Indexed In
- Open J Gate
- Academic Keys
- ResearchBible
- China National Knowledge Infrastructure (CNKI)
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- CABI full text
- Publons
- Geneva Foundation for Medical Education and Research
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2021) Volume 0, Issue 0
In-vivo and In-silico Investigations on Goron Tula (Azanza garckeana F. Hoffm), a Booster of Female Sexual Arousal and Vaginal Lubrication
Ben E. Ehigiator1*, Raphael I. Okoli2, Nkem G. Ndubueze1 and Omotayo O. Ebong112Department of Pharmacology and Therapeutics, Faculty of Basic Clinical Sciences, Ambrose Alli University, Ekpoma, Nigeria
Received: 23-Nov-2021 Published: 31-Dec-2021
Abstract
Female sexual arousal and vagina lubrication are core complexes in achieving and enjoying a desirable sexual life in females. It has been noticed that female sexual arousal disorders have been kept away from public discus and statistics because of the lack of boldness on the part of women in speaking up about it, poor collation of medical cases, and the African mentality that tends to forbid women from being outspoken on sexual satisfaction. Azanza garckeana, a fruit native to northern Nigeria, is being used in traditional settings in diverse female sexual disorders and conditions.
This study was designed to evaluate in-vivo, the female sex-enhancing claim of the plant. The study examined the vaginal width, ear twitching, and vagina lubrication in A. garckeana treated Oryctogalus cuniculus (American rabbit). Use of In-silico studies to dock previously identified secondary metabolites against targets and enzymes associated with female sexual arousal and vagina lubrication.
The results show that the aqueous extract of A. garckeana caused a significant increase in the vagina width and lubrication of Oryctogalus cuniculus and significantly enhanced vaginal muscle relaxations and vaginal secretions upon sexual stimulation. This suggests the involvement of a mechanism that involves activation of female sexual excitatory systems and inhibition of those that depress female sexual arousal, or a combination of both. These findings corroborate findings from the molecular docking studies, which suggest that gossypol may play an important role in female sexual arousal and vagina lubrication because it looks to have some affinity with the targets of consideration in this study. Therefore, gossypol may well be responsible for the sex-enhancing activity of the plant.
Keywords
Azanza garckeana; Oryctogalus cuniculus; Female sexual arousal; Molecular docking; Gossypol; Vagina lubrication
Introduction
Female sexual dysfunction refers to the persistent or recurring decrease in sexual desire or arousal, the difficulty or inability to achieve orgasm, and or the feeling of pain during sexual intercourse (dyspareunia); while Female Sexual Arousal Disorders (FASD) may refer to the recurrent inability to attain or maintain orgasm until completion of sexual activity [1]. More often than not, it is difficult to categorize female sexual dysfunction, due to the perception of many women to sex and sex-related discussions. However, the following disorders are usually considered: Sexual interest/ arousal disorder, female orgasmic disorder, and genito-pelvic pain (dyspareunia), penetration disorder (vaginismus) [2].
According to Thomas and Thurston, predictors of female sexual dysfunction include physical health, number of pre-marital partners, religion and sexual orientation, communication with partners, and attitude towards sexuality [3].
There exists also a distinct disorder known as the hypoactive sexual desire disorder, which manifests as a continuous or recurrent absence of sexual fantasies, thoughts, or desire for sexual activity leading to personal distress [4].
The beginning of sexual arousal in a woman's body is usually marked by vaginal lubrication or wetness; swelling and engorgement of the external genitals, and internal lengthening and enlargement of the vagina, although wetness of the vagina can occur without arousal due to infection or cervical mucus production around ovulation [5].
There are studies to show the degree of correlation between these physiological responses and the woman's subjective sensation of being sexually aroused. The findings are that in some cases there is a high correlation, while in others, it is surprisingly low [6]. A large component of sexual desire in females is usually responsive rather than spontaneous, indicating that women respond to physical sexual stimulation or arousal rather than fantasy [5].
Azanza garckeana (F. Hoffm) commonly known as Goron tula, (kola of Hausa in Hausa, snot-apple, slime-apple, Rhodesian tree- hibiscus belonging to the family malvaceae, native to Botswana, Burundi, Nigeria, Zambia [7]. In Nigeria, it is grown only in Tula village of Gombe state [8]. It’s a multipurpose edible fruit of tropical Africa, also an important medicinal plant commonly used in Nigeria for food and as herbal medicine [9].
A. garckeana has been reportedly used in traditional medicine for the treatment and management of more than 20 human diseases and ailments such as cough, chest pain, infertility, sexually transmitted diseases, and menstrual abnormalities [10]. Multiple classes of bioactive metabolites that include amino acids, phenols, lipids, ascorbic acid, tannins, and saponins have been isolated from A. garckeana [11] (Figure 1).
Figure 1: A. garckeana fruits.
Phytochemical analyses have revealed that A. garckeana contains various phytochemical compounds such as flavonoids, amino acids, glycosides, saponins, sterols, etc.
