Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- RefSeek
- Hamdard University
- EBSCO A-Z
- SWB online catalog
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 12, Issue 1
Interferon Production and Potentiality as a therapeutic drug for SARS-CoV-2
Pramod Yadav1* and Adhishree Yadav22School of Biotechnology, Gautam Buddha University, Yamuna Expressway, Greater Noida, Uttar Pradesh, 201312, India
Received: 06-Feb-2023, Manuscript No. PDS-23-19780; Editor assigned: 08-Feb-2023, Pre QC No. PDS-23-19780 (PQ); Reviewed: 22-Feb-2023, QC No. PDS-23-19780; Revised: 27-Feb-2023, Manuscript No. PDS-23-19780 (R); Published: 06-Mar-2023, DOI: 10.35248/2167-1052.23.12.288
Abstract
This review examines the use of interferon as a potential drug against SARS-CoV-2 and other animal coronaviruses. The production of interferon is also discussed. Despite the ongoing global pandemic of COVID-19, there are currently no effective drugs available to treat SARS-CoV-2 and its new variants. The review aims to determine if interferon could be an effective treatment option. The role and importance of interferon in viral drug development and production is summarized using recent research and literature.
Keywords
Drug development; COVID-19; RNA viruses; Nidovirales order; Viral diseases
Abbreviations
SARS-COV-2: Severe Acute Respiratory Syndrome Coronavirus 2; IFN: Interferon; RDT: Recombinant DNA Technology; O.D: Optical Density; HPSEC: High-Performance Size-Exclusion Chromatography; SDS PAGE: Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis; IL: Interleukin.
INTRODUCTION
COVID-19 is closely related to SARS which swept around the world from 2002 to 2003. That virus infected around 8,000 people and killed about 800 but it soon ran itself out, largely because most of those infected were seriously ill so it was easier to control [1]. Another coronavirus Middle East Respiratory Syndrome (MERS), cases have been occurring sporadically since it first emerged in 2012-there have been around 2,500 cases and nearly 900 deaths [2]. COVID-19 is different from these two other coronaviruses in that the spectrum of disease is broad, with around 80 percent of cases leading to a mild infection. There may also be many people carrying the disease and displaying no symptoms, making it even harder to control. So far, around 20 percent of COVID-19 cases have been classed as “severe” and the current death rate varies between 0.7 percent and 3.4 percent depending on the conditions, location, and, crucially, access to good hospital care [3,4].
Emergence and spread of coronavirus
The coronavirus has sparked a succession of events in less than a month, ranging from entrance port inspections to complete quarantine of impacted cities [5]. The timeline that follows depicts how the virus spread from a single city to turn into a worldwide health emergency [6].
Virus transmission from animal-to-human
In the Chinese largest city Wuhan (capital of the Hubei province), many people report symptoms like cough, high Fever, Fatigue, Sputum, and Dyspnea caused by a virus. Initially, the origin of this virus is liked with the wholesale market of Huanan seafood (primarily selling fish and shellfish), but later it is also linked with wild game like snakes, beavers, and porcupines. As far as the concern now, it probably originated from bats before human-to-human transmission. However, the origin of animal COVID-19 is still not clear [7].
Characterization of animal coronavirus
Chinese health officials claimed that the pathogen had been isolated. The so-called 2019-nCoV, which spreads through airborne droplets, is a member of the coronavirus family, which also includes SARS (Severe Acute Respiratory Syndrome) and MERS (Middle East respiratory illness) [8].
First fatality
It was officially reported in Wuhan city and till Dec 2022, 5247 deaths have been reported within China itself. While the worldwide 6,693,904 fatalities have been recorded retrieved, (https://www.worldometers.info/coronavirus/). COVID-19 shows a comparatively low mortality rate than SARS. Beyond its low fatality rate, the animal coronavirus has been showing one of the highest fatalities in the last pandemic that happened in the recent century. To close all boundaries of a nation for a decade is not possible in the current globalization order. For the growth and sustainability of the economy, the movement of the citizens of the nation to another nation is an absolute necessity (Figure 1). This is an alarming situation for the globe to introduce an effective drug to counter the COVID-9 along with their mutated variance of the virus [9].
