PMC/PubMed Indexed Articles
Indexed In
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2024) Volume 15, Issue 2
Inhibitory Effects of Cationic Hybrid Liposomes against Metastasis of Bile Duct Cancer In Vitro and In Vivo
Hideaki Ichihara* and Yoko MatsumotoReceived: 06-Feb-2024, Manuscript No. JCM-24-24838; Editor assigned: 08-Feb-2024, Pre QC No. JCM-24-24838 (PQ); Reviewed: 22-Feb-2024, QC No. JCM-24-24838; Revised: 29-Feb-2024, Manuscript No. JCM-24-24838 (R); Published: 07-Mar-2024, DOI: 10.35248/2157-2518.2.15.444
Abstract
Cationic Hybrid Liposomes (CL), comprising 87 mol% Dimyristoylphosphatidylcholine (DMPC), 5 mol% polyoxyethylene(21) dodecyl ether (C12(EO)21), and 8 mol% O,O’-ditetradecanoyl-N-(α–trimethylammonioacetyl) diethanolamine chloride (2C14ECl), were prepared using the sonication method. A clear CL solution with a hydrodynamic diameter of 100 nm was maintained for 4 weeks. The inhibitory effects of CL on metastasis were obtained in human bile duct cancer (HuCCT-1) cells in vitro. The inhibitory effects of CL on HuCCT-1 cell migration using scratch assay were obtained. Furthermore, it was observed that CL significantly suppressed the invasion of HuCCT-1 cells and the formation of filopodia on the cell surface in vitro. The anti-invasive effects of CL on HuCCT-1 cells were attributed to the inhibition of MMP2 (Matrix Metalloproteinase-2). In vivo, therapeutic effects were demonstrated in a mouse model of peritoneal metastasis of HuCCT-1 cells following intraperitoneal injection of CL.
Keywords
Cationic hybrid liposomes; Chemotherapy; Bile duct cancer; Metastasis; In vivo
Introduction
Bile duct cancer is a prevalent malignancy worldwide, with over one million people diagnosed annually [1-3]. A multimodal approach, combining surgical resection and adjuvant chemotherapy, is commonly employed in its treatment. Surgical resection proves most effective in achieving a complete cure for early-stage bile duct cancer.
The characteristic intrahepatic ductal extension of bile duct cancer makes the liver and adjacent organs frequent sites for early metastasis [4,5]. Although the surrounding structures can be initially affected, late-stage distant metastasis most commonly occurs in the liver, lung, and peritoneum, showing similarities in both perihilar and distal bile duct cancers [6]. Consequently, there is a need to develop a novel metastasis treatment with minimal side effects to enhance the quality of life for patients with bile duct cancer.
Bile duct cancer is a highly metastatic disease that exhibits poor responsiveness to currently available treatments. The metastasis of bile duct cancer significantly influences cell migration and invasion, involving the remodeling of the actin cytoskeleton and the formation of filopodia- and lamellipodia-like protrusions [7]. Matrix Metalloproteinases (MMPs) are widely activated and expressed in various tumors, contributing to the metastatic process. Among them, MMP2 plays a important role in facilitating cancer cell penetration through the extracellular matrix, promoting angiogenesis, and enhancing invasive characteristics. Additionally, MMP-14 has been implicated in promoting tumor invasion by activating proMMP2 [8]. MMP2 can digest extracellular matrix proteins, such as type IV collagen, a major component of the basement membrane. This enzymatic activity is critical in facilitating tumor cell invasion [9,10].
The Cationic Hybrid Liposomes (CL), which incorporate cationic lipids, were synthesized through sonication of a mixture comprising DMPC, 2C14ECl, and PEG surfactants in a buffer solution [11]. Previous studies have highlighted the noteworthy inhibitory effects of CL, incorporating cationic lipids, on the proliferation of human renal cell carcinoma (OS-RC-2) cells and colorectal cancer (HCT116), demonstrating apoptosis both in vitro and in vivo [11,12]. Notably, bile duct cancer is characterized by a high sialic acid content. Nevertheless, the metastatic impacts of CL on bile duct cancer cells and its therapeutic efficacy in murine models of Human Bile Duct Cancer (HuCCT-1) in vivo remain unexplored. In this investigation, we delved into the inhibitory effects of CL, constituted of 87 mol%dimyristoylphosphatidylcholine (DMPC), 5 mol%polyoxyethylene(21) dodecyl ether (C12(EO)21), and 8 mol %O,O’-ditetradecanoyl-N-(α–trimethyl-ammonioacetyl) diethanol-amine Chloride (2C14ECl), on the metastasis of HuCCT-1 cells both in vitro and in vivo.