Compared to previous knowledge in literature, this study was designed to appraise and investigate the aphrodisiac effect of A garckeana, and to gain insight into the potential effects by docking some of its identified compounds like Gossypol, 6,6-dimethoxygossypol, stigmasterol, e-docosyl 3-(3,4dihydroxyphenyl), acrylate: Betulinic acid, 0-naphthoquinones, Mansonones D, Mansonones E, Mansonones F, Mansonones G, Mansonones H, Azazone A, and Azazone B [12-14]. Arginine, aspartic acid, and glutamic acid, with active sites of enzymes and receptors involved in sexual function such as; Inhibition of Phosphodiesterase-5 [15,16]. Nitric oxide stimulation [17]. Activation of epithelial beta-adrenergic receptors [18]. Stimulation of Neuropeptide Y, Stimulation of melanocortin receptor 4 "MC4R", Inhibition of neprilysin, inhibition of adenosine deaminase, and stimulation of adenosine [19-22].
This study investigates in-vivo, the vaginal width, ear twitching, and vagina lubrication of A. garckeana-treated Oryctogalus cuniculus (American rabbit) and in-silico, docking identified secondary metabolites against targets and enzymes affiliated with female sexual arousal and vagina lubrication.
Materials and Methods
Acute toxicity test
The acute toxicity test (LD50) was carried out using the Organization of Economic Co-operation and Development guidelines [23].
• In-vivo studies involved the use of the Azanza garckeana aqueous extract on female Oryctolagus cuniculus (American rabbit).
• In-silico studies involved the ligand library generation of identified secondary metabolites of A. garkceana and docking with targets related to female sexual libido and lubrication, by comparing compound affinity for targets, with standards.
In-vivo studies:
Collection and identification of A. garckeana: A. garckeana, was bought from an online social media vendor and was identified at the Pharmacognosy Department of the Faculty of Pharmacy Madonna University, Elele, Rivers State Nigeria.
Preparation and administration of A. garckeana: About 387 grams of A. garckeana was weighed on an electronic weighing balance (Zhengzhou Nanbei Instrument Equipment Co. Ltd.). A. garckeana was thereafter immersed in warm water overnight, to tenderize the fruit. The soaked A. garckeana fruits were pulverised into smaller particles to enable increased surface absorption. Then the fruits were soaked again in a 500 ml beaker with about 300 ml of water added, to continue the extraction and left overnight.
Upon filteration, a slimy filtrate was obtained, which was placed in a water bath and allowed to evaporate to dryness leaving behind dried gummy aqueous extract of A. garckeana. Weighing 31.7 grams.
Experimental animals: Twelve female rabbits (Oryctogalus cunilicus), weighing between 1187 and 1190 g were obtained from the Animal Farm of the Department of Pharmacology and Toxicology, Delta State University (DELSU), Abraka. They were kept in two different rabbit hutches (two rabbits per hutch) and fed with standard animal feed and clean water and allowed to acclimatize for 1 week. The female rabbits were shared into three groups. The animals were kept in line with laid down principles for animal care as prescribed in Helsinki’s 1964 declaration. Ethical approval was obtained from the Department of Pharmacology and Toxicology of Madonna University, Nigeria-MAD/PHARM/21/212.
• Group A: Four female rabbits given water and feed only.
• Group B: Four female rabbits given 250 mg/Kg of A. garckeana extract.
• Group C: Four female rabbits given 500 mg/Kg of A. garckeana extract.
Vagina width and lubrication test: Prior to the administration of the extract, the diameters of the vagina for both sets of female rabbits were taken using a measuring thread. Group B animals were given 5 ml of aqueous extract of A. garckeana orally while those in group C, were given 10mls of aqueous extract of A. garckeana orally, and left for an hour to observe various onsets of action.
Statistical analysis: Data were analyzed, using Graph Pad Prism (5.01), one-way Analysis of Variance (ANOVA) followed by Dunnett’s multiple tests, to compare replicate means of treated groups with control and p values<0.05, 0.001 and 0.0001 were considered significant.
In-silico studies:
Ligand library generation: Identified secondary metabolites of A. garkceana employed for this study were determined from published literature and were used in the creation of the ligand library. Twenty-five (25) secondary metabolites were retrieved from NCBI Pub Chem library, in Standard Database Format (2D). The ligand library generated was imported to a docking software (Maestro) and prepared using the (Schrodinger suite version 2018-1b), as described by Brooks et al [24-26].
Protein preparation: Human PDE-5 complexed with an inhibitory coligand (2.3 Å), human endothelial nitric oxide synthase laced with activator coligand (1.76 Å), Human Adenosine deaminase in complex with an inhibitory coligand, (2.00 Å), human neuropeptide Y1 complexed with an against (2.75 Å), human neprilysin in complex with the inhibitor (2.54 Å) and human B2-adrenergic G protein-coupled receptor complexed with a stimulatory coligand (2.40 Å).
Module in Maestro 11.5 was used to prepare each protein complex. Missing hydrogen atoms, missing loop, and missing side-chains of protein structure were fixed while the added hydrogen atoms were optimized at pH 7.0. Optimized structures were then minimized using the OPLS3 force field by converging heavy atoms to Root Mean Square Deviation (RMSD) of 0.3Å [27].
2H42, 3DWB, 1NDZ, 5ZBQ, 6THP, 2RH1 respectively. Modeled Melanocortin 4 receptor, retrieved from GPCRdb. It was prepared, using the Protein Preparation Wizard as described by [27].
Pharmacokinetic parameters (ADME/TOX Prediction): The pharmacokinetic properties of the hit compounds were estimated using the Absorption, Distribution, Metabolism, Excretion, and Toxicity (ADMET) of the hit ligands, and predicted using the Qikprop module in Maestro 11.5 [24].
