Indexed In
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Access to Global Online Research in Agriculture (AGORA)
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Publons
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2022) Volume 13, Issue 11
INFLUENCE OF TUBER SECTIONS AND CHEMICAL ADDITIVES ON PHYSICOCHEMICAL PROPERTIES OF PREGELATINIZED CASSAVA (Manihot esculenta Crantz) FLOUR
Iheagwara Marcquin Chibuzo*Received: 26-Feb-2022, Manuscript No. JFPT-22-15710; Editor assigned: 28-Mar-2022, Pre QC No. JFPT-22-15710(PQ); Reviewed: 14-Mar-2022, QC No. JFPT-22-15710; Revised: 26-Apr-2022, Manuscript No. JFPT-22-15710(R); Published: 03-May-2022, DOI: 10.35248/2157-7110.22.13.961
Abstract
Effect of tuber sections and some processing conditions on the physicochemical properties of pre gelatinized bitter cassava tuber (Manihot esculenta Crantz) flour was investigated. Flour samples were generated as fresh, boiled and steamed from the head, middle, tail and the whole tuber. All the samples were subjected to some physicochemical analyses such as pH, Water Absorption Capacity (WAC), Oil Absorption Capacity (OAC), Swelling Index (SI), Total Soluble Solids (TSS), Gelling Point Temperature (GPT) and Boiling Point Temperature (BPT) in order to assess the effect due to pre-milling heat treatment and post milling rehydration properties of flour in different steeping solution of varying concentrations. The result showed that proximate composition and physicochemical properties of flour from the sections and whole did not differ significantly (p<0.05). Other influencing factors such as Tuber Processing Methods (TPM) effected significant differences (p<0.05) on the physicochemical properties except for BPT. Effect of Tuber Processing Duration (TPD) were statistically (p=0.05) in all the test parameters except for pH. Steeping Solution Type (SST) showed no statistical difference for BPT and SI while Steeping Solution Concentration (SSC) affected significant difference (p<0.05) for SI, TSS and pH. Parameter Analytical Temperature (PAT) 30°C gave the least value for OAC, SI, TSS and WAC and was significantly different (p<0.05) from 50°C and 80°C temperature profiles.
Keywords
Cassava; Physicochemical; Flour; Tuber sections; Chemical additives
Introduction
Cassava (Manihot esculenta Crantz) is a widely cultivated tuber crop in Nigeria [1]. Cassava cultivation enjoys the advantage of adaptation to environmental conditions, limited skill application, inputs and can store underground up to 24 months [2-5]. The tuber is the main economically useful portion of the cassava plant and it is highly perishable. The high perishable tendency of the tuber makes it to start rotting some few days after harvest. The spoilage makes it unfit not only for edible purpose but also for processing into any useful product. Cassava has two main varieties, the sweet cassava variety, classified for its low cyanide content and the bitter cassava variety with high cyanide content which makes it lethal when consumed raw [6-8]. Cassava is a good source of high carbohydrate and a commonly sourced staple food in many tropical countries. Rich in carbohydrate, it is found to contain very low amounts of protein and fat, and appreciable level of vitamin C (35 mg/100 g fresh root), vitamin A and significant quantity of calcium [9,10]. In Nigeria, cassava tubers are boiled and eaten as abacha while fermented and toasted to obtain gari. As cassava flour, it could be prepared into eba. The flour can be used in making noodles, biscuits and confectionaries as well as composite flour in bread making [11-14]. The limitation in the utilization of cassava is the presence of poisonous cyanogenic glucosides, linamarin and lotaustralin which can be reduced or eliminated through combination of one or more processing techniques such as cutting, steeping, fermentation, boiling, steaming, toastinggs, drying etc [15-18]. Since cassava is predominantly carbohydrate, it is eaten in combination with other foods and can be enriched or fortified by blending its products such as cassava flour with soybeans and fortifying gari with vitamin A. Aside domestic consumption, cassava has been greatly utilized in the industry especially, food, textile and pharmaceutical industries [19,20]. A convenient form in which these industries can utilize cassava is the processed flour. It serves as raw material base for industrial purposes, having good advantages of availability, ease of handling and utilization, storage stability and reduction in processing time. Yet there is a continuous search to expand utilization and this has prompted several researches on cassava. The technical interest is to explore diverse modification techniques or processes which could enhance the physicochemical properties of the flour in food operation systems. Several modification approaches have been attempted including pre-gelatinization. However, there is also need to do a section by section tuber modification adopting pre gelatinization technique in order to assess the impact on the physicochemical properties of the various tuber sections.
