Indexed In
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Access to Global Online Research in Agriculture (AGORA)
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Publons
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2020) Volume 11, Issue 2
Influence of storage condition and added vitamin E on oxidation stability of edible sunflower and nigger seed oils produced and sold around Bahir Dar city, Ethiopia
Zelalem Gizachew*Received: 19-Dec-2019 Published: 20-Mar-2020, DOI: 10.35248/2157-7110.20.11.821
Abstract
In human nutrition, fats are physiologically important food constituents but also the components most liable to oxidative degradation. This study was carried out in order to probe the extent of oxidative alterations in Sunflower Oil (SFO) and Nigger Oil (NO), subjected to daylight and dark storage conditions with, and without added vitamin E, over a period of 5 weeks storage. The magnitude of oxidative changes was monitored by the periodical measurement of acid value, peroxide value, and induction time throughout storage time. The acid value of sunflower oil increased from initial value 4.5 mg KOH/g to (24.7, 19.64, 16.83, and 12.34) mg KOH/g, and of nigger oil from 6.2 mg KOH/g to (21.3, 17.95, 16.83 and 11.22) mg KOH/g for oil samples kept in daylight, dark, daylight+vitamin E, dark+vitamin E for 5 weeks. Like the acid value, the peroxide value of sunflower oil increased from 2.2 meq O2/kg to (27.2, 16.6, 13.2, and 7.2) meq O2/kg, and of nigger oil from 2.0 meq O2/kg to (17.2, 13.2, 8.8 and 4.6) meq O2/kg for oil samples kept in the same conditions with that mentioned in acid value. Unlike the acid value, the induction time of sunflower oil decreased from 1.97 h to 1.51 h, 1.65 h, 1.77 h, and 1.85 h, and of nigger, oil decreased from 2.05 h to 1.60 h, 1.73 h, 1.81h, and 1.92 h for oil samples stored in daylight, dark, daylight+vitamin E, dark+vitamin E for 5 weeks. However, the induction time of fresh sunflower and nigger oils containing added vitamin E increased to 2.11 h and 2. 13 h. Changes in acid value, peroxide value and induction time obtained shows that the oxidative deterioration levels of oils were different between storage conditions. The results of the present study show that light acts as a major catalyst in accelerating the development of rancidity in oils. Also, the addition of vitamin E to oil can increase the oxidation stability of oils during storage. In conclusion, this study has been able to show storage in the light can affect oil stability and minimizes the potency of vitamin E in oils, fats or fat-containing products. Therefore, storing in dark (packaging with material protect light) and supporting with antioxidants is the best way to maintain the quality of oils during storage and domestic uses.
Keywords
Storage conditions; Vitamin E; Induction time; Oxidation; Sunflower oil; Nigger oil
Introduction
Background
Oil is a very important resource, much in demand everywhere in the world and is used in a variety of ways [1]. Oils extracted from plant sources are an integral part of human foods, being essential for health. Industrially, they play an important role in the development of different products such as pharmaceuticals, cosmetics, paints and most importantly, food [2].
Edible oils are lipid materials composed of glycerides, Fatty Acids (FAs) and some other compounds in small quantities, like hydrocarbons, alcohols, phenols, tocopherols, phospholipids [3,4]. Vegetable oils are obtained from oil-containing seeds, fruits, or nuts by different pressing methods, solvent extraction or a combination of these [5]. There are numerous vegetable oils derived from various sources. These include soybean oil, cottonseed oil, sunflower oil, palm oil, coconut oil, castor oil, rapeseed oil, and others. Their yields, different compositions and by extension their physical and chemical properties determine their usefulness in various applications aside edible uses. The physicochemical properties of fats and oils are influenced by the degree of unsaturation, the length of the carbon chain and the type, quantity, distribution on the triglycerol and isomeric form of FA.
Triacylglycerols (TAG) are the most abundant constituent in fats and oils. In vegetable oil, for example, TAG comprises more than 95% of the total lipid. The majority of the FAs occurring in common edible fats and oil are straight-chain compounds with an even number of carbon atoms, and they may be saturated (SFA), monounsaturated (MUFA) or polyunsaturated (PUFA). Unsaturated FAs in fats and oil are susceptible to oxidative processes in the presence of catalytic systems such as light, heat, enzymes, metals, metalloproteins, and microorganisms, giving rise to the development of off-flavors and loss of Essential Fatty Acids (EFAs), fat-soluble vitamins, and other bio-actives. Oils may undergo auto-oxidation, photo-oxidation, thermal oxidation, and enzymatic oxidation under different conditions, most of which involve some type of free radical or oxygen species [6].
Lipid oxidation is one of the major reasons that foods deteriorate and is caused by the reaction of fats and oils with molecular oxygen leading to off-flavors that are generally called rancidity. The hydroperoxides produced by lipid oxidation can decompose into various smaller molecules such as aldehydes, ketones, alcohols, and carboxylic acids. Some of these volatile products influence flavor, even at very low concentrations, in which both the oil and the food prepared from it become unpalatable [7]. Lipid oxidation not only produces rancid flavor but also can lower the nutritional value of food by the formation of oxidation products, which may play a role in the development of disease and can be harmful to humans [8].