Material and Methods
Preparation of hybrid liposomes
The CL was prepared by sonicating a mixture containing DMPC (purity>99%; NOF Co. Ltd., Tokyo, Japan), micellar molecules C12(EO)21 (Nikko Chemicals Co. Ltd., Tokyo, Japan), and 2C14ECl (DC-6-14; Sogo Pharmaceutical Co. Ltd., Tokyo, Japan) in a 5% glucose solution at 45°C with 300 W. The resulting mixture was then filtered through a 0.20 μm filter.
Dynamic light scattering measurements
The diameter of CL was measured using a light scattering spectrometer (ELS-8000, Otsuka Electronics, Osaka, Japan) equipped with a He-Ne laser (633 nm) at a 90° scattering angle.
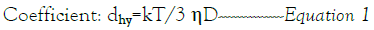
The diameter (dhy) was calculated using the Stokes-Einstein formula (Equation 1), where κ represents the Boltzmann constant, T is the absolute temperature, η denotes viscosity, and D stands for the diffusion.
Cell culture
HuCCT-1 cells were obtained from the American Type Culture Collection (Manassas, VA, USA). HuCCT-1 cells were cultured in RPMI-1640 medium (Gibco, Gaithersburg, MD), supplemented with 1 unit/ml penicillin, 50 μg/ml streptomycin, and 10% fetal bovine serum (HyClone Laboratories, Logan, UT). The cells were maintained in a 5% CO2 humidified incubator at 37°C.
Assessment of growth inhibition by CL
The 50% inhibitory concentration (IC50) affecting the growth of tumor cells was determined utilizing the WST-8 assay [2-(2- Methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tet razolium Sodium Salt; 5-(2,4-disulfophenyl)-3-(2-methoxy-4- nitrophenyl)-2-(4-nitrophenyl)-2H-tetrazolium, inner salt, mono sodium salt] (Cell Counting Kit-8, Dojindo Laboratories, Kumamoto, Japan). Cells (5.0 × 104 cells/ml) were seeded in 96-well plates and cultured in a 5% CO2 humidified incubator at 37°C for 24 h. Subsequently, cells were cultured for an additional 48 h following the addition of DMPC (0.1-5 mM), HL21, or CL (0.1-2 mM based on the DMPC concentration). The WST-1 solution was introduced, and the cells were incubated for 3h. Absorbance was measured at 450 nm using a spectrophotometer (Emax; Molecular Devices Co., California, USA). The inhibitory effects of CL on tumor cell growth were assessed using Amean/Acontrol, where Amean and Acontrol represent the absorbance of water-soluble formazan in the presence and absence of CL, respectively.
Invasion assay in vitro
The in vitro assessment of cancer cell invasion employed a BiocoatTM MatrigelTM invasion chamber (BD Biosciences, NJ, and USA) equipped with cell culture inserts featuring an 8 μm pore size membrane and a thin layer of Matrigel basement membrane matrix. In brief, 450 μL of HuCCT-1 cells (4.0×104 cells/mL) were re-suspended in a serum-free medium containing either a 5% glucose solution (control) or HL (100, 200, 300 μM). This cell suspension was added to the cell culture insert of a BiocoatTM MatrigelTM invasion chamber. Subsequently, 0.7 ml of medium supplemented with 10% FBS served as a chemoattractant and was added to the outer chamber. The cells were incubated at 37°C in a humidified 5% CO2 environment for 24 h.
To quantify tumor cell invasion, non-invading cells were gently removed from the upper surface of the membrane using a cotton-tipped swab. Cells on the lower surface of the membrane were fixed with ethanol and stained with crystal violet. Subsequently, the cells were photographed under a light microscope (ECLIPSE TS100; Nikon, Tokyo, Japan). Microscopic fields on the lower surface of the membrane were counted to determine the number of cells that successfully invaded through the Matrigel layer and the membrane. The results are expressed as the number of invaded cells per microscopic field.