Materials and Methods
Materials
Plant materials: Improved local variety (Nwaocha) of bitter cassava (Manihot esculenta Crantz) harvested after 18 months of planting from Federal University of Technology, Owerri (FUTO) farms was used for the study.
Chemicals: All the chemicals used for the experimental study were of analytical grade and were obtained from FUTO chemical store.
Methods
Generation of samples: The cassava tubers were peeled, washed and cut into three parts of head, middle and tail sections in the proportion of 30% head, 40% middle and 30% tail across the length of each tuber cutting. Each section was processed into raw flour by cutting into 2 cm thick chips, dried, milled and sieved through a 500 micron sieve to obtain fresh flour. A second batch of the tuber sections were cut into 5 cm chunk and processed by submerged boiling while samples were drawn at 0, 30 and 60 min, cooled, sliced into 2 cm thickness, dried and finished as the fresh. A third batch of the tuber sections were also cut into 5 cm chunk and processed by steaming through a wire mesh suspended on a boiling water at atmospheric condition while samples were drawn at 0, 30 and 60 min respectively, cooled, sliced into 2 cm thickness, dried and finished as the fresh. Whole cassava flour was also obtained from whole tuber and processed as fresh, boiled and steamed as described above.
Proximate analysis: The method described by Nielsen and AOAC were adopted for the proximate analysis determination.
Functional properties: Standard methods of AOAC were adopted for the pH determination. The Swelling Index (SI), Blue Value Index (BVI), and solubility determinations were conducted according to the method described by. The method described by was adopted for Water Absorption Capacity (WAC) and Oil Absorption Capacity (OAC) respectively. The method of was adopted for the Gelling and Boiling Point Temperatures (GPT and BPT).
Data analysis: The data from the analyses were subjected to statistical analysis. They were computed into means and standard deviations. The obtained means were subjected to analysis of variance (ANOVA) to test if any significant difference(s) existed using Duncan Multiple Range Test (DMRT) at requisite level of confidence.
Result and Discussion
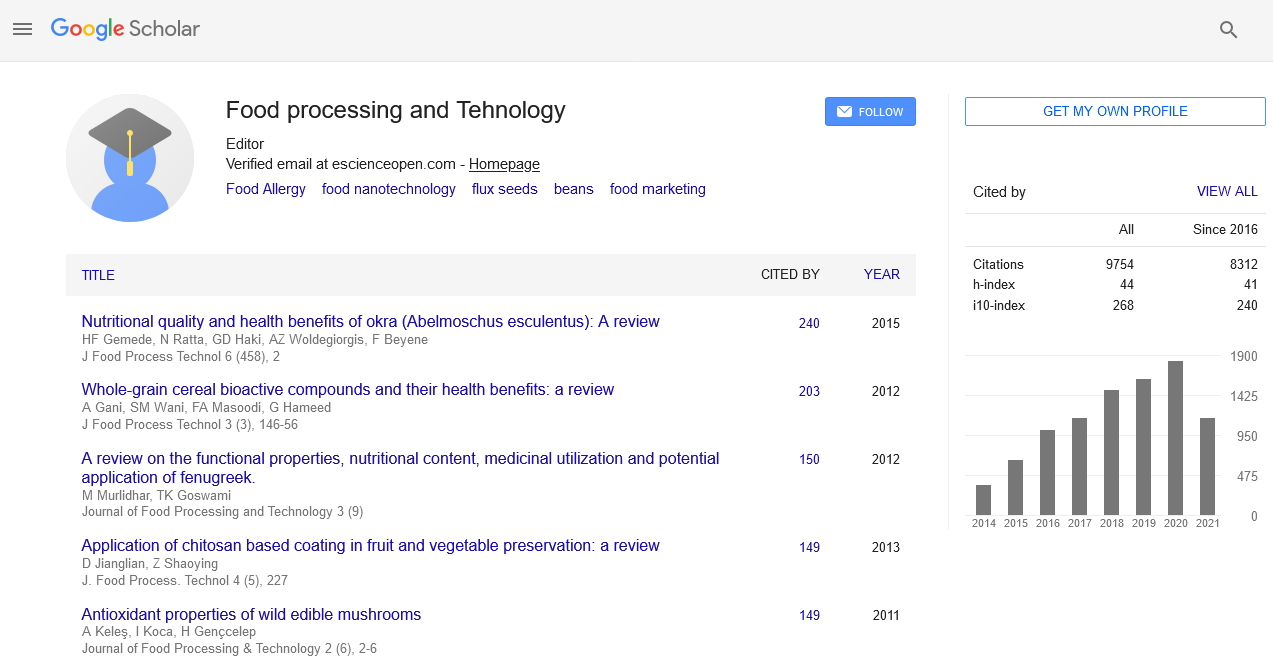
Proximate composition
Proximate composition of cassava flour as affected by section of root tuber is shown in Table 1. Across the sections of the root tuber and whole, there was no significant difference (p<0.05) in the tested parameters. This indicates that the distribution of these components across the tuber length follows a similar pattern. No section indicated an emerging sequence that could suggest it has predominant influence on the whole tuber. This view agrees with the report of on sweet potato flour. Hence the usual practice of processing the whole tuber into cassava flour should continue.