Results
Results
Acute toxicity test
Table 1 shows the acute toxicity test (LD50) results, using the method Organization of Economic Co-operation and Development guidelines [23].
| Dose | No of Deaths |
|---|---|
| 10 mg/Kg | 0/4 |
| 1000 mg/Kg | 0/4 |
| 2000 mg/Kg | 0/4 |
| 5000 mg/Kg | 0/4 |
Table 1: Results of acute toxicity tests in female rabbits.
Clinical toxicity symptoms such as respiratory distress, salivation, weight loss, and change in the physical appearance of hair as well as maternal mortality were not observed at any period of the experiment. The LD50 is expected to exceed 5000 mg/Kg. Doses were chosen in agreement with the work done by Yusuf, et al. using the said plant on wound healing [8].
Observable arousal sign in female rabbits
As shown in Table 2, the observable arousal signs of vaginal lubrication, ear twitching, and agility of the female rats in experimental groups were significantly higher compared to the control.
| Observable arousal signs | Untreated n | Treated 250 mg/Kg | Treated 500 mg/Kg |
|---|---|---|---|
| Vagina Lubrication | - | + | + |
| Ear Twitching | - | - | + |
| Agility | - | + | + |
| +: Presence, -: Absence | |||
Table 2: Effect of A. garkeana on observable arousal sign in female rabbits. Animals per group (n)=4.
Table 3 shows the width change, measured with a thread. It was observed that the vaginal width increased significantly on the administration of the extract.
| Parameter | Untreated | Treated 250 mg/Kg | Treated 500 mg/Kg |
|---|---|---|---|
| vaginal diameter (cm) pre/post difference | 0.00 ± 0.00 | 0.36 ± 0.033*** | 0.50 ± 0.037*** |
| Effect of A. garkeana on relative vaginal diameter. Animals per group (n)=4. The values are mean ± SEM.; ***p<0.05; when compared with the control group. (One-way ANOVA followed by Dunnett’s multiple comparison test). | |||
Table 3: Effect of A. garkeana on observable distention of vaginal diameter 1 hour post-treatment compared to pre-treatment.
Scores of identified phytochemical compounds docked into the substrate-binding sites of Phosphodiesterase 5
Among the compounds docked into the substrate-binding sites of PDE-5 in comparison with an inhibitory coligand. Results showed that (-)-6-methoxygossypol and Gossypol, 6,6'- dimethoxygossypol presented with a high affinity for this target (Figure 2).
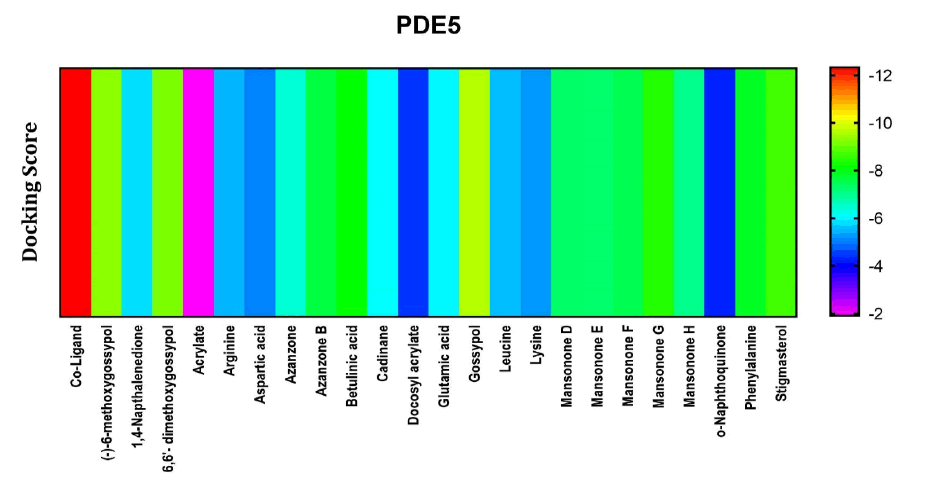
Figure 2: Heat map representation of docking results for compound interaction with PDE-5.
The free energy of binding of phytochemicals of A. garckeana docked into the substrate-binding sites of PDE-5 represented in the form of a heat map. (The scale is a spectrum from purple (-2 kcal/mol) to red (-12 kcal/mol).
Scores of identified phytochemical compounds docked into the substrate-binding sites of Endothelial nitric oxide synthase
Among the compounds docked into the substrate-binding sites of endothelial nitric oxide synthase, (an enzyme responsible for the production of endothelial nitric oxide) which was in comparison with a stimulatory coligand. Results showed that gossypol looked to have the highest affinity for the enzyme indicating that it may well have some viable activity on stimulation of endothelial nitric oxide synthase (Figure 3).