Fusion and accumulation of CL into the cell membrane
The fusion and accumulation of CL, including a fluorescent probe (1-palmitoyl-2-[12-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino] dodecanoyl]-sn-glycero-3-phosphocholine, NBDPC; Avanti Polar Lipids, Alabama, U.S.A.), into the membrane of HuCCT-1 cells were performed using a confocal laser microscope (TCS-SP; Leica Microsystems, Berlin, Germany).
Cells (2.0 × 105 cells/mL) were cultured in a 5% CO2 humidified incubator at 37°C for 24 h. Subsequently, the cells were treated with cardiolipin (CL) ([DMPC]=0.45 mM,[C12(EO)21]=0.027 mM, [2C14ECl]=0.044 mM,[NBDPC]=0.022 mM) containing fluorescence-labeled lipids for 4 h and were observed using a confocal laser microscope equipped with a 488 nm Ar laser line (detection at 505-555 nm).
Scratch wound assay in vitro
The motility of HuCCT-1 cells was evaluated through the scratch wound assay. Cells were initially seeded into tissue culture dishes at a concentration of 2.5 × 105 cells/ml and cultured in a medium supplemented with 10% FBS until nearly confluent cell monolayers were formed. Subsequently, these monolayers were meticulously wounded using a 200 µl sterile pipette tip, and any residual cellular debris was eliminated through PBS(-) washes. The injured monolayers were then incubated in a medium containing 10% FBS, with or without the addition of 100 μM HL. After a 19-hour incubation period, cell images were captured using a light microscope (EVOS fl, Life Technologies, CA, USA). The migration area was quantified using ImageJ software (version 1.46r; National Institutes of Health, Bethesda, MD, USA).
Assessment of MMP-2 by ELISA
HuCCT-1 cells were initially seeded at a density of 5.0 × 104 cells per well and incubated for 24 hours. Subsequently, the cells were treated with CL (0.15 μM) for an additional 24 hours. Following the treatment, the cells underwent a series of steps: they were washed with PBS(-), trypsinized, centrifuged at 1100 rpm for 5 minutes, and then fixed with 4% formaldehyde for 10 minutes. To enable permeabilization, a solution of 0.1% triton X in PBS (-) was applied to the cells for 10 minutes at room temperature. Following this, the cells were subjected to two washes and then incubated with an anti-MMP2 rabbit monoclonal antibody (Abcam plc, Cambridge, UK) at a concentration of 5 μg/ml. This incubation took place at room temperature for 30 minutes. Subsequently, the cells were exposed to Alexa Fluor 488® Goat Anti-Mouse IgG antibody at a concentration of 10 μg/ml in a humidified box at 4°C for 1 hour. After this incubation period, the cells were washed again and analyzed using a flow cytometer.
Therapeutic assessment of CL using peritoneal metastases model mice of human bile duct cancer in vivo
The mice were handled in accordance with the guidelines for animal experimentation under Japanese law. All animal experiments received approval from the Committee on Animal Research of Sojo University. BALB/c-R/J mice, generously provided by Prof. Okada (Kumamoto University, Japan), were utilized [13]. Mice were bred under specific conditions, including a 100% fresh ventilatory change 14 times per hour, a temperature maintained at 25 ± 1°C, humidity set at 50 ± 10%, and a light/ dark cycle of 12 hours. The mice were randomly grouped based on body weight, employing the stratified randomization method, with five mice in each group. HuCCT-1 cells (5.0 × 106) were intraperitoneally injected into BALB/c-R/J mice (n=5). Subsequently, CL (136 mg/kg/d for DMPC, 65.7 mg/kg/d for C12(EO)21, 65.7 mg/kg/d 2C14ECl) was administered intravenously once daily for 14 days. Tumor nodules were macroscopically counted, and the collected mesenteries were weighed.
Statistical analysis
Results are presented as mean ± S.D. Data were statistically analyzed using Student’s t-test. A p value of less than 0.05 was considered to represent a statistically significant difference.
Results and Discussion
Physical properties of CL
We prepared a mixture containing DMPC (purity>99%; NOF Co. Ltd., Tokyo, Japan), C12(EO)21 (Nikko Chemicals Co. Ltd., Tokyo, Japan), and 2C14ECl (DC-6-14; Sogo Pharmaceutical Co. Ltd., Tokyo, Japan) by sonicating it using a bath-type sonicator in a 5% glucose solution, as described previously [11-12]. The sample solutions were stored at 37°C.