| Parameter (%db) | Section of root tuber | |||
|---|---|---|---|---|
| Head | Middle | Tail | Whole | |
| Carbohydrate * | 73.41 ± 0,05a | 72.83 ± 0.03a | 71.83 ± 0.07a | 72.57 ± 0.05a |
| Protein | 5.85 ± 0.02a | 6.15 ± 0.02a | 7.02± 0.01a | 6.74 ± 0.05a |
| Fat | 3.75 ± 0.01a | 4.04 ± 0.04a | 3.87 ± 0.07a | 3.86 ±0 0.01a |
| Ash | 0.80 ± 0.02a | 0.95 ± 0.00a | 0.90 ± 0.01a | 0.93 ± 0.01a |
| Fiber | 1.66 ± 0.01a | 1.40 ± 0.00a | 1.37 ± 0.02a | 1.65 ± 0.00a |
| Moisture | 14.53 ± 0.04a | 14.53 ± 0.08a | 15.01 ± 0.02a | 14.25 ± 0.01a |
*Determined by difference
Means with the same superscripts along rows are not significantly different (p<0.05)
Table 1: Proximate composition of cassava flour as affected by section of root tuber.
Physicochemical properties
Effect of section of root tuber on physicochemical properties of cassava flour: Table 2 showed the effect of section of root tuber on the physicochemical properties of cassava flour. No significant difference (p<0.05) was noted on the mean values of the tested physicochemical properties across the section of the root tuber and whole. It can be inferred from the result that no section of the root tuber had over bearing influence on all the tested properties over the other. This suggests that the section of the cassava tuber from which flour is generated is not a critical factor that controls the physicochemical properties of cassava flour. It also reflects that granular formation, density and distribution across the tuber length probably follow a similar pattern. This may not be the case with other tubers. Therefore, processing whole tuber is time advantageous as it will eliminate time wasted in cutting tuber into sections.
| Properties | Section of root tuber | |||
|---|---|---|---|---|
| Head | Middle | Tail | Whole | |
| pH | 8.12 ± 2.16a | 8.11 ± 2.16a | 8.11 ± 2.15a | 8.11 ± 2.16a |
| Gelling point temperature (℃) | 60.53 ± 3.71a | 60.53 ± 3.82a | 60.09± 3.22a | 60.55 ± 3.87a |
| Boiling point temperature (℃) | 72.24 ± 2.38a | 73.69 ± 3.71a | 71.61 ± 3.35a | 72.10 ± 2.41a |
| Swelling index | 2.53 ± 0.88a | 2.58 ± 0.87a | 2.56 ± 0.83a | 2.57 ± 0.88a |
| Total soluble solids (%) | 13.23 ± 2.91a | 13.20 ± 2.44a | 13.16 ± 2.36a | 13.19 ± 2.38a |
| Water absorption capacity (ml/g) | 2.12 ± 0.79a | 2.20 ± 0.79a | 2.22 ± 0.78a | 2.21 ± 0.78a |
Means with the same superscripts along rows are not significantly different (p<0.05)
Table 2: Physicochemical properties of cassava flour as affected by section of root tuber.