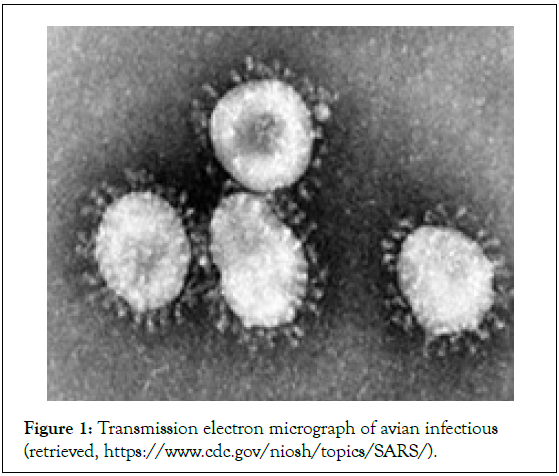
Figure 1: Transmission electron micrograph of avian infectious (retrieved, https://www.cdc.gov/niosh/topics/SARS/).
City quarantined
Chinese authorities halt travel to and from Wuhan, which has a population of more than 10 million. Other Hubei province cities also impose similar travel restrictions, impacting a total of around 50 million individuals [10].
Hospitals overwhelmed
As time passed from the first human encounter with animal coronavirus, the transmission of COVID-9 from human to human was continuously increasing in Wuhan city in a very short time lamps. It badly impacted the availability of doctors and hospital staff per patient in the hospital. Medicine, PPT kits, beds, ventilators, oxygen cylinders, and other medical supplies were becoming inadequate for the treatment of infected patients. In response to these insufficiencies, in the 1st week of February 2020, the Chinese government urgently planned 1000 plus facilities of beds and other accessories by breaking the grounds of the Huoshenshan Hospital (retrieved https://www.bbc.com/news/in-pictures-51280586). At the same time, public health experts and researchers were working day and night to break down the basic protocols and essential facts to prevent the super spread of this never before the seen virus. In addition, the biggest challenge in the world is to trace the infected patient, especially those who are infected but do not show any symptoms of COVID-9 at all [11].
Interferon therapy as antiviral drug medication
What are Interferons?
Interferon represents a family of cytokines, which is of central importance in the innate immune response to virus infections [12]. All interferon’s act as secreted ligands of specific cell surface receptors, eliciting the transcription of hundreds of interferon stimulated genes whose protein products have antiviral activity, as well as antimicrobial, ant proliferative/antitumor, and immunomodulatory effects [13]. Expression of type I and III interferon’s is induced in virtually all cell types upon recognition of viral molecular patterns, especially nucleic acids, by cytoplasmic and endosomal receptors, whereas type II interferon is induced by cytokines such as IL-12, and its expression is restricted to immune cells such as T cells and NK cells [14]. The effectiveness of the interferon system in counteracting viral infections is reflected by the multitude of inhibitors of interferon induction or interferon action that are encoded by many viruses, preventing their eradication and resulting in the continued coexistence of viruses and vertebrates [15]. The unique biological functions of interferon have led to their therapeutic use in the treatment of diseases such as hepatitis, multiple sclerosis, and certain leukemia [16]. In humans and other animals, the distinct protein and IFN genes (more than 25) have been recognized. They are generally categorized into three classes: IFN Type I, II, and III. IFNs belonging to all three classes are important for fighting viral infections and for the regulation of the immune system [17].
Types of interferon
Based on the type of receptor through which they signal, human interferons have been classified into three major types [18].
Type I Interferon: All IFNs type I bind to IFN α/β Receptor (IFNAR) which is made of two chains IFNAR 1 and IFNAR 2. It is the category of a specific cell surface receptor complex. The common and isolated human IFN α, β, ε, κ, and ω are considered type I interferons [19]. In general, type I interferons are produced when the body recognizes a virus that has invaded it [20]. They are produced by fibroblasts and monocytes. However, the production of type I IFN-α is prohibited by another cytokine known as Interleukin-10 [21]. Once type I IFN is released which binds to their specific receptor of targeted cells, leads to the translation of a specific protein (Figure 2). Later this protein stops the production and replication of viral DNA and RNA. Overall, IFN-α can be used to treat hepatitis B and C infections, while IFN-β can be used to treat multiple sclerosis [22].