To prevent the decomposition of oils, then, one should find substances that react with lipid free radicals and/or oxygen. Either reaction will arrest the oxidative process just described. A rather large number of substances have been found that meet either or both of these criteria and these substances are named as antioxidants. The most widely used antioxidants are free radical scavengers that remove reactive radicals formed in the initiation and propagation steps of oxidation [9].
Several natural or synthetic phenols can compete, even at low concentrations, with lipid molecules as hydrogen donors to hydroperoxy and alkoxy radicals, producing hydroperoxides and alcohols and an unreactive radical [9,10]. These stabilized radicals do not initiate or propagate the chain reaction. The best known and most effective primary antioxidants among natural substances are polyphenols. They are most effective in foods that contain little natural antioxidants, while they are much less effective in vegetable oils enriched in antioxidants naturally.
Several methods have been used to evaluate the extent of oxidative deterioration, which are related to the measurement of the concentration of primary or secondary oxidation products. The Peroxide Value (PV), Acid Value (AV) and Rancimattest are used to evaluate the extent of oxidative deterioration in this thesis. PV is related to the concentration of hydroperoxide, while the AV is related to extent of hydrolyzed FA. Rancimat test is also used to measures the formation of volatile acids by monitoring the change in electrical conductivity when effluent from oxidizing oils is passed through water [11].
Edible oil is part of everyday food expenditure in urban areas as well as in most of the rural areas. In Ethiopia, edible oils are mainly produced from cottonseed, rapeseed, nigger seed, sesame seed, palm, and vegetables. Informal oil processors, mainly in the rural area, produce around 85% of all domestically produced oil, while formal oil processors have a market of less than 15%, mainly in the urban region [12]. In Bahir Dar market different kinds of imported and domestically produced oils are sold. Out of domestically produced oils, Nigger Seed Oil (NO) and Sunflower Oil (SFO) are major oils produced in the city.
Warm ambient temperatures silently encourage the onset of oxidation. Oil processers package and store oils in a way exposed to oxidation. There are several shopkeepers and wholesale dealers in the city who store their oils and fat supplies in open air and light for publicity and also for the lack of storage space. Also, there is no information on the containers of these oils regarding their production and expiry dates. It is thus very important to evaluate the effects of photo-oxidation of lipids on the quality of oils and use alternative methods to increase the oxidation stability of oils.
The primary objective of the present work was to investigate the influence of storage condition and addition of vitamin E on oxidation stability of domestically produced NO and SFO by measuring AV, PV, and Induction Time (IT) for each samples of oils stored in dark and daylight with and without the addition of vitamin E starting from the first days of production to 5 weeks of storage. Checking the influence of storage condition and addition of vitamin E on oxidation stability of NO and SFO and studying methods on how the shelf life of NO and SFO will be improved during domestic use, helps the producers and users to know the time of deterioration and use suggested methods.
Objectives and significance of the study
Significance of the study: Edible vegetable oils are considered as a concentrated source of energy for human beings and carriers of oil-soluble Vitamins which supply the essential fatty acids that are required for a wide range of biological and physiological functions. The oxidative changes during storage and domestic use make oils unsuitable for consumption. Oxidative stability is an important indicator of oil quality and shelf-life. This study is expected to increase awareness among the population regarding the influence of storage conditions and added vitamin E on the oxidation stability of domestically produced NO and SFO by adapting different oxidation detection methods. Furthermore, the study is important to see the quality and shelf life of domestically produced edible oils.
Overall objective: The general objective of this study is to evaluate the influence of storage condition and added Vitamin E on oxidation stability of NO and SFO produced and sold around Bahir Dar city, Ethiopia.
Specific objectives:
• To evaluate the oxidative deterioration of sample oils during storage
• To estimate changes in free fatty acid during storage conditions
• To measure changes in peroxide value during storage conditions
• To measure the changes in the induction time of oils during storage conditions
• To compare oxidation stability of SFO and NO by measuring acid value, peroxide value and induction time
• To estimate the effect of added vitamin E on the stability of oils
• To compare the effect of light on oils stored with and without added vitamin E
• To compare the influence of added vitamin E on the oil kept away from sunlight and exposed to daylight
Experimental Methods
Materials and methods
Standards and reagents: All chemicals and reagents used in this work were analytical grade and were used with no further purification. Ethanol, phenolphthalein, potassium hydroxide (KOH), glacial acetic acid, potassium iodide (KI), sodium thiosulfate (Na2S2O3), starch were all obtained from Brulux, India. Chloroform was obtained from WINLAB LIMITED, United Kingdom. Vitamin E capsules, GELTEC PVT. LTD was purchased from the pharmacy. Distilled water was used throughout the work.
Equipment: The equipment used in this work are electronic balance (OHAUS, Switzerland), 743 Metrohm Rancimat (Methrom, Herisau, Switzerland), micro burettes, pipettes, conical flasks, thermometer, stirrer, stands, heating device.