The inference on the result of physicochemical properties of cassava flour agrees with the proximate composition, which principally is an influencing factor on the physicochemical response of cassava flour.
Effect of processing methods on physicochemical properties of cassava flour: Tuber processing method afforded the possibilities of generating flour samples as fresh, boiled and steamed. The impact of these pre milling treatments on the physicochemical properties of cassava flour is shown in Table 3.
| Properties | Tuber processing method | ||
|---|---|---|---|
| Boiled | Steamed | Fresh | |
| pH | 8.05 ± 2.16a | 8.09 ± 2.15b | 8.20 ± 2.16c |
| Gelling point temperature (℃) | 56.47 ± 1.90a | 59.87 ± 3.40b | 65.21± 1.66c |
| Boiling point temperature (℃) | 69.96 ± 1.93a | 73.32 ± 2.47 a | 74.26 ± 3.58a |
| Swelling index | 3.26 ± 0.88a | 2.96 ± 0.17b | 1.36 ± 0.17c |
| Total soluble solids (%) | 15.12 ± 1.13a | 13.76 ± 1.27b | 5.06 ± 0.96c |
| Water absorption capacity (ml/g) | 2.83 ± 0.07a | 2.63 ± 0.13a | 1.08 ± 0.05b |
Means with the same superscripts along rows are not significantly different (p<0.05)
Table 3: Physicochemical properties of cassava flour as affected by tuber processing method.
The pH of different flour samples generated through the processing methods is statistically significant (p<0.05). Although, the pH of the samples from fresh, boiled and steamed are in the regime of alkalinity, the fresh sample had the highest pH of 8.20 as against steamed and boiled with respective 8.09 and 8.05. The data showed slight reduction in pH from fresh to boil.
Gelling point temperatures for all the samples (fresh, boiled and steamed) differ significantly (p<0.05). The fresh sample had the highest gelling point 65.21°C which is within the range reported by Moorthy and Ramanujam, while the boiled sample had the least value of 56.46°C. It should be noted that both boiled and steamed flour samples are already pregelled samples that are being tested for gelling point; and as expected, the more severely propelled sample according to Iwuoha will exhibit the least gelatinization temperature level. Flour sample with low temperature gelling points may be found useful in food operation system requiring lower temperature application.
The boiling point temperature behavior of the flour samples still followed similar pattern of the gelling point, with the fresh sample having the highest mean value of 74.26°C and the boiled sample 69.96°C. This indicates that the hydrothermal pretreatment of the boiled sample ensured its earliest response to boil than the flour sample obtained from other processing method. However, the variation in the three processing method on boiling point temperature of flour were statistically insignificant (p>0.05).
The swelling index of the flour sample generated from the flour processing method were significantly different (p<0.05). Boiled sample had the highest swelling index of 33.26 followed by steamed sample with 2.96 and the least value of 1.36 for fresh sample. This data clearly demonstrate that pre-gelatinization modifies the structure of carbohydrate moiety in the flour. The heat tampering during the process brings about cleavage of the weak intermolecular hydrogen bonds and exposure of the hydrophilic ends which in ambient aqueous environment becomes attracted to water; the flour absorbs water and swell. However, the data still suggest that the extent of swelling is influenced by the nature of processing. Hydrothermal effect of boiling ensured greater severity on bond cleavage and hydroxyl ion proliferation than steaming which is only semi dry heat. This implies that boiled flour sample is expected to swell more than the steamed counterpart. The slight swelling of the fresh flour sample is expected, since it is the native state and the limited hydrophilic sites will permit water up-take in proportion to the relative humidity of the atmosphere.
From this result it can be inferred that processing methods is a critical factor of swelling index of flour. Flour with high swell ability can be incorporated into food system to control volume or size. Volume control is an important feature to both processors and consumers of especially baked products.