We measured the apparent mean hydrodynamic diameter (dhy) of the CL based on dynamic light scattering [11-12]. The hydrodynamic diameter (dhy) of CL was 100 nm with a single and narrow size distribution, which remained stable for more than one month. In contrast, DMPC liposomes were unstable and precipitated after 14 days. The absorbance of HL21 (95 mol% DMPC/5 mol% C12(EO)21) gradually increased (>400 nm) over time. These results suggest that CL with a diameter smaller than 100 nm can avoid detection by the Reticular Endothelial System (RES); therefore, they should be suitable for intravenous injection, both in vivo and for clinical applications [14].
Inhibitory effects of CL on the growth of HuCCT-1 cells
We investigated the IC50 of Chloroquine (CL) in inhibiting the growth of human bile duct cancer (HuCCT-1) cells through an in vitro WST-8 assay. The IC50 values of chloroquine (280 µM, p<0.05, compared to DMPC and HL21) against HuCCT-1 cells were significantly lower than those of DMPC liposomes (>1000 µM) and HL21 (400 µM). Importantly, chloroquine exhibited robust inhibitory effects on human bile duct cancer cells (HuCCT-1).
Inhibition of migration in HuCCT-1 cells by CL
The study investigated the anti-migratory effects of CL, which are associated with the suppression of motility in HuCCT-1 cells. The analysis of the anti-migratory effect of CL on HuCCT-1 cells was conducted using the scratch wound assay, and the findings are presented in Figure 1. Notably, the scratched area of HuCCT-1 cells treated with CL remained significantly larger compared to the control group treated with DMPC and HL21 cells [15,16].
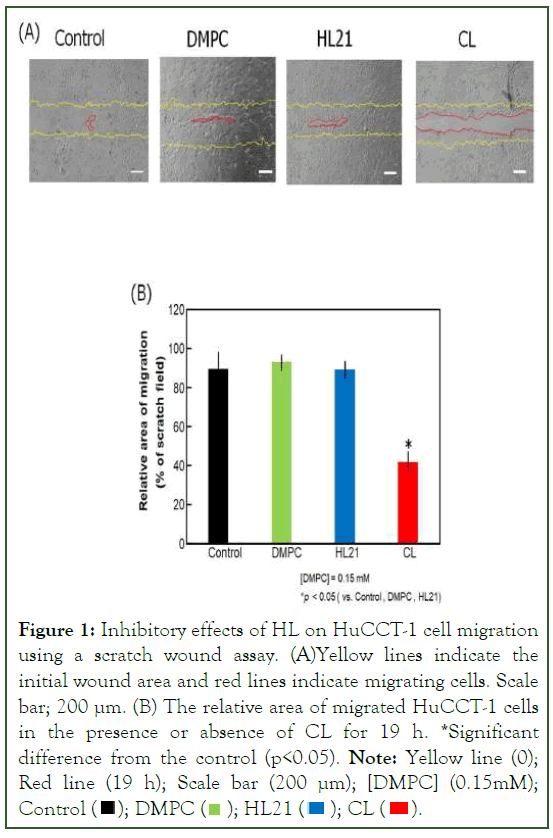
Figure 1: Inhibitory effects of HL on HuCCT-1 cell migration
using a scratch wound assay. (A)Yellow lines indicate the
initial wound area and red lines indicate migrating cells. Scale
bar; 200 μm. (B) The relative area of migrated HuCCT-1 cells
in the presence or absence of CL for 19 h. *Significant
difference from the control (p<0.05). Note: Yellow line (0); Red line (19 h); Scale bar (200 μm); [DMPC] (0.15mM); Control 
To further elucidate the anti-migration effects of CL, experiments were conducted at low concentrations (0.15 mM) to ensure that it did not impact cell growth or inhibit IC50 values (0.38 mM) (Figure 1A). Results indicated that CL markedly inhibited HuCCT-1 cell migration in comparison to cells treated with glucose (control), DMPC, and HL21. The inhibitory effects of CL on HuCCT-1 cell migration were statistically significant when compared with the control groups treated with glucose, DMPC, and HL21 (p<0.05) (Figure 1B).
In summary, these findings strongly suggest that CL effectively suppresses the migration of HuCCT-1 cells.