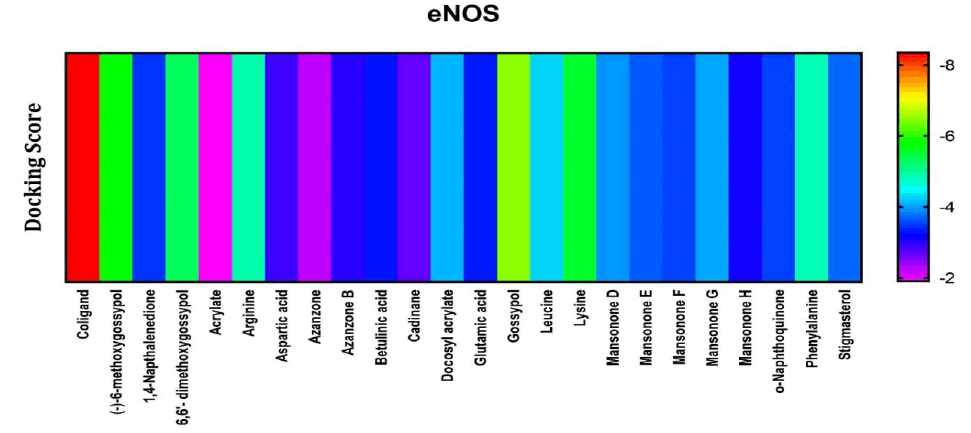
Figure 3: Heat map representation of docking results for compound interaction with Human Endothelial nitric oxide.
The free energy of binding of phytochemicals of A. garckeana docked into the substrate-binding sites of Human Endothelial Nitric Oxide is represented in the form of a heat map. The scale is a spectrum from purple (-2 kcal/mol) to red (-8 kcal/mol).
Scores of identified phytochemical compounds docked into the substrate-binding sites of β2 adrenergic receptor
Among the compounds docked into the substrate-binding sites of β2 adrenergic in comparison with a stimulatory coligand. Results showed that the azazone and mansonone compounds presented with high affinity for this target. Hence are likely to contribute to the female arousal effect by this mechanism of action (Figure 4).
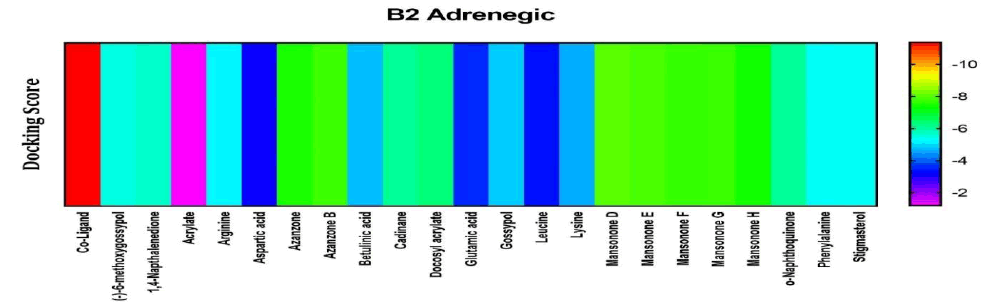
Figure 4: Heat map representation of docking results for compound interaction with β2 adrenergic.
The free energy of binding of phytochemicals of A. garckeana docked into the substrate-binding sites of β2 adrenergic represented as a heat map. (The scale is a spectrum from blue (-2 kcal/mol) to red (-10 kcal/mol).
Scores of identified phytochemical compounds docked into the substrate-binding sites of neuropeptide Y
Among the compounds docked into the substrate-binding sites of neuropeptide y, one of the most prevalent peptides in the body [28]. It plays a role in increasing dopamine levels. Dopamine is an important neurotransmitter that plays a vital role in sexual arousal; has a wide-reaching effect on the central nervous system and periphery. It also has the potency of inhibiting cortisol and ACTH release with a stress-protective effect, in comparison with a stimulatory coligand. The Result showed that Gossypol looked to have the highest affinity for the receptor (Figure 5).
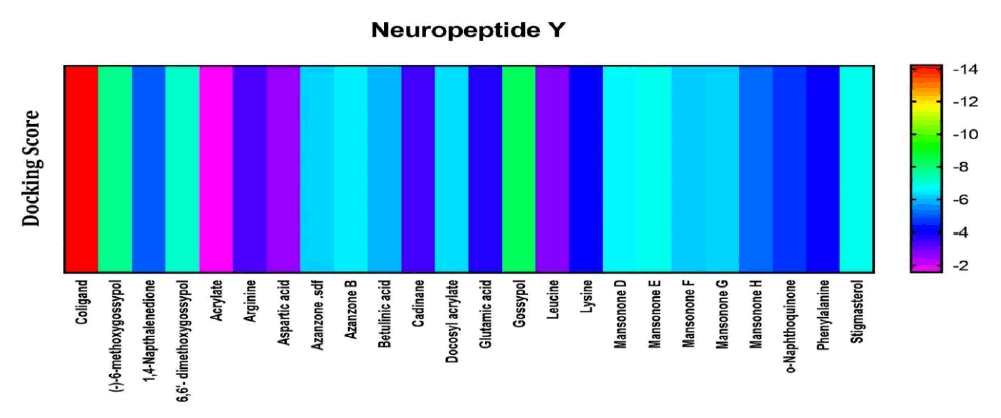
Figure 5: Heat map representation of docking result for compound interaction with Neuropeptide Y.
The free energy of binding of phytochemicals of A. garckeana docked into the substrate-binding sites of Neuropeptide Y is represented in the form of a heat map. (The scale is a spectrum from purple (-2 kcal/mol) to red (-14 kcal/mol).