The total soluble solids (%db) data obtained under ambient environment indicated that there was a statistical difference (p<0.05) among the flour samples generated from the various processing methods. The boiled sample had the highest solubility of 15.12%, steamed 13.76% and the least fresh 5.06%. This further suggests that processing method is a crucial factor in cassava flour processing and also that boiling imparted doneness and softness to the tuber tissues and cells which resulted in greater solubility.
The water absorption capacity (ml H2O/g, db) in response to the processing method showed that flour samples from boiled treatment had highest value of 2.83 ml/g followed by steamed treatment with 2.63 ml/g. These mean values were statistically different from freshly treated sample which had least mean value for water absorption. Water absorption capacity increases with proliferation of hydrophilic sites which is more posited in flour sample generated from boiled and steamed programme than the fresh. The severity of heat treatment which is dependent upon types of processing adopted together and the length of exposure of the tuber to such treatment are possible factors that could initiate greater biophysical readjustment in the structure matrix of the tuber, thus enhancing their capability to hold more water. Similar view was expressed by Iwuoha. A comprehensive evaluation of the three processing methods showed that a clear sequence emerged across the tested properties among the processing methods. Though boiling as well as steaming clearly influences the physicochemical properties, boiling appears to be a better strategic working tool in the manipulation/control of the physicochemical properties. The additional benefits of boiling over the other methods are that it ensures greater reduction in the cyanide content of the flour. Also agreed that boiling, chipping, steeping, and sun-drying and milling yielded safe flour from cyanide intoxication. Flour generated from it will serve as a better drying/dusting/anti-caking/bulking agent than the fresh cassava flour.
Effect of tuber processing duration on physicochemical properties of cassava flour: Table 4 shows the effect of tuber processing duration on the physicochemical properties of cassava flour. The pH of the flour sample obtained from the various time exposure of processing showed that significant difference exists for pH of flour at different processing time. However, it slightly dropped as the processing time is increased from 0 to 60 minutes. The pH of the flour samples at the different time exposure were in the regime of alkalinity. Reported similar trend for samples obtained via steaming at prevailing atmospheric condition over time. The longer the exposure time the more the severity/intensity of the heat treatment on the sample and the tendency of the starch granules to rupture leaching out into the solution.
| Properties | Tuber processing duration | ||
|---|---|---|---|
| 0 | 30 | 50 | |
| pH | 8.09 ± 0.02a | 8.07 ± 0.02b | 8.05 ± 0.03c |
| Gelling point temperature (℃) | 58.55 ± 5.46a | 58.73 ± 5.19a | 57.48 ± 4.12a |
| Boiling point temperature (℃) | 74.47 ± 4.52a | 73.92 ± 6.22a | 72.98 ± 3.71a |
| Swelling Index | 2.65 ± 0.47a | 2.74 ± 0.09a | 2.81± 0.24a |
| Total soluble solids (%) | 12.67 ± 1.39a | 14.03 ± 1.34a | 15.09 ± 1.57a |
| Water absorption capacity (ml/g) | 3.00 ± 0.54a | 3.09 ± 0.26a | 3.24 ± 0.30a |
Means with the same superscripts along rows are not significantly different (p<0.05)<
Table 4: Physicochemical properties of cassava flour as affected by tuber processing duration.
The gelling point of flour generated from different time exposure showed no significant difference (p<0.05). Similar trend was observed for other physiochemical properties. This suggest that time of processing is not a critical factor that influence the physicochemical properties of flour. Depending on the intended interest, any of the time schedules can be selected by a processor and that is after a comparative analysis of energy economy and modification requirements of flour properties have been assessed.