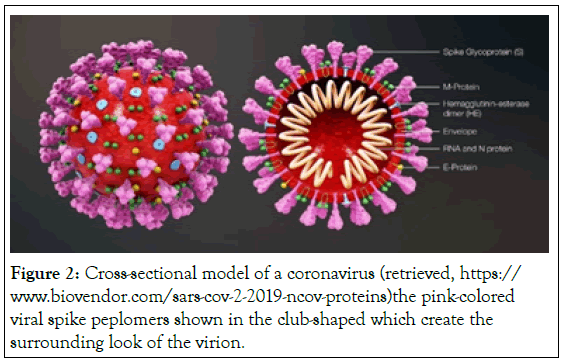
Figure 2: Cross-sectional model of a coronavirus (retrieved, https://www.biovendor.com/sars-cov-2-2019-ncov-proteins) the pink-colored viral spike peplomers shown in the club-shaped which create the surrounding look of the virion.
Type II Interferon (IFN-γ in humans): In the immune systems, interleukin-12 activates IFN II and that’s why it is also called immune interferon. Furthermore, type II interferons are released by Cytotoxic T cells and T helper cells, type 1 specifically [23]. They do, however, prevent type 2 T helper cell multiplication. The prior causes the Th2 immune system response to be inhibited and the immune system response of Th1 to be further stimulated, which results in the growth of debilitating disorders (like multiple sclerosis). IFN type II binds to IFNGR, which consists of IFNGR1 and IFNGR2 chains [24].
Type III Interferon: It is stimulated the signal via a receptor complex made of IL10R2 and IFNLR1, and it is also known as CRF2-4 and CRF2-12 respectively. Although discovered more recently than type I and type II IFNs, recent information demonstrates the importance of type III IFNs in some types of virus or fungal infections [25].
Various research studies reports and books conclude that IFN type I and IFN type II are mainly responsible for activating and regulating the immune response (Figure 3). Expression of type I and III IFNs can be induced in virtually all cell types upon recognition of viral components, especially nucleic acids, by cytoplasmic and endosomal receptors, whereas type II interferon is induced by cytokines such as IL-12, and its expression is restricted to immune cells such as T cells and NK cells [26].
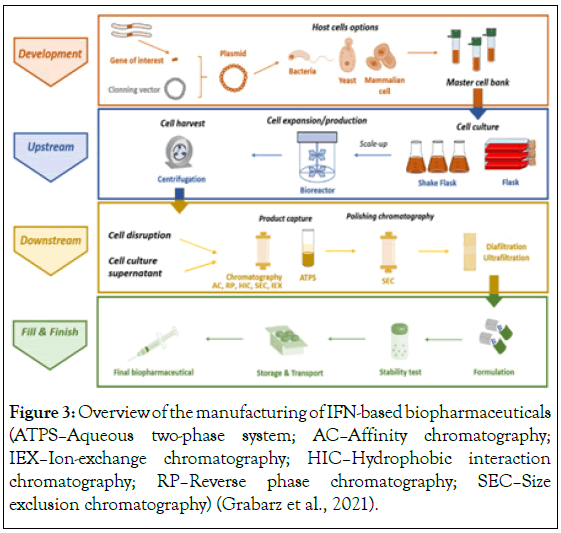
Figure 3: Overview of the manufacturing of IFN-based biopharmaceuticals (ATPSâ??Aqueous two-phase system; ACâ??Affinity chromatography; IEXâ??Ion-exchange chromatography; HICâ??Hydrophobic interaction chromatography; RPâ??Reverse phase chromatography; SECâ??Size exclusion chromatography) (Grabarz et al., 2021).