Suppression of the pseudopodia formation in HuCCT-1 cells by CL
Pseudopodia formation by tumor cells plays a crucial role in the migration and invasion of surrounding tissues [17,18]. Therefore, we investigated the suppressive effect of CL on pseudopodium formation in HuCCT-1 cells using confocal laser microscopy. The results are presented in Figure 2. Few pseudopodia were observed on the surface of HuCCT-1 cells treated with CL (0.15 mM). In contrast, many pseudopodia were observed on cells treated with glucose (control), DMPC, or HL21. These findings suggest that CL blocks the actin cytoskeleton, thereby inhibiting the migration of HuCCT-1 cells.

Figure 2: Micrographs showing the pseudopodium in HuCCT-1 cells treated with CL for 19 h using a confocal laser microscope. Scale bar; 120 μm.
Suppression of invasion of CL in HuCCT-1 cells by CL
The invasion of tumor cells represents a critical early stage in the progression of tumor metastasis. In this study, we explored the anti-invasive effects of CL on HuCCT-1 cells through a Matrigel invasion chamber assay. Figure 3, illustrates the obtained results. Notably, even at low concentrations (0.15 mM), CL demonstrated anti-invasive effects without adversely impacting cell growth or inhibition, as evidenced by an IC50 value of 0.28 mM. Comparing the treatment groups, including control, DMPC, and HL21 cells, with those treated with CL, a significant inhibition of invasion in HuCCT-1 cells was observed (Figure 3A). Furthermore, the dose-dependent nature of this inhibition is depicted in Figure 3B, where the invasion rate of HuCCT-1 cells decreased markedly with increasing CL concentrations. Remarkably, these anti-invasive effects of CL were notable at concentrations below the IC50 value, indicating its efficacy in impeding invasion without compromising cell viability. In summary, treatment with CL exhibited a substantial and dose-dependent reduction in the invasion of HuCCT-1 cells when compared to control, DMPC, and HL21-treated cells.
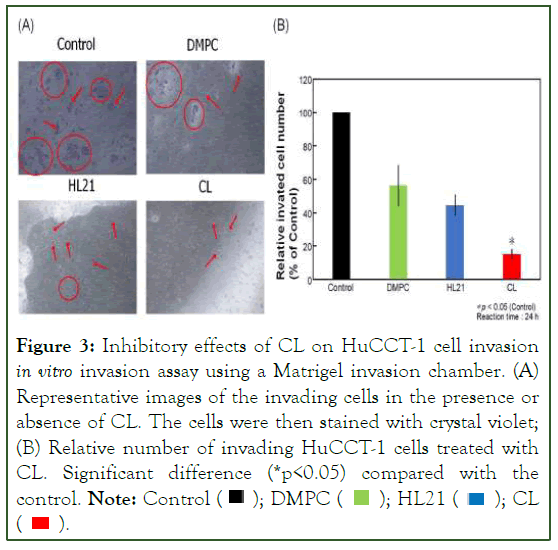
Figure 3: Inhibitory effects of CL on HuCCT-1 cell invasion in vitro invasion assay using a Matrigel invasion chamber. (A) Representative images of the invading cells in the presence or
absence of CL. The cells were then stained with crystal violet;
(B) Relative number of invading HuCCT-1 cells treated with
CL. Significant difference (*p<0.05) compared with the control. Note: Control  CL
CL 
Inhibitory effects of MMP-2 by CL on invasion
We investigated the impact of CL on matrix metalloproteinase-2 (MMP-2) activation in HuCCT-1 cells using ELISA. The results are illustrated in Figure 4. The reduction in the relative protein expression of MMP-2 in HuCCT-1 cells treated with CL was less pronounced compared to cells treated with glucose (control), DMPC, or HL21. Currently, we are exploring the inhibitory effects of HL on the activation of MMPs such as MT1-MMP and MMP-9 in HuCCT-1 cells in vitro. These findings suggest that CL inhibits the inversion of HuCCT-1 cells in vitro.