Effect of steeping solution type on physicochemical properties of cassava flour: Table 5 shows mean values of physicochemical properties of cassava as affected by steeping solution type. The pH of flour sample from the different steeping solution differed significantly from each other, except for sodium chloride and sucrose which where statistically equivalent (p=0.05). The two salt types are neutral salts.
| Properties | Steeping solution type | |||
|---|---|---|---|---|
| Sodium Carbonate | Sodium Chloride | Sodium Citrate | Sucrose | |
| pH | 11.81 ± 0.06a | 6.58 ± 0.05b | 7.40 ± 0.07c | 6.07 ± 0.05b |
| Gelling point Temperature (℃) | 58.70 ± 4.53a | 61.90 ± 4.10b | 59.74 ± 2.72c | 60.39±3.73b |
| Boiling point temperature (℃) | 70.54 ± 1.91a | 75.41 ± 2.95a | 71.21 ± 3.41a | 72.89±2.80a |
| Swelling index | 2.68 ± 0.91a | 2.56 ± 0.88a | 2.58± 0.82a | 2.56± 0.80a |
| Total soluble solids (%) | 15.35 ± 1.93a | 13.05 ± 1.89b | 14.47 ± 1.63ac | 13.56±1.28b |
| Water absorption capacity (ml/g) | 2.23 ± 0.81a | 2.15 ± 0.78b | 2.18 ± 0.77b | 2.16 ± 0.78b |
Means with the same superscripts along rows are not significantly different (p<0.05)<
Table 5: Physicochemical properties of cassava flour as affected by steeping solution type.
Sodium chloride had the least influence while sodium carbonate had the highest effect forsuch parameter as water absorption capacity, swelling index, total soluble solids. The obvious explanation of this is that sodium carbonate is a basic salt and, as reported, increases alkalinity of the medium which promotes swelling power and solubility. Sodium carbonate also induces hydration and tenderization effect and can break down the starch molecule creating stronger hydrophilic interaction. For some parameter such as gelling point and boiling point, sodium chloride favors increased gelling and boing point than other salts though the response is not statistically different (p<0.05) on boiling point while sodium carbonate impacts lower gelling and boiling points since it facilitate granular swelling and water absorption. Hence in terms of choice, sodium carbonate may be preferred because of its techno economic advantages.
Effect of steeping solution concentration on physicochemical properties of cassava flour: The result as shown in Table 6 showed the impact of varying steeping solution concentration in enhancing the physicochemical properties of flour. Higher concentration of solute favor increase gelling and boiling points, on the account that the solute partly binds water and make it unavailable for the hydration of the flour. This is in agreement with the report of while it promotes higher values for total soluble solids for all the steeping solution concentration. Swelling index and water absorption capacity reduced with increased concentration for all solute except sodium carbonate which expectedly promoted swelling index and water absorption capacity and total soluble solids. Generally, there is no significant difference due to solute concentration for all the properties other than pH and as such, it is suggested that lower concentration be applied for economic and health reason whenever solute addition is required to avoid excessive loading of chemical.
| Properties | Steeping solution concentration (%m/v) | |||
|---|---|---|---|---|
| 0 | 5 | 10 | 30 | |
| pH | 6.77 ± 0.07a | 8.38 ± 0.07b | 8.56 ± 0.06c | 8.75 ± 0.07d |
| Gelling Point Temperature (℃) | 59.04 ± 4.83a | 59.47 ± 4.50a | 60.70 ± 4.16a | 61.53 ± 2.95a |
| Boiling point temperature (℃) | 71.19 ± 4.78a | 72.39 ± 1.37a | 72.92 ± 2.94a | 73.58 ± 3.40a |
| Swelling index | 2.24 ± 0.73a | 2.18 ± 0.79a | 2.16± 0.79b | 2.13 ± 0.75c |
| Total soluble solids (%) | 15.35 ± 1.87a | 13.05 ± 1.65b | 14.47 ± 1.60b | 13.56 ± 1.40c |
| Water absorption capacity (ml/g) | 2.60 ± 0.87a | 2.15 ± 0.83a | 2.51 ± 0.88a | 2.43 ± 0.87a |
Means with the same superscripts along rows are not significantly different (p<0.05)
Table 6: Physicochemical properties of cassava flour as affected by steeping solution concentration.