Application of interferon as therapy
There is no absolute antidote or effective medication available in the treatment of COVID-19 in India. Thus, interferon therapy can be given. Type 1 interferon i.e., alpha and beta interferon therapy can be used to treat the novel coronavirus pandemic. Alpha and Beta interferons are produced by the entire virus-infected nucleated cells of our body. Interferons are natural antiviral proteins but sometimes it is a man-made version of proteins (use of Recombinant DNA Technology) that are used as a drug to treat many diseases. The drugs equip the body to tackle viruses, preventing them from multiplying in the body given to COVID patients with the least side effects [27]. IFN-β has been tested on 60 patients who were 65 years old and infected with COVID-9 by a group of doctors belonging to the United States Council for Automotive Research (USCAR) in the year 2021. In this clinical trial, sixty individuals who were recently infected (within 72 hours) with SARS-CoV-2, are randomly selected (both male and female) and 11μg of interferon β1a has been spontaneously injected at days 1, 3, 7, and 10. IFN- β1a is marketed as the trade name of Rebif®, Merck KGaA, Darmstadt, Germany which is usually used for the treatment of Multiple Sclerosis (MS) (especially for the relapsing forms) [28]. After the successful administration of IFN-β1a and intensive care, the patients show some speedy recovery from the COVID-9 infection in comparison to the control. Although, this data is too small, but it shows that the interferon β1a along with other types of IFN has significant potential to be used as a novel drug for the treatment of SARS-CoV-2 infection and other viral diseases.
Production of Interferons as a therapeutic agent
Interferons are produced by living cells both in vivo as well as cultured cells. Interferon production and its antiviral activity are required for the expression of cellular genes, and these functions are blocked by inhibitors of transcription and translation. One important characteristic of interferon is its host specificity; therefore exogenous interferon intended for use in man must be produced in human cell culture [29]. Sources of human cells or cell lines that can be obtained in large quantities for interferon production are peripheral blood leukocytes, placenta, embryos, skins, lymphoid cells, and transformed cancerous cell lines [30]. Interferon type 1 i.e., interferon alpha 2b can be used in the treatment of COVID-9 as it is an antiviral drug and is reliable and effective. It is produced at the commercial level with help of RDT which stands for Recombinant DNA Technology. Mammalian or human cell lines in a serum-based medium are required to achieve it [31]. Hence in this procedure desired interferon is produced and purified with affinity chromatography packed with anti-alfa 2b antibodies. Hence interferon products can be eluted and purified [32].
Methodology
Designing and cloning of interferon genes
The codon sequences of the IFN genes, which are available in online database sequences and have been previously discovered and designed by the farmer’s research can be used for insertion for the application through vector (pET28a expression vector) insertion in the bacteria. E. coli is a natural choice for the commercial production of interferon (Castro et al., 2021). In case when the codon sequence of the IFN genes is online not available, it can design in any advanced molecular laboratory [33].
Amplification of IFN genes
To make multiple copies of the IFN gene, PCR is used in-vitro conditions. To complete this operation typically, reverse and forward primers, Taq polymerase, MgCl2 dNTP, and PCR buffer are required. Further, this DNA sequence is utilized on agarose gel electrophoresis under UV rays.
Selection of vector and Bacterial strain
Only an expression vector is used in the production of IFN and the most preferred vector is the pET28a expression vector. DH5α and BL21 (DE3) strain of E. coli is used in the amplification of plasmid and molecular cloning respectively.
Culture media and protein synthesis
Luria-Broth culture media is used to culture E. coli and other media are also used concerning microorganisms that are intended to insertion of the insertional gene in the vector. For example, a serum-based culture medium can be used which has growth factors, amino acids, vitamins, etc. Impellers Based Bioreactors are used, equipped with pH, temperature controller, DO probe, oxygen, antifoaming agent supplier, cooling jackets, etc. [34]. The bioreactor can also be used with an optimal supply of culture media. Once the exponential growth of bacteria was started, the expression of the recombinant IFN within the bacterial cells was also started with help of the expression vector (expression system).
Purification of recombinant IFN protein
For the best outcomes, affinity chromatography and ion exchange or gel filtration are used to separate our desired interferon. In Affinity chromatography, the column is packed with Anti alpha-2b antibodies in the stationary phase, or the column can be packed with nickel ions in a resin and bind with an intracellular interferon tag with Histidine. Once IFN is isolated, the columns are eluted with a decreasing slope gradient of pH (8 to 4) with the help of an elution buffer through gradient elution. This will give 99.9% purity; hence desired interferon alpha can be obtained.