Scores of identified phytochemical compounds docked into the substrate-binding sites of the Melarnocortin 4 receptor
Among the compounds docked into the substrate-binding sites of MC4R in comparison with a stimulatory coligand. Results showed that (-)-6-methoxygossypol, Gossypol, and stigmasterol, presented with high affinity for this target. Hence, they are likely to contribute to the female arousal effect via this mechanism of action (Figure 6).
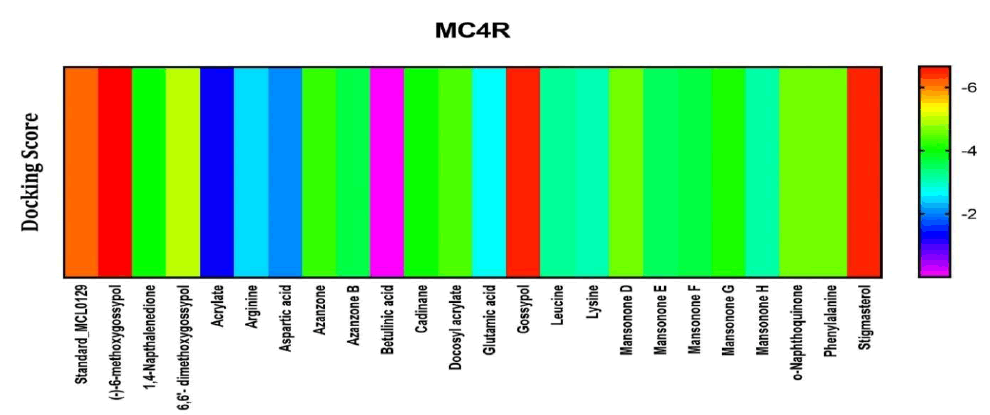
Figure 6: Heat map representation of docking results for compound interaction with Melarnocortin 4.
The free energy of binding of phytochemicals of A. garckeana docked into the substrate-binding sites of Melarnocortin 4 receptor is represented in the form of a heat map. (The scale is a spectrum from blue (-2 kcal/mol) to red (-6 kcal/mol).
Scores of identified phytochemical compounds docked into the substrate-binding sites of Neprilysin
Among the compounds docked into the substrate-binding sites of neprilysin in comparison with an inhibitory coligand, results showed that none of the compounds presented with high affinity for this target (Figure 7).
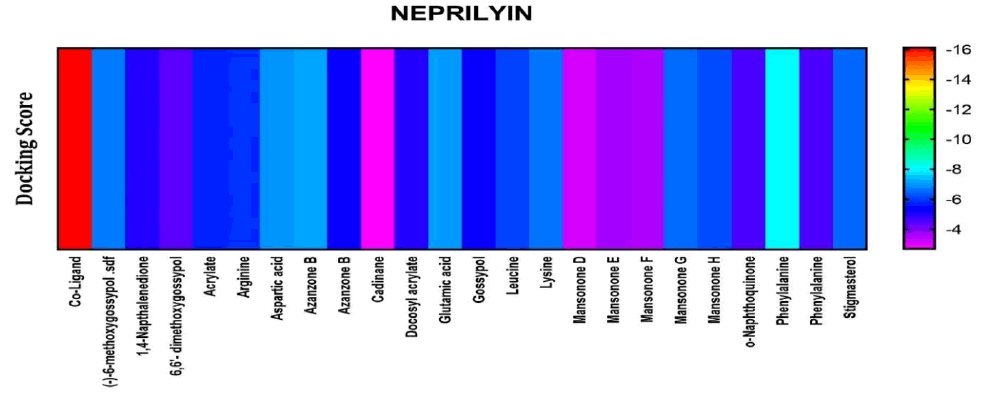
Figure 7: Heat map representation of docking results for compound interaction with Neprilysin.
The free energy of binding of phytochemicals of A. garckeana docked into the substrate-binding sites of Neprilysin represented in the form of a heat map. (The scale is a spectrum from blue (-4 kcal/mol) to red (-16 kcal/mol).
Scores of identified phytochemical compounds docked into the substrate-binding sites of adenosine deaminase
Among the compounds docked into the substrate-binding sites of adenosine deaminase in comparison with a coligand, gossypol looked to have the highest affinity for the enzyme. Indicating that it may well have some viable activity on inhibition of adenosine deaminase. Results showed that Phenylalanine, lysine, leucine, stigmasterol, and glutamic acid also look to have good affinities for the enzyme (Figure 8).
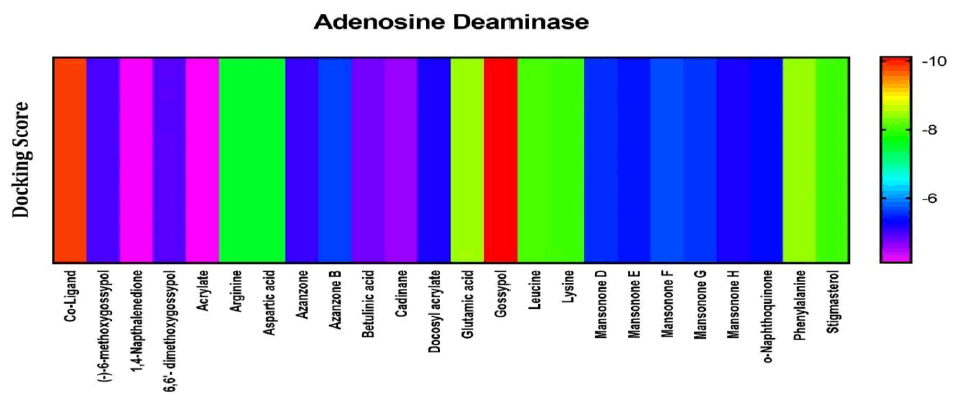
Figure 8: Heat map representation of docking results for compound interaction with Adenosine deaminase.