Effect of parameter application temperature on physicochemical properties of cassava flour: The rehydration properties of the cassava flour were tested in oil and aqueous solution respective at temperature of 30°C, 50°C and 80°C. The result of the effect is shown in Table 7. The oil absorption capacity (ml oil/g db) increased slightly over the temperature profile. The effect on 30℃ differed significantly (p<0.05) from 50°C and 80°C which were statistically equivalent. Obviously at temperature of 50°C and above, the thermal effect on the flour component especially protein possibly undergo structural change i.e. denaturation leading to the uncoiling of the hydrophobic ends of it which then interact with the oil. More so, amylose-lipid interaction may also contribute to the oil absorption capacity according to Swinkle. However, hydrophobic ends appears to be limited in a predominantly carbohydrate system. Flour with low oil absorption capacity may not serve as meat extenders.
| Properties | Parameter application temperature (°C) | ||
|---|---|---|---|
| 30 | 50 | 80 | |
| Water absorption capacity (ml/g) | 1.03 ± 0.12a | 1.15 ± 0.11b | 1.22 ± 0.11b |
| Swelling Index | 2.18 ± 0.48a | 2.63 ± 0.53b | 2.69 ± 0.55b |
| Total soluble solids (%) | 10.64 ± 0.44a | 16.46 ± 1.48b | 18.19 ± 1.01b |
| Water absorption capacity (ml/g) | 2.58 ± 0.85a | 3.32 ± 0.93b | 3.51 ± 0.86b |
Means with the same superscripts along rows are not significantly different (p < 0.05)
Table 7: Physicochemical properties of cassava flour as affected by parameter application temperature.
The swelling power of flour at different temperature profile in the aqueous solution showed that 80°C application had the highest mean value of 2.69 ml H2O/g db while the 30°C application had 2.18 ml H2O/gdb. However, 50°C and 80°C temperature application were statistically equivalent (p=0.05) and differed significantly from 30°C. This has demonstrated that rehydrating flour in aqueous solution of higher temperature improves the swelling power. This observation agrees with the findings of that swelling of starch is temperature dependent. This is because at higher temperature starch molecule in the flour may undergo structural realignment resulting in the cleavage of hydrogen bond. The free hydroxyl group becomes attracted to the water molecule bringing about hydration of the starch moiety and resultant volume increase.
The total soluble solids also follow the same sequence increasing with temperature and recorded highest solubility at 80℃ than at 50℃ and 30℃. However, solubility at 50℃ and 80℃ did not differ significantly (p=0.05).
Solubility is associated with the dissolution of the amylose fraction of the starch component which is slight at lower temperature but with increase temperature, the amylopectin fraction become contributory resulting to higher values obtained at higher temperature. Other minor dissolvable component of the flour other than the starch moiety may also be contributory to the aggregate effect. Flour with high solubility may be required in some operating food system to serves as thickening agent or gelling agent.
The water absorption capacities (ml H2O/g db) of the flour at different rehydration temperature also follow similar sequence. The application at 50℃ and 80℃ did not differ significantly (p>0.05). However, water absorption increased with increasing temperature which further buttresses the fact that at higher temperature more hydrophilic sites are created through the liberation of free hydroxyl group from the amylose cleavage. There is no statistical difference between application at 50℃and 80℃. Processor may for economic reason choose 50℃since lesser energy is expended in this application.
Conclusion
This investigative study revealed that heat treatment showed improvement in physicochemical properties of properties of processed cassava flour over the fresh flour. Rehydration properties of flour in different steeping solution and at varying concentration as an influencing factor on physicochemical properties have been shown to be significantly impactful. Data obtained will provide baseline information for expandable utilization of cassava, while characteristics of the physicochemical properties of these flours will guide/maximize the usage of flour into suitable products.
REFERENCES
- Ojo MO, Ariahu CC, Chinma EC. Proximate, Functional and Pasting Properties of Cassava Starch and Mushroom (Pleurotus Pulmonarius) Flour Blends. Am J Food Technol. 2017;5(1):11-18.
- El-Sharkawy MA. Cassava biology and physiology. Plant Mol Biol. 2003; 53(5):621-641.
- Uchechukwu-Agua AD, Caleb OJ, Opara UL. Postharvest handling and storage of fresh cassava root and products: A review. Food Bioproc Tech. 2015;8(4):729-748.
- Anikwe M, Eze J, Ibudialo A. Influence of lime and gypsum application on soil properties and yield of cassava (Manihot esculenta Crantz.) in a degraded Ultisol in Agbani, Enugu Southeastern Nigeria. Soil and Tillage. 2016;158:32-38.