Validation of recombinant IFN protein
DS-PAGE which stands for Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis is often used to check the purity and concentration of the IFN proteins. Digital gel doc, reading machine of SDS-PAGE, analyses the test sample with help of the band’s density and with [6,16]. Lowry and Bradford’s assays are also used to determine the concentration of IFN protein by calculating the relative value of the test sample with the standard samples HPSEC which stands for High-Performance Size-Exclusion Chromatography is used to concentrate the isolated recombinant IFN proteins [35].
Validation of Antiviral activity of IFN protein
To check the effectiveness of the recombinant IFN protein, spectrophotometric observance-based tests are used. In this test, a human cell line in two different cultures (which can vary with the requirements) was infected with SARS-CoV-2 for 2 hours and the then recombinant IFN protein was added in one test culture (test sample) while others are left untouched (blank sample). O.D. has been recorded initially, followed by after 2 hours and the end of the test. By comparing the O.D. value of the test sample with the blank sample, data has been drawn.
Discussion
This study found that the use of interferon in viral infection show fewer side effect, is highly accurate, and easy to administer. As the failure of COVID-9 vaccination in many countries like China against the new variants of SARS-CoV-2, looking forward to a new category of drugs is necessary to control the second wave of the pandemic within a few years. As the literature suggest the use of interferon for the viral disease is going to be a new trend in the clinical biology and research field. Selection of a right cell lines will be reducing the overall production cost by reducing the maintenance, contamination, culture media cost.
pET28a expression vector is the key part and the DH5α and BL21 (DE3) strain of E. coli is most useful in the production. As we scale up the IFN.
Recombinant protein production, cost of IFN drugs will be reduced which will be helpful for developing and under developed nations.
Overall, the results of these studies suggest that interferon may be effective in the treatment or prevention of COVID-19 caused by SARS-CoV-2. More research is needed to determine the potential role of interferon in the management of this disease. It is important to note that these studies were conducted early in the COVID-19 pandemic, and more recent research may have found different results. It is also important to follow the recommendations of public health authorities and healthcare providers for the prevention and treatment of COVID-19. In humans, IFNs are to be used as treatment and prophylaxis for interferon therapy is not new although the introduction of RDT in the production of IFN is the latest technology. IFNs were used many centuries ago as a transfer of immunized plasma of one patient to another patient; for example, during the pandemic of Spanish influenza H1N1 in the year 1918-20 (Ripoll et al., 2021). If, in various viral diseases, then, the knowledge of the relative capacity of various cells to produce human interferon should contribute toward the development of an economical method of production. This drug will be effective with the least side effects. It will be a lifesaving drug worldwide and the Economy will be more productive and better.
Conclusion
Exploring therapies for SARS-CoV-2 is relevant and urgent, especially considering the inequitable access to SARS-CoV-2 vaccines, with some countries reporting <30% of their population vaccinated. IFN therapies’ unique administration schedule (e.g., can be administered once, weekly, or more in severe cases), limited adverse effects, and minimal follow-up make IFN a compelling treatment option in populations with fewer or lower-skilled providers and limited access to pharmaceuticals. However, the high diversity of intervention types and delivery methods and the absence of consistent outcome measures make it difficult to synthesize the available data. Ahead of patients being capable of benefiting from outcomes of therapeutic uses of IFN, standardized reporting and outcomes for interferon trials are critically required to ease evidence synthesis and production.
Challenges
Antibiotics and other viral medicine are mostly preferred for the treatment of COVID-9 as for the current scenario aspects. Vaccines and monoclonal antibodies are also proved their utility in the treatment of SARS-CoV2. But due to the new dimension of drugs, highly variable outcomes, variables in types of administration, the variable effect of interferon with late and early intervention, and less exploration of IFN type, are restricted to use of it in a wide range of applications for the treatment of the viral infection. Before starting the large clinical application of IFN, it is urgently required standardized the outcome measures. The research community needs to do a more detailed and focused study on types of interferon and their specific clinical uses.
Declarations
Ethical approval and consent to participate
Not applicable
Consent for publication
Yes, the author is agreed to publish his manuscripts according to journal publication guidelines.
Availability of data and materials
Not applicable
Competing interests
The author declared no conflict of interest.
Funding
The author declared no funding was granted to conduct this study.