The free energy of binding of phytochemicals of A. garckeana docked into the substrate-binding sites of Adenosine deaminase is represented in the form of a heat map. (The scale is a spectrum from blue (-6 kcal/mol) to red (-10 kcal/mol) (Table 4).
| Entry Name | mol MW | donorHB | accptHB | QPlogPo/w | HOA | ROF |
|---|---|---|---|---|---|---|
| (-)-6-methoxygossypol | 532.589 | 3 | 6.5 | 4.41 | 1 | 1 |
| 1,4-Napthalenedione | 158.156 | 0 | 4 | 0.908 | 3 | 0 |
| 1'-OH-4-Keto-gamma-carotene | 568.881 | 1 | 2.75 | 11.804 | 1 | 2 |
| 6,6'- dimethoxygossypol | 546.616 | 2 | 6.5 | 5.094 | 1 | 2 |
| Acrylate | 72.063 | 1 | 2 | 0.128 | 2 | 0 |
| Arginine | 174.202 | 7 | 5 | -3.529 | 1 | 1 |
| Aspartic acid | 133.104 | 3 | 4 | -3.886 | 1 | 0 |
| Azanzone | 244.29 | 1 | 4.75 | 1.75 | 3 | 0 |
| Azanzone B | 258.273 | 1 | 5.5 | 1.315 | 3 | 0 |
| Betulinic acid | 456.707 | 2 | 3.7 | 6.345 | 1 | 1 |
| Cadinane | 208.386 | 0 | 0 | 7.049 | 1 | 1 |
| Docosyl acrylate | 380.653 | 0 | 2 | 8.909 | 1 | 1 |
| Glutamic acid | 147.13 | 4 | 5 | -3.015 | 1 | 0 |
| Gossypol | 518.562 | 4 | 6.5 | 3.667 | 1 | 2 |
| Leucine | 131.174 | 3 | 3 | -1.521 | 2 | 0 |
| Lysine | 146.189 | 5 | 4 | -3.187 | 1 | 0 |
| Mansonone D | 242.274 | 0 | 4.75 | 1.646 | 3 | 0 |
| Mansonone E | 242.274 | 0 | 4.75 | 1.708 | 3 | 0 |
| Mansonone F | 240.258 | 0 | 4.5 | 1.765 | 3 | 0 |
| Mansonone G | 244.29 | 1 | 4.75 | 1.854 | 3 | 0 |
| Mansonone H | 258.273 | 1 | 5.5 | 1.354 | 3 | 0 |
| o-Naphthoquinone | 158.156 | 0 | 4 | 0.663 | 3 | 0 |
| Phenylalanine | 165.191 | 3 | 3 | -1.145 | 2 | 0 |
| Stigmasterol | 412.698 | 1 | 1.7 | 7.564 | 1 | 1 |
| MW: Molecular Weight; R.V.=130-725; donorHB: Estimated number of hydrogen bonds that would be donated by the solute to water molecules in an aqueous solution; R.V.=0.0-6.0; accptHB: Estimated number of hydrogen bonds that would be accepted by the solute from water molecules in an aqueous solution; R.V.=2.0-20.0; QPlogPo/w: Predicted octanol/water partition coefficient; HOA: Human Oral Absorption level 1, 2, 3; 1=low, 2=medium, 3=high; ROF: The number of violations of Lipinski’s rule of five | ||||||
Table 4: Showing the pharmacokinetic/toxicological properties of compounds present in A. garckeana.
Discussion
This study evaluated and validated the claim of high efficacy of A. garckeana, in the treatment of female sexual arousal disorder. Sexual dysfunction affects about 30%-40% of women and the lack of sexual desire is the most common complaint among them [29]. In women, sexual dysfunction tends to increase as women age, but can affect women at any stage of life [30]. It may be chronic or temporary. Factors that can lead to arousal disorders include, use of some medications such as antidepressants, drugs of addiction and alcohol abuse, conditions that impair blood flow to the vagina, gynecological conditions such as, ovarian cyst, endometrial hormonal changes, chronic disease states, and debilitating conditions such as menopausal changes, vaginal atrophy, and conditions that lead to it. These conditions may contribute to vaginismus, painful sex (dyspareunia), and low sexual desire [31].
Manifestations of hypoactive female sexual arousal disorder may include low libido or loss of interest in sexual activity, lack of pleasurable sensations even when the sexually sensitive areas are stimulated, difficulty getting pleasure from actual sexual activities, few or no sexual fantasies at all [32]. More often than not, male sexual dysfunction and its remedy is given much more attention, while, very little attention is paid to female sexual dysfunction and its possible remedies.
In this study, the increase in vagina width in treated groups proves without doubt, that A. garckeana has a unique property of increasing vaginal lubrication and inducing sexual arousal and justifies its use in the traditional medicine setting to treat female sexual arousal problems.