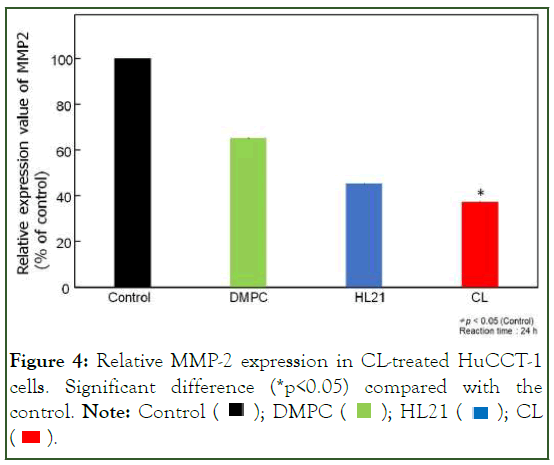
Figure 4: Relative MMP-2 expression in CL-treated HuCCT-1
cells. Significant difference (*p<0.05) compared with the control. Note: Control  CL
CL 
Therapeutic effects of CL for peritoneal metastases model mice of bile duct cancer in vivo
We investigated the therapeutic effects of intravenous CL treatment on tumor growth in a peritoneal metastasis model of bile duct cancer. The results are presented in Figure 5. Model mice treated with CL exhibited a significant reduction in mesenteric weight (p<0.05) compared to the control group (Figure 5A). The number of tumor nodules in the mesenteries of CL-treated model mice was significantly lower than that in the control group (Figure 5B). Notably, there was a substantial decrease in the number of tumor nodules on the mesentery in CL-treated mice compared to the control group (Figure 5C). Remarkable therapeutic effects were observed in a mouse model of peritoneal metastasis of bile duct cancer following intravenous CL treatment, without the use of additional drugs.
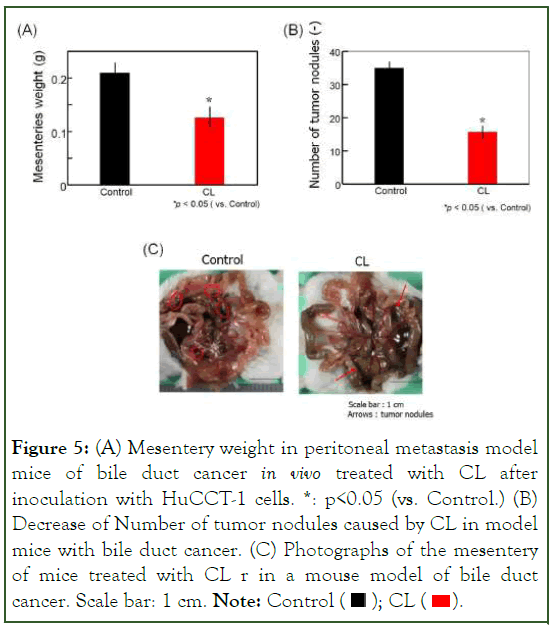
Figure 5: (A) Mesentery weight in peritoneal metastasis model
mice of bile duct cancer in vivo treated with CL after
inoculation with HuCCT-1 cells. *: p<0.05 (vs. Control.) (B)
Decrease of Number of tumor nodules caused by CL in model
mice with bile duct cancer. (C) Photographs of the mesentery
of mice treated with CL r in a mouse model of bile duct cancer. Scale bar: 1 cm. Note: Control 
Conclusion
We demonstrated the invasive inhibition of HuCCT-1 cells by CL. The noteworthy aspects of this study are as follows: (a) The IC50 value of CL for the growth of HuCCT-1 cells was significantly lower than that of DMPC liposomes and HL21. (b) We determined the inhibitory effects of HL on HuCCT-1 cell migration using the scratch wound assay. (c) We observed suppressive effects of CL on pseudopodium formation in HuCCT-1 cells. (d) The anti-invasive effects of CL on HuCCT-1 cells were achieved through the inhibition of MMP2. (e) Using a scratch wound assay, we investigated the inhibitory effects of HL on HuCCT-1 cell migration. (f) In a peritoneal metastases mouse model of bile duct cancer, remarkable therapeutic effects were observed with intravenous treatment of CL without additional drugs. The results of this study may offer advantages for the clinical application of chemotherapy in patients with bile duct cancer.
Acknowledgment
We thank Hiromitsu Takaki for technical assistance. We would like to thank Editage (www.editage.jp) for English language editing.
References
- Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70(3):145-164.
[Crossref] [Google Scholar] [PubMed]
- ECIS-European Cancer Information System. 2020.
- Nakachi K, Konishi M, Ikeda M, Mizusawa J, Eba J, Okusaka T, et al. A randomized Phase III trial of adjuvant S-1 therapy vs. observation alone in resected biliary tract cancer: Japan Clinical Oncology Group Study (JCOG1202, ASCOT). Jpn J Clin Oncol. 2018;48(4):392-395.
[Crossref] [Google Scholar] [PubMed]
- Wang X, Yu GY, Chen M, Wei R, Chen J, Wang Z. Pattern of distant metastases in primary extrahepatic bile‐duct cancer: A SEER‐based study. Cancer Med. 2018;7(10):5006-5014.
