cydn) of patients receiving lignocaine preconditioning before heparin neutralisation experienced less fluctuations than that of the patients receiving normal saline preconditioning. Cydn is the ratio of the change in lung volume to the change in pressure over a tidal breath, with the pressure measured at moments of zero flow during breathing; its fall usually implies a narrowing of subtending alveoli. Meanwhile, the plasma TXB2 level of patients receiving lignocaine preconditioning was lower than that of patients receiving normal saline preconditioning, while the level of 6-keto-PGF1a of patients receiving lignocaine preconditioning was higher. Post protamine pulmonary function (OI, RI, and A-aDO ) was unchanged when compared with the baseline in patients receiving lignocaine preconditioning, but this worsened in patients receiving normal saline preconditioning. RI is the ratio of P (A-a) DO and PaO and it can reflect the function of pulmonary ventilation and oxygen exchange. OI reflect the effects of respirator pressure on oxygenation.
Severe protamine-mediated pulmonary hypertension is more likely associated with the anaphylactoid reaction. Protamine is known to be immunogenic, and the large complex formed by protamine binds to heparin in a charger-dependent manner, which are also immunogenic. The neutralization of heparin with protamine in patients undergoing CPB procedure activates the complement: the system through two cascades, protamine and protamine/heparin complexes can directly activate the complement system in the lung through the classical way when protamine was administered into the pulmonary circulation. Lignocaine may not avoid the so feared catastrophic anaphylactic reactions to protamine. The anti-inflammatory effect of lidocaine is well-recognized in literature. These include the inhibition of granulocyte adherence, the inhibition of neutrophil function, including chemotaxis and superoxide anion release [16], and the inhibition of the release of cytokines and proteolytic enzymes [17], which have a certain protective effect on vascular endothelial cells. Meanwhile, lignocaine is known to inhibit aggregation and the activation of platelets and decrease the serum concentration of TXB2 [18].
This study was designed to investigate the potential cardiopulmonary benefits of lignocaine preconditioning before the neutralization of protamine. Looking at available clinical literature lignocaine 2 mg/kg is considered haemodynamically safe, so we decided on this dosage of lignocaine [10,19]. Regarding haemodynamic function, the present study indicated that lignocaine preconditioning before the neutralization of protamine can effectively reduce pulmonary vasoconstriction response, and enhance the resistance of the blood vessel wall to the harmful stimulation of protamine, reducing the stress responses and spasm reaction of the pulmonary vascular and tracheal smooth muscle caused by the direct stimulation of protamine. At the time point of one-minute pre-protamine administration, the values of PAP and Paw were significantly higher in the B group than that in the A group, while the values of MAP in the C group were significantly lower. Meanwhile, Paw was significantly higher in the D group than that in the C group, while the values of Cydn were significantly lower in the D group. Furthermore, when the values of one-minute pre-protamine administration were compared with that of three-minute post-protamine administration, patients in the B and D groups exhibited a mean increase in PAP and Paw. Furthermore, when these were compared with the values at three-minute and four-minute post-protamine administration, patients in the B and D groups exhibited a mean decrease in Cdyn. The differences in pulmonary circulation indexes between the two routes of prevention (lignocaine/normal saline) were even more impressive and clinically relevant when considering the number of patients in each group who experienced advise pulmonary vascular reactions. Although without statistically significant difference, substantially more patients in the B and D groups (3 and 5, respectively) experienced advise pulmonary vascular reactions at this time point, when compared to patients in the A and C groups (0 and 1, respectively). Furthermore, the incidence of protamine adverse reactions in the lignocaine group (A and C groups) was lower than that in the normal saline group (B and D group).
Regarding to pulmonary inflammatory response indexes, the present study indicated that lignocaine preconditioning before the neutralisation of protamine effectively reduces TXB2 release and increases 6-keto-PG-F1a generation to inhibit the inflammatory response in the lung. At the time point of the 20th-minute post–protamine administration, the plasma TXB2 levels in the B and D groups were higher than that in the A and C groups, and the levels of 6-keto-PGF1a in the B and D groups were lower. Our findings of pulmonary inflammatory response indexes were similar to Jerath A. et al. in their study they used inhaled prostacyclin for the successful management of severe protamine-induced pulmonary hypertension [20].
Regarding pulmonary function, the present study indicated that lignocaine preconditioning before the neutralisation of protamine may improve respiratory function. Even though the baseline values were compared with that at one-minute pre- protamine administration, the values of OI in the C group exhibited a mean increase, and the values of RI and A-aDO in the B, C, and D groups exhibited a mean decrease, but the values of RI and A-aDO in the C group at the time point of the 20-minute post–protamine administration recovered to baseline.
Conclusion
All physicians should alert to severe pulmonary vasoconstriction induced by protamine in cardiac surgery. The primary symptom was hypotension shortly after protamine infusion, combined with increasing in PAP. The use of lignocaine preconditioning before the neutralization of heparin exerts its effect through the inhibition of TXB2 release and generation of 6-keto-PG-F1a, in order to inhibit the inflammatory responses in the lung and pulmonary vasoconstriction responses, which are caused by the direct stimulation of protamine. By reducing the occurrence rate of pulmonary vascular adverse reactions induced by protamine, lignocaine improves respiratory function and plays a certain role in lung protection.