The mechanism of A. garckeana female sexual arousal action is unknown, but it is likely to involve the activation of female sexual excitatory systems and inhibition of those that depress female sexual arousal, or a combination of both. These systems might include either one or more of the following: the enhancement or potentiation of the physiological actions of adenosine through the L-Arginine-Nitric oxide syntheses pathway, which induces relaxation of the female corpora and increases levels of PDE-5 in the female vagina, clitoral and labial smooth muscles, accounting for the lengthening of the width of the vagina or the activation of Melanocortin receptor. This receptor has been associated with central control of sexual behavior and is known to increase penile erection in males and receptivity in females.
Over 20 compounds had previously been identified in the plant as indicated above. Upon molecular docking with related targets to the subject of interest, it was observed that gossypol looked to have potential drug activity on the targets of consideration. It is pertinent to say that, molecular docking is one of the most frequently used methods in structure-based drug design, due to its ability to predict the binding conformation of small-molecule ligands to the appropriate binding sites. Characterization of the binding behavior play important role in the rationale designs of drug and it helps to elucidate fundamental biochemical processes [33]. Gossypol is a polyphenolic aldehyde present in A garckeana [12,13].
This compound may well be responsible for the sexual arousal and vaginal lubrication properties of the plant, as it showed a highaffinity level to the various targets of concern.
However, previous research works have shown that gossypol may produce sterility or act as a contraceptive in males, as it is likely to inhibit spermatogenesis [34-36]. In females, gossypol administration may promote degeneration of ovarian follicles, but it is unknown whether this effect is direct or indirect [37].
The female aphrodisiac activity of this plant is somewhat a novel discovery in terms of scientific documentation, as all claims laid to it are anecdotal. This study is proposed to draw attention to the sexual arousal and vaginal lubrication properties of A. garckeana seed even though it justifies the use of A. garckeana seed as a female aphrodisiac both socially and in traditional medicine.
Conclusion
This study has shown that the extract of A. garckeana significantly enhances vaginal muscle relaxations and vaginal secretions upon sexual stimulation in rabbits, by a mechanism that may likely involve activation of female sexual excitatory systems and inhibition of those that depress female sexual arousal, or a combination of both. It is, therefore, logical to infer that it enhances the same in humans, and the likely active component for this activity in the plant is gossypol.
Conflict of Interest Statement
The authors declare that there is no conflict of interest.
Acknowledgement
We are grateful to all the researchers at the Pharmacology and Pharmacognosy Department of Madonna University, Centre for Bio-computing and Drug Development, Adekunle Ajasin University, Ondo state, for their support and team spirit, without whom efforts, this work would not have been possible.
REFERENCES
- Salonia A, Munarriz RM, Naspro R, Nappi RE, Briganti A, Chionna R, et al. Women's sexual dysfunction: A pathophysiological review. BJU Int. 2004;93:1156-1164.
- Shafer LC. Sexual disorders and sexual dysfunctions in Massachusetts General Hospital. Handbook of General Hospital Psychiatry. 6th Edi. 2010:pp.323-335.
- Thomas HN, Thurston RC. A biopsychosocial approach to women's sexual function and dysfunction at midlife: A narrative review. Maturitas. 2016;87:49-60.
- Basson R. Women's sexual function and dysfunction: current uncertainties, future directions. Int J Impot Res. 2008;20:466-78.
- Soucasaux N. Psychological dynamics of the gynecologist-patient relationship. Museum of Menstruation and Women's Health. 1st Edi. 2010:pp.10-2006.
- Rellini AH, McCall KM, Randall PK, Meston CM. The relationship between women's subjective and physiological sexual arousal. Psychophysiology. 2005;42:116-124.
- Maroyi A, Cheikh Y. Traditional knowledge of wild edible fruits in southern Africa. A comparative use pattern in Namibia and Zimbabwe. Ind J Tradition Knowledge. 2017;16:385-392.
- Yusuf AA, Lawal B, Sani S, Garba R, Mohammed BA, Oshevire DB. Pharmacological activities of Azanza garckeana (Goron Tula) grown in Nigeria. Clinical Phytoscience. 2020; 60:4-11.
- Ahmed RH, EL-Hassan MS, El-Hadi HM. Potential capabilities of Azanza garckeana fruits aqueous extract on the enhancement of iron absorption in Wistar albino rat. Int J Adv Rese Biol Sci. 2016;3:245-250.
- Glew RS, Vanderjagt DJ, Chuang LT, Huang YS, Millson M, Glew RH. Nutrient content of four edible wild plants from West Africa. Plant Foods Hum Nutr. 2005;60:187-193.
- Akinnifesi FK, Kwesiga FR, Mhango J, Mkonda A, Chilanga T, Swai R. Domesticating priority for Mombo indigenous fruit tree as a promising livelihood option for smallholders farmers in southern Africa. Acta horta. 2004;632:15-30.
- Masila VM, Midiwo JO, Zhang J, Gisacho BM, Munayi R, Omosa LK, et al. Anti-vancomycin-resistant Enterococcus faecium and E. faecalis activities of (-)-gossypol and derivatives from Thespesia garckeana. Nat Prod Commun. 2015;10:613-616.