Sample collection: Fresh Sunflower Oil (SFO), obtained from Meseret Virgin Seed Oil Processor, and Nigger Oil (NO), obtained from Mehari Edible Oil Processor were used for the study. The containers of the oil samples were wrapped with aluminum foil and stored in a refrigerator at 4°C until analysis.
Sample preparation: Oil samples were taken from the refrigerator and transferred to 250 ml conical flasks. An equal volume of 200 ml of SFO and NO, SFO+vitamin E (200 mg/kg of oil), NO+vitamin E (200 mg/kg of oils) [13], were added to two groups of 250 ml conical flasks (one group wrapped with aluminum foil). The flasks were closed and subjected to different storage conditions: (i) in daylight, (ii) at dark place, and (iii) in daylight with added vitamin E, (iv) in dark with added vitamin E.
Analytical determinations
Acid value: The AV is the number of mg of KOH necessary to neutralize the free acid in l g of sample. Each oil sample (1.0 g) was weighed and dissolved with 50 ml of ethanol in a conical flask. Two drops of phenolphthalein indicator were added and titrated to pink endpoint (which persisted for 15 minutes) with 0.1N KOH. AV was calculated (Equation 1) [14].
Acid value = 56.1 × V × C/m (1)
Where V is the volume in ml of standard KOH solution used, C is the exact concentration in KOH solution used (0.1 N); m is the mass in grams of the test portion (1g). 56.1 is the equivalent weight of KOH.
Peroxide value: PV was evaluated according to AOCS Official Method Cd 8-53 [15]. 5 g oil sample was weighed into a conical flask and 30 ml of a solvent mixture of glacial acetic acidchloroform in the ratio of 3:2, respectively, was added to the oil sample. 0.5 ml saturated potassium iodide (KI) solution was added to the solution and allowed to stand for 1 minute thereafter, 30 ml of distilled water was added and titrated with 0.01 N sodium thiosulfate (Na2S2O3) solution using starch indicator until the yellow color was discharged. A blank was prepared alongside the oil samples. PV was calculated (Equation 3).
Peroxide value = 10 (V1-V2)/m (2)
Where: V1 volume of Na2S2O3 for determination of test sample in ml, V2 volume of Na2S2O3 for determination of blank solution in ml and m is mass of test portion in g (5 g).
Rancimat test: The oxidative stability of the investigated oil samples was studied using the Rancimat test. The test was performed on a 743 Rancimat apparatus (Methrom, Herisau, Switzerland). The oil samples (3 g) were subjected to a temperature of 120°C at an airflow rate of 20 L/h. The determination was based on the conductometric detection of volatile acids. The results were expressed as the Induction Time (IT) in hours. IT represents the time needed for the decomposition of hydroperoxides produced by oil oxidation [16].
Statistical data analysis: All the parameters were determined in three trials and the results obtained from each determination was presented as mean value ± Standard Deviation (SD). Tests for significance in variations were conducted by One-Way Analysis Of Variance (ANOVA) and the means were compared using Tukey Honestly Significant Difference test (Tukey HSD). Variations were considered significant at p<0.05. For the case of the Rancimat test, the median value was taken.
Results and Discussions
Changes in acid value (AV) during storage
AV is a measure of the FFAs in oil. Normally, FAs are found in the TAG form, however; during processing, the FAs may be hydrolyzed into FFA. Production of FFA is the best predictor of fat deterioration and the presence of FFA could be used to monitor the extent of oils abused [17]. The higher the AV found, the higher the level of FFA which results in decreased oil quality. The acceptability level of virgin oils is below 4.0 mg KOH/g (measured in potassium hydroxide per gram) [18].
The changes observed in AV during 5 weeks of storage were depicted in Table 1. The results show that under daylight and dark storage of oils, the formation of FFA gradually increased as storage time increased. The changes in AV of SFO and NO stored with and without added vitamin E was not significant (at p<0.05) under different conditions. At the end of 5 weeks of storage, the AV of SFO and NO samples stored at daylight>dark>daylight+vitamin E>dark+vitamin E storage condition.