- Chipeta MM, Shanahan P, Melis R, Sibiya J, Benesi IR. Early storage root bulking index and agronomic traits associated with early bulking in cassava. Field Crops Res. 2016;198:171-178.
- Oluwole OSA, Onabolu AO, Mtunda K, Mlingi N. Characterization of cassava (Manihot esculenta Crantz) varieties in Nigeria and Tanzania, and farmers’ perception of toxicity of cassava. J Food Compost Anal. 2007;20(7):559-567.
- Bradbury EJ, Duputié A, Delêtre M, Roullier C, Narváez-Trujillo A, Manu-Aduening JA, et al. Geographic differences in patterns of genetic differentiation among bitter and sweet manioc (Manihot esculenta subsp. esculenta; Euphorbiaceae). Am J Bot. 2013;100(5):857-866.
- Mtunguja MK, Laswai HS, Kanju E, Ndunguru J, Muzanila YC. Effect of genotype and genotype by environment interaction on total cyanide content, fresh root, and starch yield in farmer‐preferred cassava landraces in Tanzania. Food Sci Nutr. 2016;4(6):791-801.
- Charles AL, Sriroth K, Huang TC. Proximate composition, mineral contents, hydrogen cyanide and phytic acid of 5 cassava genotypes. Food Chem. 2005;92 (4):615-620.
- Enidiok SE, Attah LE, Otuechere CA. Evaluation of moisture, total cyanide and fiber contents of garri produced from cassava (Manihot utilissima) varieties obtained from Awassa in Southern Ethiopia. Pak J Nutr. 2008;7(5):625-629.
- Shittu TA, Aminu RA, Abulude EO. Functional effects of xanthan gum on composite cassava-wheat dough and bread. Food Hydrocoll. 2009;23(8):2254 - 2260.
- Abass A, Awoyale W, Alenkhe B, Ndavi M, Asiru B, Manyong V, et al. Can Food Technology Innovation Change the Status of a Food Security Crop? A Review of Cassava Transformation into “Bread” in Africa. Food Rev Int. 2016;1:1-16.
- Serventi L, Jensen S, Skibsted LH, Kidmose U. Addition of enzymes to improve sensory quality of composite wheat–cassava bread. Eur Food Res Technol. 2016;242(8):1245-1252.
- Oladunmoye OO, Aworh OC, Ade-Omowaye B, Elemo G. Substitution of wheat with cassava starch: effect on dough behavior and quality characteristics of macaroni noodles. Nutr Food Sci. 2017;47(1):1-5.
- Simonyan K. Cassava post harvest processing and storage in Nigeria: A review. Afr J Agric Res. 2014;9(53):3853-3863.
- Ferraro V, Piccirillo C, Pintado ME. Cassava (Manihot esculenta Crantz) and Yam (Discorea spp.) Crops and their Derived Foodstuffs: Safety, Security and Nutritional Value. Crit Rev Food Sci Nutr. 2015;56(15):1-15.
- Anyanwu CN, Ibeto CN, Ezeoha SL, Ogbuagu NJ. Sustainability of cassava (Manihot esculenta Crantz) as industrial feedstock, energy and food crop in Nigeria. Renew Energy. 2015;81:745-752.
- García-Segovia P, Urbano-Ramos A, Fiszman S, Martínez-Monzó J. Effects of processing conditions on the quality of vacuum fried cassava chips (Manihot esculenta Crantz). LWT-Food Sci. 2016;69:515-521.
- Hasmadi M, Harlina L, Jau-Shya L, Mansoor AH, Jahurul MHA, Zainol MK. Physicochemical and functional properties of cassava flour grown in different locations in Sabah, Malaysia. Food Res. 2020;4(4):991–999.
- Sandoval AA, Fernández QA. Physicochemical characterization of two cassava (Manihot esculenta Crantz) starches and flours. Sci Agropecu. 2013;1: 19-25.
Citation: Chibuzo IM (2022) Influence of Tuber Sections and Chemical Additives on Physicochemical Properties of Pregelatinized Cassava (Manihot Esculenta Crantz) Flour. J Food Process Technol. 13:961.
Copyright: �??�?�© 2022 Chibuzo IM. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.