Authors’ contributions
PY searched and analysis all the data and facts of the review paper and was a major contributor in writing the manuscript. AY prescribed the other activity like reviewing, revising, formatting, and proofreading. All authors read and approved the final manuscript.
Acknowledgements
PY searched and analysis all the data and facts of the review paper and was a major contributor in writing the manuscript. AY prescribed the other activity like reviewing, revising, formatting, and proofreading. All authors read and approved the final manuscript.
References
- Grabarz F, Lopes AP, Barbosa FF, Barazzone GC, Santos JC, Botosso VF, et al. Strategies for the production of soluble interferon-alpha consensus and potential application in arboviruses and SARS-CoV-2. Life. 2021;11(6):460.
[Crossref] [Google Scholar] [PubMed]
- Ripoll JG, van Helmond N, Senefeld JW, Wiggins CC, Klassen SA, Baker SE, et al. Convalescent plasma for infectious diseases: historical framework and use in COVID-19. Clin Microbiol Newsl. 2021;43(4):23-32.
[Crossref] [Google Scholar] [PubMed]
- Understanding vaccination progress by country. 2022.
- Hopkins J. Coronavirus resource center. 2020.
- Aricò E, Castiello L, Bracci L, Urbani F, Lombardo F, Bacigalupo I, et al. Antiviral and immunomodulatory interferon-beta in high-risk COVID-19 patients: a structured summary of a study protocol for a randomised controlled trial. Trials. 2021;22:1-4.
[Crossref] [Google Scholar] [PubMed]
- Asselin-Paturel C, Trinchieri G. Production of type I interferons: plasmacytoid dendritic cells and beyond. J Exp Med. 2005;202(4):461-465.
[Crossref] [Google Scholar] [PubMed]
- Barrat FJ, Crow MK, Ivashkiv LB. Interferon target-gene expression and epigenomic signatures in health and disease. Nat Immunol. 2019;20(12):1574-1583.
[Crossref] [Google Scholar] [PubMed]
- Bonilauri P, Rugna G. Animal coronaviruses and SARS-COV-2 in animals, what do we actually know?. Life. 2021;11(2):123.
[Crossref] [Google Scholar] [PubMed]
- Chen K, Liu J, Cao X. Regulation of type I interferon signaling in immunity and inflammation: A comprehensive review. J Autoimmun. 2017;83:1-11.
[Crossref] [Google Scholar] [PubMed]
- De Weerd NA, Nguyen T. The interferons and their receptors—distribution and regulation. Immunol Cell Biol. 2012;90(5):483-491.
[Crossref] [Google Scholar] [PubMed]
- Domańska-Blicharz K, Woźniakowski G, Konopka B. Animal coronaviruses in the light of COVID-19. J Vet Res. 2020;64(3):333-345.
[Crossref] [Google Scholar] [PubMed]
- Domingo JL. What we know and what we need to know about the origin of SARS-CoV-2. Environ Res 2021;200
[Crossref] [Google Scholar] [PubMed]
- Fensterl V, Sen GC. Interferons and viral infections. BioFactors. 2009;35(1):14-20.
[Crossref] [Google Scholar] [PubMed]
- Friedman RM. Clinical uses of interferons. Br J Clin Pharmacol. 2008;65(2):158-162.
[Crossref] [Google Scholar] [PubMed]
- George PM, Badiger R, Alazawi W, Foster GR, Mitchell JA. Pharmacology and therapeutic potential of interferons. Pharmacol Ther. 2012;135(1):44-53.
[Crossref] [Google Scholar] [PubMed]
- Geraghty RJ, Capes-Davis A, Davis JM. Guidelines for the use of cell lines in biomedical research. Br J Cancer. 2014;111(6):1021-1046.
[Crossref] [Google Scholar] [PubMed]
- Gutierrez-Merino J, Isla B, Combes T, Martinez-Estrada F, Maluquer De Motes C. Beneficial bacteria activate type-I interferon production via the intracellular cytosolic sensors STING and MAVS. Gut Microbes. 2020;11(4):771-788.
[Crossref] [Google Scholar] [PubMed]
- Jhuti D, Rawat A, Guo CM, Wilson LA, Mills EJ, Forrest JI. Interferon treatments for SARS-CoV-2: challenges and opportunities. Infect Dis Ther. 2022;11(3):953-972.