| Storage Time (weeks) | Storage Conditions | Maximum recommended value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| NORS | NODS | NOARS | NOADS | SFORS | SFODS | SFOARS | SFOADS | ||
| 0 | 6.20 ± 0.46 | 6.2 ± 0.46 | 6.20 ± 0.46 | 6.20 ± 0.46 | 4.50 ± 0.26 | 4.50 ± 0.26 | 4.50 ± 0.26 | 4.50 ± 0.26 | 4 |
| 1 | 10.47 ± 0.53 | 8.60 ± 0.26 | 6.73 ± 0.26 | 6.45 ± 0.23 | 10.66 ± 0.24 | 9.06 ± 0.13 | 7.30 ± 0.26 | 5.40 ± 0.00 | |
| 2 | 12.16 ± 0.70 | 10.84 ± 0.24 | 7.30 ± 0.00 | 6.80 ± 0.24 | 13.46 ± 0.46 | 10.10 ± 0.26 | 7.85 ± 0.26 | 5.95 ± 0.46 | |
| 3 | 17.76± 0.24 | 13.27 ± 0.69 | 10.10 ± 0.26 | 8.42 ± 0.24 | 20.20 ± 0.46 | 17.40 ± 0.24 | 13.18 ± 0.46 | 9.72 ± 0.70 | |
| 4 | 20.10 ± 0.26 | 15.15 ± 0.92 | 12.56 ± 0.26 | 9.60 ± 0.46 | 22.44 ± 0.70 | 21.32 ± 0.46 | 16.83 ± 0.70 | 11.22 ± 0.46 | |
| 5 | 21.32 ± 0.46 | 17.95 ± 0.24 | 16.64 ± 1.15 | 11.22 ± 0.69 | 24.7 ± 0.60 | 23.56 ± 0.70 | 17.95 ± 0.46 | 12.34 ± 0.70 | |
Table 1: Changes in AV (mg KOH/g of oil) of SFO and NO during storage. SFORS: Sunflower Oil Room Stored; SFODS: Sunflower Oil Dark Stored; SFOARS: Sunflower Oil+Antioxidant Room Stored; SFOADS: Sunflower Oil+Antioxidant Dark Stored; NORS: Nigger Oil Room Stored; NODS: Nigger Oil Dark Stored; NOARS: Nigger Oil+Antioxidant Room Stored; NOADS: Nigger Oil+Antioxidant Dark Stored.
The initial AV of NO and SFO presented in this study show a higher value than Codex Standard for Named Vegetable Oils (CODEX-STAN210-1999) (4.0 mg KOH/g). The observation of high initial AV for NO (6.20 ± 0.46 mg KOH/g) indicates a high formation of FFAs. Probably, this is due to oil seeds moisture content and storage periods [19]. Kalua et al. discussed that there were changes in oil quality during cold temperature storage of fruits [20].
As can be seen in Table 1, greater changes in AV were observed for oils subjected to daylight compared to dark storage. This is might be attributed to the elevated rate of hydrolysis (caused by a variety of agents; the presence of moisture in the oil, elevated temperature (above room temperature) and photo-oxidation of the oil. The effects of light on AV of NO and SFO during storage were illustrated in Figure 1.
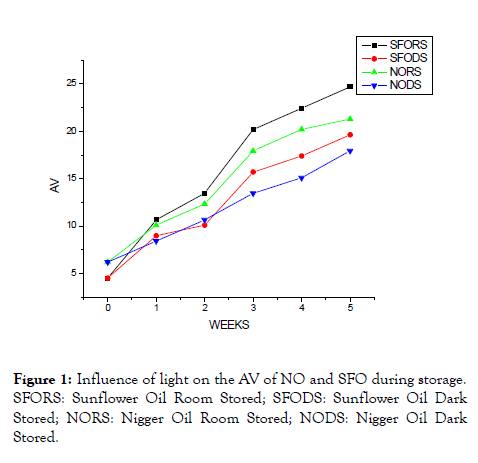
Figure 1: Influence of light on the AV of NO and SFO during storage. SFORS: Sunflower Oil Room Stored; SFODS: Sunflower Oil Dark Stored; NORS: Nigger Oil Room Stored; NODS: Nigger Oil Dark Stored.
Light is much more important than temperature in lipid oxidation. When oil-containing foods are exposed to light, they develop off-flavors more rapidly than when protected from the light because light accelerates the rate of oxidation. The AV of SFO stored at daylight was higher (4.50 ± 0.26-24.70 ± 0.6) mg KOH/g than NO (6.20 ± 0.46-21.30 ± 0.46) mg KOH/g stored in similar condition. The low change in AV of NO is probably due to the higher content of saturated palmitic acid, which is less prone to oxidation than unsaturated fatty acids [21].
The NO and SFO samples kept at dark place show a decrease in the FFA content by 3.55 ± 0.22 and 5.06 ± 0.1 mg KOH/g compared to NO and SFO samples exposed to daylight. From the samples stored in a dark place, higher change in AV was noted for SFO than NO. This might be related to the presence of high PUFAs in SFO, especially linolenic acid (18: 3n-3), which is susceptible to oxidation, hydrolysis, and thermal degradation [22]. It may be postulated that light absorption (effect of photosensitizers) was greater in SFO as compared to NO. This result is in line with works reported on sunflower and rapeseed oil under dark storage by Abramovic and Abram [23].
Effect of light on AV of NO and SFO stored with added vitamin E
Vitamin E functions as an antioxidant by serving as free-radical terminators and scavenging singlet oxygen molecules. The α- tocopherol concentration is an important factor that influences α-tocopherol antioxidant activity in bulk oils. Studies in purified TAGs obtained from SFO showed that antioxidant activity α-tocopherol was greater at lower concentrations (<700 ppm) and it loses efficacy at higher concentrations due to its participation inside reactions [24]. Figure 2 shows the AV changes of oils stored under daylight and dark after the addition of 200 mg/kg vitamin E.