[Crossref] [Google Scholar] [PubMed]
- Nath MC, Torbenson MS, Erickson LA. Perihilar cholangiocarcinoma. Mayo Clin Proc. 2018;93(3):397-398.
[Crossref] [Google Scholar] [PubMed]
- Yu K, Kei N, Kuniharu Y, Hiroshi Y, Hiroki H. Lymph nodes metastasis is a risk factor for bone metastasis from extrahepatic cholangiocarcinoma. Hepatogastroenterology. 2012;59:1758-1760.
[Crossref] [Google Scholar] [PubMed]
- Razumilava N, Gradilone SA, Smoot RL, Mertens JC, Bronk SF, Sirica AE, et al. Non-canonical Hedgehog signaling contributes to chemotaxis in cholangiocarcinoma. J Hepatol. 2014;60(3):599-605.
[Crossref] [Google Scholar] [PubMed]
- Soubani O, Ali AS, Logna F, Ali S, Philip PA, Sarkar FH. Re-expression of miR-200 by novel approaches regulates the expression of PTEN and MT1-MMP in pancreatic cancer. Carcinogenesis. 2012;33(8):1563-1571.
[Crossref] [Google Scholar] [PubMed]
- Avcı E, Arıkoğlu H, Kaya DE. Investigation of juglone effects on metastasis and angiogenesis in pancreatic cancer cells. Gene. 2016;588(1):74-78.
[Crossref] [Google Scholar] [PubMed]
- Chiba H, Nagai H, Ohdaira T, Yasuda Y, Saito K. Immunohistochemical study of characteristics of bile duct dysplasia evaluated on the basis of expression of metastasis/invasion-related factors and p53. J Hepatobiliary Pancreat Surg. 2004;11:409-416.
[Crossref] [Google Scholar] [PubMed]
- Umebayashi M, Makizono T, Ichihara H, Matsumoto Y, Ueoka R. Inhibitory effects of cationic hybrid liposomes on the growth of human renal cell carcinoma. Anticancer Res. 2010;30(2):327-337.
[Crossref] [Google Scholar] [PubMed]
- Hino M, Ichihara H, Matsumoto Y, Ueoka R. Anti-tumor effects of cationic hybrid liposomes against colon carcinoma along with apoptosis in vitro. Biol Pharm Bull. 2012;35(11):2097-2101.
[Crossref] [Google Scholar] [PubMed]
- Ono A, Hattori S, Kariya R, Iwanaga S, Taura M, Harada H, et al. Comparative study of human hematopoietic cell engraftment into BALB/c and C57BL/6 strain of rag-2/jak3 double-deficient mice. J Biomed Biotechnol. 2011;2011.
[Crossref] [Google Scholar] [PubMed]
- Allen TM, Hansen C, Martin F, Redemann C, Yau-Young A. Liposomes containing synthetic lipid derivatives of poly (ethylene glycol) show prolonged circulation half-lives in vivo. Biochim Biophys Acta. 1991;1066(1):29-36.
[Crossref] [Google Scholar] [PubMed]
- Yamasaki HI. Hybrid liposomes inhibit the growth and angiogenesis in human breast cancer model. J Carcinog Mutagen. 2015;6(01).
- Kuwabara K, Ichihara H, Matsumoto Y. Inhibitory effects and anti-invasive activities of trehalose liposomes on the proliferation of lung carcinoma cells. J Carcinog Mutagen. 2017;8(01).
- Fujimoto K, Iwasaki C, Kawaguchi H, Yasugi E, Oshima M. Cell membrane dynamics and the induction of apoptosis by lipid compounds. FEBS Lett. 1999;446(1):113-116.
[Crossref] [Google Scholar] [PubMed]
- Lacour S, Hammann A, Grazide S, Lagadic-Gossmann D, Athias A, Sergent O, et al. Cisplatin-induced CD95 redistribution into membrane lipid rafts of HT29 human colon cancer cells. Cancer Res. 2004;64(10):3593-3598.
[Crossref] [Google Scholar] [PubMed]
Citation: Ichihara H, Matsumoto Y (2024) Inhibitory Effects of Cationic Hybrid Liposomes against Metastasis of Bile Duct Cancer in Vitro and In Vivo. J Carcinog Mutagen. 15:444.
Copyright: © 2024 Ichihara H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