[Crossref] [Google Scholar] [PubMed]
- Kaur G, Dufour JM. Cell lines: valuable tools or useless artifacts. Spermatogenesis. 2012;2(1):1-5.
[Crossref] [Google Scholar] [PubMed]
- Khanna NR, Gerriets V. Interferon. InStatPearls [Internet] 2021. StatPearls Publishing.
- Khorramdelazad H, Kazemi MH, Azimi M. Type-I interferons in the immunopathogenesis and treatment of coronavirus disease 2019. Eur J Pharmacol. 2022;927:175051.
[Crossref] [Google Scholar] [PubMed]
- Liechti T, Iftikhar Y, Mangino M. Immune phenotypes that are associated with subsequent COVID-19 severity inferred from post-recovery samples. Nat Commun. 2022;13(1):7255.
[Crossref] [Google Scholar] [PubMed]
- Lin FC, Young HA. Interferons: success in anti-viral immunotherapy. Cytokine Growth Factor Rev. 2014;25(4):369-376.
[Crossref] [Google Scholar] [PubMed]
- López De Padilla CM, Niewold TB. The type I interferons: basic concepts and clinical relevance in immune-mediated inflammatory diseases. Gene. 2016;576(1):14-21.
[Crossref] [Google Scholar] [PubMed]
- Lotfi M, Hamblin MR, Rezaei N. COVID-19: transmission, prevention, and potential therapeutic opportunities. Clin Chim Acta. 2020;508:254-266.
[Crossref] [Google Scholar] [PubMed]
- Negishi H, Taniguchi T, Yanai H. The interferon (IFN) class of cytokines and the IFN regulatory factor (IRF) transcription factor family. Cold Spring Harb Perspect Biol. 2018;10(11):a028423.
[Crossref] [Google Scholar] [PubMed]
- Odendall C, Voak AA, Kagan JC. Type III IFNs are commonly induced by bacteria-sensing TLRs and reinforce epithelial barriers during infection. J Immunol. 2017;199(9):3270-3279.
[Crossref] [Google Scholar] [PubMed]
- Petrosillo N, Viceconte G, Ergonul O, Ippolito G, Petersen E. COVID-19, SARS, and MERS: are they closely related? Clin Microbiol Infect. 2020;26(6):729-734.
[Crossref] [Google Scholar] [PubMed]
- Piper JM, Wen TT, Xenakis EM. Interferon therapy in primary care. Prim Care Update Ob Gyns. 2001;8(4):163-169.
[Crossref] [Google Scholar] [PubMed]
- Risbridger GP. Human cell lines as tools of our trade: “laying it on the (cell) line”. Mol Endocrinol. 2015;29(1):1-2.
[Crossref] [Google Scholar] [PubMed]
- Scantamburlo T, Cortés A, Dewitte P, Van der Eycken D, De Wolf R, Martens M. COVID-19 and tracing methodologies: A lesson for the future society. Health Technol (Berl). 2021;11(5):1051-1061. doi:10.1007/s12553-021-00575-1.
[Crossref] [Google Scholar] [PubMed]
- Segeritz CP, Vallier L. Cell culture: growing cells as model systems in vitro. Basic Sci Methods Clin Res. 2017:151-172.
- Spiteri G, Fielding J, Diercke M. First cases of coronavirus disease 2019 (COVID-19) in the WHO European Region, 24 January to 21 February 2020. Euro Surveill. 2020;25(9):2000178.
[Crossref] [Google Scholar] [PubMed]
- Taylor MW. Interferons. Viruses Man Hist Interact. 2014:101-119.
[Crossref]
- Castro LS, Lobo GS, Pereira P, Freire MG, Neves MC, Pedro AQ. Interferon-Based Biopharmaceuticals: Overview on the Production, Purification, and Formulation. Vaccines (Basel). 2021;9(4):328.
Citation: Yadav P, Yadav A (2023) Interferon Production and Potentiality as a therapeutic drug for SARS-CoV-2. Pharmacoepidemiol. Drug Saf. 12:288.
Copyright: © 2023 Yadav P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