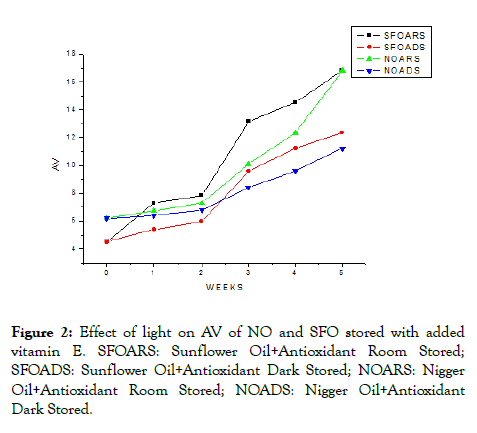
Figure 2: Effect of light on AV of NO and SFO stored with added vitamin E. SFOARS: Sunflower Oil+Antioxidant Room Stored; SFOADS: Sunflower Oil+Antioxidant Dark Stored; NOARS: Nigger Oil+Antioxidant Room Stored; NOADS: Nigger Oil+Antioxidant Dark Stored.
During the storage time, the AV of samples stored with vitamin E increased slowly and steadily (Figure 2). The effect of vitamin E was high in maintaining the formation of FFA during the first two weeks, and it starts to lose its capacity as storage time increased. The added vitamin E decreased the AV of NO and SFO samples kept at daylight by (4.47 ± 0.69 and 6.75 ± 0.14) mg KOH/g compared to NO and SFO samples stored in daylight without added vitamin E. Similarly, the vitamin E decreased the AV of NO and SFO stored at the dark place by (6.73 ± 0.45 and 11.22 ± 0.00) mg KOH/g from NO and SFO stored in similar condition without added vitamin E by protecting them against deterioration caused by oxidation which leads to rancidity. This effect shows the antioxidant activity of vitamin E in increasing the shelf life of oils.
Changes in Peroxide Value (PV) during storage
PV is a measure of oxidation during storage and the freshness of the lipid matrix. The PV indicates the level of oxidation during production and storage. One of the most important parameters that influence lipid oxidation is the degree of unsaturation of its FAs. When double bonds of unsaturated fats are oxidized, peroxides are among the oxidation products formed. Change in PV during storage was shown in Table 2.
| Storage time (weeks) | Storage Conditions | Maximum recommended value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| NORS | NODS | NOARS | NOADS | SFORS | SFODS | SFOARS | SFOADS | ||
| 0 | 2.20 ± 0.047 | 2.20 ± 0.047 | 2.20 ± 0.047 | 2.20 ± 0.047 | 2.00 ± 0.16 | 2.00 ± 0.16 | 2.00 ± 0.16 | 2.00 ± 0.16 | 15 |
| 1 | 5.20 ± 0.094 | 3.60 ± 0.16 | 2.20 ± 0.16 | 2.20 ± 0.094 | 7.80 ± 0.00 | 4.40 ± 0.16 | 2.20 ± 0.047 | 2.00 ± 0.00 | |
| 2 | 9.60 ± 0.16 | 6.20 ± 0.094 | 3.60 ± 0.094 | 2.46 ± 0.094 | 12.20 ± 0.094 | 8.93 ± 0.19 | 7.20 ± 0.047 | 2.80 ± 0.16 | |
| 3 | 14.20 ± 0.094 | 9.60 ± 0.16 | 6.40 ± 0.094 | 3.00 ± 0.16 | 19.40 ± 0.16 | 10.40 ± 0.047 | 11.20 ± 0.16 | 3.46 ± 0.25 | |
| 4 | 16.33 ± 0.25 | 10.66 ± 0.19 | 8.13 ± 0.41 | 3.73 ± 0.25 | 24.33± 0.047 | 15.20 ± 0.094 | 12.20 ± 0.00 | 6.40 ± 0.16 | |
| 5 | 17.02 ± 0.094 | 13.20 ± 0.094 | 8.80 ± 0.16 | 4.60 ± 0.047 | 27.20 ± 0.81 | 16.60 ± 0.16 | 13.20 ± 0.047 | 7.20 ± 0.25 | |
Table 2: Changes in PV (meq O2/kg) of NO and SFO. SFORS: Sunflower Oil Room Stored; SFODS: Sunflower Oil Dark Stored; SFOARS: Sunflower Oil+Antioxidant Room Stored; SFOADS: Sunflower Oil+Antioxidant Dark Stored. NORS: Nigger Oil Room Stored; NODS: Nigger Oil Dark Stored; NOARS: Nigger Oil+Antioxidant Room Stored; NOADS: Nigger Oil+Antioxidant Dark Stored.
Peroxides are possibly (not directly) responsible for the taste and odor of rancid fats, their concentration as represented by the PV is often useful in assessing the extent to which the rancidity has advanced. Rancid taste often begins to be noticeable when the PV is above 20 meq/kg [25]. At the beginning of the experiment, the PV of SFO and NO was (2.00 ± 0.16 and 2.20 ± 0.047) meq O2/kg. This value falls in the range considered as satisfactory and in agreement with the maximum Codex standard PV (15 meq O2/Kg) [18] for virgin vegetable oil.