Limitations
This study includes a fairly small sample size, from one centre and a lack of insight into the mechanisms. Future studies with larger sample size, at multiple centres as well as involving laboratory studies, will be conducted to further prove our results and to elucidate the mechanism.
Conflicts of Interest
The authors declare no conflicts of interest.
REFERENCES
- Boer C, Meesters MI, Veerhoek D, Vonk ABA. Anticoagulant and side-effects of protamine in cardiac surgery: A narrative review. British J Anaesth. 2018; 120(5): 914-927.
- Tiwari D, Saraogi A, Gehlot R. Life-threatening protamine allergic reaction in a patient with extra-adrenal pheochromocytoma undergoing off-pump coronary artery bypass grafting. Ann Card Anesth. 2016; 19: 351-353.
- Comunale ME, Maslow A, Robertson LK, Haering JM, Mashikian JS, Lowenstein E. Effect of site of venous protamine administration, previously alleged risk factors, and preoperative use of aspirin on acute protamine-induced pulmonary vasoconstriction. J Cardiothorac Vasc Anesth. 2003; 17: 309-313.
- Bruins P, te Velthuis H, Eerenberg-Belmer AJ, Yazdanbakhsh AP, de Beaumont EM, Eijsman L, et al. Heparin-protamine complexes and C-reactive protein induce activation of the classical complement pathway: Studies in patients undergoing cardiac surgery and in vitro. Thromb Haemost. 2000; 84: 237-243.
- Kimmel SE, Sekeres M, Berlin JA, Ellison N. Mortality and adverse events after protamine administration in patients undergoing cardiopulmonary bypass. Anesth Analg. 2002; 94: 1402-1408.
- Singh S, Zanwar YN, Okyere I. Does meticulous blood glucose control during cardiopulmonary bypass improves outcome in paediatric patients? EAS J Anesthesiol Crit Care. 2020; 2(4): 151-159.
- Guan Z, Shen X, Zhang YJ, Li XG, Gao YF, Tan J, et al. Use of epoprostenol to treat severe pulmonary vasoconstriction induced by protamine in cardiacsurgery. Medicine (Baltimore) 2018; 97: e10908.
- Whitman GJ, Martel D, Weiss M, Pochanapring A, See WM, Hopeman A, et al. Reversal of protamine-induced catastrophic pulmonary vasoconstriction by prostaglandin E1. Ann Thorac Surg. 1990; 50: 303-305.
- Ralley FE. The use of nitric oxide for managing catastrophic pulmonary vasoconstriction arising from protamine administration. Anesth Analg. 1999; 88: 505-507.
- Kulsum SS, Singh SH, Annamalai A A, Mahrous DE. Can sodium channel blocker lidocaine attenuate haemodynamic responses to endotracheal intubation in patients with coronary artery disease effectively? J Anesthesiol. 2013; 1 (3): 27-35.
- Ding XF, Zhong LL, Zhang B, Li J. Protective effects of lidocaine on hyperoxia-exposed type 11 alveolar epithelial cells from premature rats. Zhongguo Dang Dai Er Ke Za Zhi. 2011; 13: 313-316.
- Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019; 53(1): 1801913.
- Morel DR, Zapol WM, Thomas SJ, Kitain EM, Robinson DR, Moss J, et al. C5a and thromboxane generation associated with pulmonary vaso-and broncho-constriction during protamine reversal of heparin. Anesthesiol. 1987; 66: 597-604.
- Singh S, Mahrous DE. Conventional ultrafiltration versus combined conventional and modified ultrafiltration on clinical outcomes of pediatric cardiac surgery. J Anesth Clin Res. 2019; 10 (12): 1-6.
- Koster A, Fischer T, Praus M, Haberzettl H, Kuebler WM, Hetzer R, et al. Hemostatic activation and inflammatory response during cardiopulmonary bypass: Impact of heparin management. Anesthesiology. 2002; 97: 837-841.
- Cassuto J, Sinclair R, Bonderovic M. Anti-inflammatory properties of local anesthetics and their present and potential clinical implications. Acta Anaesthesiol Scand. 2006; 50: 265-282.
- Feng G, Liu S, Wang GL, Liu GJ. Lidocaine attenuates lipop-olysaccharide induced acute lung injury through inhibiting NF-kappaB activation. Pharmacology. 2008; 81: 32-40.
- Satoh K, Kamada S, Kumagai M, Sato M, Kuji A, Joh S. Effect of lidocaine on swine lingual and pulmonary arteries. J Anesth. 2015; 29: 529-534.
- Singh S, Laing EF, Owiredu WKBA , Singh A, Annamalai A. A Study of the efficacy of cardiac antidysrhythmic drugs in attenuating haemodynamic responses to laryngoscopy. J Anesthesiol. 2013; 1(1): 1-8.
- Jerath A, Srinivas C, Vegas A. The successful management of severe protamine-induced pulmonary hypertension using inhaled prostacyclin. Anesth Analg. 2010; 110: 365-369.
Citation: Singh S, Annamalai A (2021) Is Lignocaine Preconditioning Effective to Treat Severe Pulmonary Vasoconstriction Induced by Protamine during Cardiac Surgery? J Surg Anesth. 5:148.
Copyright: © 2021 Singh S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