- Mutindi MV. Phytochemical investigation of Harrisonia abyssinica and Thespesia garckeana for antiplasmodial and antimicrobial compounds. M.Sc. Thesis, University of Nairobi. 2014.
- Letcher RM, Shirley IM. O-naphthoquinones from the heartwood of Azanza garckeana. Phyto Chem. 1992;31:4171-4172.
- Nkafamiya II, Ardo BP, Osemeahon SA, Akinteriwa A. Evaluation of nutritional, non-nutritional elemental content and amino acid profile of Azanza garckeana. British J App Sci Tech. 2015;12:1-10.
- Ehigiator BE, Adesida AS, Omotuyi IO. Chicoric Acid, a phytochemical Compound of Solenostemon monostachyus: Possible drug candidate for the relief of erectile dysfunction. Int J Engg App Sci Tech. 2020;4:509-518.
- Levin ER, Rosen GF, Cassidenti DL, Yee B, Meldrum D, Wisot A, et al. Role of vascular endothelial cell growth factor in Ovarian Hyperstimulation Syndrome. J Clin Invest. 1998;102:1978-1985.
- Sun Q, Huang J, Yang DL, Cao XN, Zhou WL. Activation of β-adrenergic receptors during sexual arousal facilitates vaginal lubrication by regulating vaginal epithelial Cl(-) secretion. J Sex Med. 2014;11:1936-1948.
- Antonijevic IA, Murck H, Bohlhalter S, Frieboes RM, Holsboer F, Steiger A. Neuropeptide Y promotes sleep and inhibits ACTH and cortisol release in young men. Neuropharmacology. 2000;39:1474-1481.
- Pfaus JG, Sadiq A, Spana C, Clayton AH. The neurobiology of bremelanotide for the treatment of hypoactive sexual desire disorder in premenopausal women. CNS Spectr. 2021;2021:1-9.
- Zhuang CW, Qiongying MY, Yu J. Angiotensin receptor-Neprilysin inhibition is superior to angiotensin receptor inhibition alone for the treatment of sexual dysfunction in female spontaneously hypertensive rats. J Hypertension. 2021;39:1-10.
- Stücker O, Pons C, Neuzillet Y, Laemmel E, Lebret T. Effects of adenosine monophosphate used in combination with L-arginine on female rabbit corpus cavernosum tissue. Sex Med. 2014;2:1-7.
- OECD. Guidance for testing of chemicals. Acute Oral Toxicity- Acute Toxic Class Method. 2001;17:423.
- Schrödinger Release 2018-1c: Maestro, Schrödinger, LLC, New York, NY, 2018.
- Schrödinger Release 2018-1b: LigPrep, Schrödinger, LLC, New York, NY, 2018.
- Brooks WH, Daniel KG, Sung SS, Guida WC. Computational validation of the importance of absolute stereochemistry in virtual screening. J Chem Inf Model. 2008;48:639-645.
- Sastry GM, Adzhigirey M, Day T, Annabhimoju R, Sherman W. Protein and ligand preparation: parameters, protocols, and influence on virtual screening enrichments. J Comput Aided Mol Des. 2013;27:221-234.
- Kask A, Harro J, von Hörsten S, Redrobe JP, Dumont Y, Quirion R. The neurocircuitry and receptor subtypes mediating anxiolytic-like effects of neuropeptide Y. Neurosci Biobehav Rev. 2002;26:259-283.
- Basson R. Women's sexual dysfunction: Revised and expanded definitions. CMAJ. 2005;172:1327-1333.
- Frank JE, Mistretta P, Will J. Diagnosis and treatment of female sexual dysfunction. Am Fam Physician. 2008;77:635-642.
- Hamilton LD, Meston CM. Chronic stress and sexual function in women. J Sex Med. 2013;10:2443-2454.
- Goldstein I, Kim NN, Clayton AH, DeRogatis LR, Giraldi A, Parish SJ, et al. Hypoactive sexual desire disorder: International Society for the Study of Women's Sexual Health (ISSWSH) expert consensus panel review. Mayo Clin Proc. 2017;92:114-128.
- Kitchen DB, Decornez H, Furr JR, Bajorath J. Docking and scoring in virtual screening for drug discovery: methods and applications. Nat Rev Drug Discov. 2004;3:935-949.
- Waller DP, Zaneveld LJD, Fong HHS. In-vitro spermicidal activity of gossypol, Science Direct. 1980; 22:183-187.
- Coutinho EM, Athayde C, Atta G, Gu ZP, Chen ZW, Sang GW, et al. Gossypol blood levels and inhibition of spermatogenesis in men taking gossypol as a contraceptive. A multicenter, international, dose-finding study. Contraception. 2000;61:61-67.
- Coutinho EM. Gossypol: A contraceptive for men. Contraception. 2002;65:259-263.
- Luz VB, Gadelha ICN, Cordeiro LAV, Melo MM, Soto-Blanco B. In-vitro study of gossypol's ovarian toxicity to rodents and goats. Toxicon. 2018;145:56-60.
Citation: Ehigiator BE, Okoli RI, Ndubueze NG, Ebong OO (2021) In-vivo and In-silico Investigations on Goron Tula (Azanza garckeana F. Hoffm), a Booster of Female Sexual Arousal and Vaginal Lubrication. J Trop Dis doi:10.35248/2329-891X.21.9.304.
Copyright: © 2021 Ehigiator BE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