The PV of oil samples stored for 5 weeks registered a progressive increase with the increment of storage period. From Table 2 it was observed that changes in PV of SFO and NO stored under different conditions with and without added vitamin E were significant (p<0.05). At the end of 5 weeks of storage, the PV of SFO and NO samples stored at daylight>dark>daylight+vitamin E>dark+vitamin E storage condition.
Light accelerates oxidation more than temperature. The change in PV of NO and SFO kept at daylight was significantly (p<0.05) higher than the PV value of NO and SFO stored at dark. Lower change in PV of NO compared to SFO during daylight storage was probably due to higher content of saturated palmitic acid, which is less prone to oxidation than unsaturated fatty acids, linolenic acid, linoleic acid, etc. [21]. The higher PV of SFO is mostly because SFO has an appreciable amount of unsaturated FA to fix oxygen and easily oxidized. These findings are similar to the work of Huang et al. [22], who reported that high PUFAs, especially linolenic acid (18:3n-3), are prone to oxidation, hydrolysis, and thermal degradation.
The absence of light lowered the PV of NO and SFO by (4.00 ± 0.00 and 10.6 ± 0.65) meq O2/kg when compared to NO and SFO stored in daylight. This finding shows the oxidation process during periods of storage was affected by light. A lot of literature states that faster oxidation occurs due to exposure to light [26]. The absence of light minimized the hydroperoxide formation and also synergistically supported by minor components found in oils which acts as an antioxidant in the dark [27]. Such decreases in PVs had been reported by Neff et al. [28].
Effect of light on PV of NO and SFO stored with added vitamin E
Vitamin E is the most important lipophilic antioxidant in vegetable oils. It acts as an antioxidant by donating a hydrogen atom to peroxyl radicals of unsaturated lipid molecules, forming a hydroperoxide and a tocopheroxyl radical, which reacts with other peroxyl or tocopheroxyl radicals forming more stable adducts [29].
The PV of SFO and NO which contain additional vitamin E were significantly (p<0.05) different from SFO and NO samples stored without added vitamin E. The PV of NO and SFO stored in daylight and dark with added vitamin E was significantly lower than NO and SFO stored in similar conditions without added vitamin E. This is due to antioxidant ability of added vitamin E to retard the formation of hydroperoxides which further decomposed to produce a different kind of volatile compounds. Furthermore, vitamin E decreased the formation of FFA which is more susceptible to oxidation than the glycerol esters of these FA. The PV for oil samples stored with the addition of Vitamin E at dark and daylight was depicted in Figure 3.
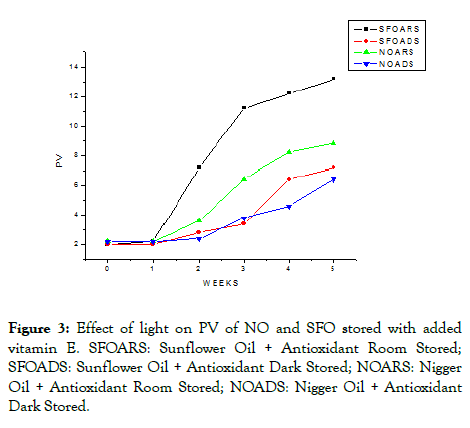
Figure 3: Effect of light on PV of NO and SFO stored with added vitamin E. SFOARS: Sunflower Oil + Antioxidant Room Stored; SFOADS: Sunflower Oil + Antioxidant Dark Stored; NOARS: Nigger Oil + Antioxidant Room Stored; NOADS: Nigger Oil + Antioxidant Dark Stored.
Compared to NO and SFO kept in daylight without added vitamin E, the formation of hydroperoxides of NO and SFO samples stored at daylight containing the added vitamin E decreased by (8.40 ± 0.07 and 14.00 ± 0.76) meq O2/kg. The effect of added vitamin E was high in hindering the oxidation of SFO stored in daylight. This is due to the presence of photosensitizer and PUFA, which was accelerated by exposure to UV light irradiation [30].
As illustrated in Figure 3, the sample containing vitamin E stored in dark exhibited lower change in PV than in oil samples stored in daylight. This shows the antioxidant activity of vitamin E was affected by daylight. Antioxidants in the presence of light act as singlet oxygen quencher (formed due to photosensitizer when exposed to light) and free radical scavenger. During storage at dark, added vitamin E and minor components which may be found in the oil samples such as chlorophyll, phospholipids, and others act as an antioxidant [31]. Bandarra et al. reported that tocopherols show a synergistic effect with phospholipids for enhancing the oxidative stability of oils [32].
This observation helps to suggest that SFO and NO has a high content of unsaturated fatty acids, linoleic (C18:2) and oleic acid (C18:1), which are responsible for oxidative rancidity [33]. Thus, oxidation is retarded even in highly unsaturated oil if proper storage conditions are chosen and as long as antioxidants are present.
Rancimat test
The susceptibility of oils to oxidation was measured by Rancimat and expressed by Induction Time (IT). The Rancimat measures the increase in the conductivity of distilled water resulting from the trapped volatile oxidation products produced when the oil product is heated under a flow of air. IT represents the time needed for the decomposition of hydroperoxides produced by oil oxidation [34]. The Rancimat test is a commonly used procedure in the food industry for the examination of the oxidative stability of edible oils and the prediction of their shelf life. Gordon and Mursi [35] reported for rapeseed oil that the IT of 1 h determined at 100°C was equivalent to 2 days storage at 20°C. Maszewska [36] found that an IT of 1 h at 120°C was equal to 5 months storage at 12°C. Table 1 depicts the IT of SFO and NO during 0 and at the end of 5 weeks storage.
As shown in Table 3, the IT of fresh NO was found to be higher than the IT of SFO. This is due to an increased degree of unsaturation (high PUFA contents) negatively affects the oxidative stability of oil determined by the Rancimat test [37]. According to Ramadan and Morsel [38], the PUFA contents of SFO is higher than NO.
| Type of oil | Storage condition | Storage periods (weeks) | IT(h) | Type of oil | Storage condition | Storage periods (weeks) | IT(h) |
|---|---|---|---|---|---|---|---|
| Niger oil (NO) | NOF | 0 | 2.05 | Sunflower Oil (SFO) | SFOF | 0 | 1.97 |
| NOAF | 0 | 2.13 | SFOAF | 0 | 2.11 | ||
| NORS | 5 | 1.59 | SFORS | 5 | 1.51 | ||
| NODS | 5 | 1.73 | SFODS | 5 | 1.65 | ||
| NOARS | 5 | 1.81 | SFOARS | 5 | 1.77 | ||
| NOADS | 5 | 1.92 | SFOADS | 5 | 1.85 |
Table 3: Induction Time (IT) of the fresh and stored sample with and without vitamin E. NOF: Nigger Oil Fresh; NOAF: Nigger Oil Fresh +Antioxidant; NORS: Nigger Oil Room Stored; NODS: Nigger Oil Dark Stored; NOARS: Nigger Oil+Antioxidant Room Stored; NOADS: Nigger Oil+Antioxidant Dark Stored; SFOF: Sunflower Oil Fresh; SFOAF: Sunflower Oil Fresh+Antioxidant; SFORS: Sunflower Oil Room Stored; SFODS: Sunflower Oil Dark Stored; SFOARS: Sunflower Oil+Antioxidant Room Stored; SFOADS: Sunflower Oil+Antioxidant Dark Stored.
The added vitamin E increased the IT of fresh NO and SFO. Lipid soluble vitamin E reduced lipid oxidation by retarding the formation of lipid hydroperoxides and decreasing the concentration of individual volatile oxidation products. Okpuzor et al. [14] reported vitamin E protects 103-108 PUFA molecules against oxidation at low peroxide levels.
As can be observed in Table 3, the IT of oil samples decreases as storage time increased. This can be explained in two ways. Firstly, the antioxidative components originally present in NO and SFO degraded with time, thus losing their antioxidant properties. Secondly, the oxidation products increase with time of storage as shown in Tables 1-3. In a study on virgin olive oil [39,40], after 12 months of storage in darkness at ambient temperature, a decrease in total phenolic content and 100 % loss of tocopherol was established, while the IT (by Rancimat test at 120°C) was about 50 % of the initial (10 h) value.
Oil quality deterioration greatly increased in the oils containing more unsaturation and less natural antioxidants indicating lesser stability. As presented in Table 3, the IT oil samples stored with added vitamin E was higher than IT of oil samples stored without added vitamin E in all storage conditions. The effect of added vitamin E in increasing the oxidation stability of tested oils was high on SFO. Matthaus [40] in his investigation performed on some vegetable oils with different degrees of unsaturation found a high correlation between total tocopherol content and IT determined by the Rancimat method at 120°C.
The differences in the oxidation of oils stored in different conditions were also observed from changes in AV and PV measured during storage and induction time. The highest AV and PV were measured for NO and SFO samples stored under daylight, but lower induction time was recorded. A critical concentration of degradable volatile products under the Rancimat test conditions and the deterioration of the oil were reached faster in oils stored in daylight.
Conclusion and Recommendation
Conclusion
Oxidative stability is an important indicator of oil quality and shelf-life. The oxidative changes during storage and domestic use make oils unsuitable for consumption. The present investigation is an overview of changes in acid value, peroxide value and induction time of sunflower oil and nigger oil from 0 days to 5 weeks of storage.
The results observed during the study show that prolonged storage of sunflower oil and nigger oil at ambient (25-33)°C temperatures can lead to oxidative deterioration of oil samples. Initially, both oil samples show high acid value from the recommended value and significantly increased during storage. Higher change in acid value observed for a sample stored in daylight than dark. Also, additions of vitamin E decreased the formation of free fatty acids in sunflower oil and nigger oil.
The initial peroxide value of both oil samples is in agreement with Codex standard guideline value. The peroxide value significantly increased during storage. Higher peroxide value was observed for samples stored in daylight than samples stored in dark. Also, the addition of vitamin E decreased the change in the peroxide value of sunflower oil and nigger oil. The added vitamin E prohibited the formation of hydroperoxide more in the dark than daylight storage.
The nigger oil showed higher induction time than sunflower oil during the Rancimat test. The addition of vitamin E increased the induction time of sunflower and nigger oil. Even though the induction time of sample oil decreased during storage there was deference between storage conditions. Sample which contains vitamin E and stored in dark conditions showed lower decreases in induction time than daylight storage when measured in Rancimat.
The high content of primary oxidation products and the presence of FFAs might profoundly lower the oxidative stability of the oil. Even if the oxidation stability of nigger oil was found to be higher than oxidative stability of sunflower oil from the acid value, peroxide value and induction time obtained, both NO and SFO are prone to oxidation during storage.
In general, the results of the present study show that light acts as a major catalyst in accelerating the development of rancidity in oils. However; the addition of vitamin E to oil can increase the oxidation stability of oils during storage. Storing in dark (packaging with material protect light) and supporting with antioxidants is the best way to maintain the quality of oils during storage and domestic uses.
Recommendation
The investigations presented in this study suggest:
• Better packing and storage conditions can lead to an improvement in the oxidative stability of vegetable oils and other related products containing fats and oils
• Lipid oxidation products make the oil unfit for human health; therefore, to minimize the oxidation phenomenon, some antioxidants should be added to increase the storage and shelflife of oils and oil products
• Producers, shopkeepers, and users shouldstore oils and oil products in dark places protected from light
• The government or any responsible body should follow that local oil producers have put the production and expiry dates of domestically produced oils to safeguard the health of people
Acknowledgment
Bahir Dar University is kindly acknowledged for laboratory facility and financial support to conduct this research work. Jibrel Abdulkadir is highly appreciated for his effort in conducting the laboratory works. Dr. Zelalem is also kindly appreciated for his constructive comments and guidance during the work. He is also thankful for editing the manuscript.
REFERENCES
- Pryde EH, Carlson KD. Trends in industrial usage for vegetable oils-symposium overview. J Am Oil Chem Soc. 1985;62:916-917.
- Al-Attar AM. Physiological effects of some plant oils supplementation on streptozotocin-induced diabetic rats. Res J Med Med Sci. 2010;5:55-71.
- Gordon MH, Griffith RE. Steryl ester analysis as an aid to the identification of oils in blends. Food Chem. 1992;43:71-78.
- Wood GM, Slack PT, Rossell JB, Mann PJ, Farnell PJ. Spanish toxic oil syndrome (1981): Progress in the identification of suspected toxic components in simulated oils. J Agric Food Chem. 1994;42:2525-2530.
- Bennion M, Scheule BM. Introductory foods. 10th ed. Prentice-Hall Inc., Upper Saddle River, New Jersey, USA, 1995.
- Vercellotti JR, St. Angelo AJ, Spanier AM. Lipid oxidation in foods: An overview. ACS Publications. 1992:1-11
- Richards A, Wijesundera C, Salisbury P. Evaluation of oxidative stability of canola oils by headspace analysis. J Am Oil Chem Soc. 2005;82:869-874.
- Muik B, Lendl B, Molina-Díaz A, Ayora-Cañada MJ. Direct monitoring of lipid oxidation in edible oils by Fourier transform Raman spectroscopy. Chem Phys Lipids. 2005;134:173-182.
- Ullah J, Hamayoun M, Ahmad T, Ayub M, Zafarullah M. Effect of light, natural and synthetic antioxidants on stability of edible oil and fats. Asian J Plant Sci. 2003;2:1192-1194.
- Imaida K, Fukushima S, Shirai T, Ohtani M, Nakanishi K, Ito N. Promoting activities of butylated hydroxyanisole and butylated hydroxytoluene on 2-stage urinary bladder carcinogenesis and inhibition of γ-glutamyl transpeptidase-positive foci development in the liver of rats. Carcinogenesis. 1983;4:895-899.
- Casimir CA, David BM. Food lipids chemistry, nutrition and biotechnology, revised and expanded. 2nd ed 2002;1:1-3.
- Wijnands JH, Gurmesa ND, Lute JC, Van Loo EN. Ethiopian soya bean and sunflower value chains: Opportunities and challenges. LEI Wageningen UR, 2011.
- Wagner KH, Elmadfa I. Effects of tocopherols and their mixtures on the oxidative stability of olive oil and linseed oil under heating. Eur J Lipid Sci Technol. 2000;102:624-629.
- Okpuzor J, Okochi VI, Ogbunugafor HA, Ogbonnia S, Fagbayi T, Obidiegwu C. Estimation of cholesterol level in different brands of vegetable oils. Pak J Nutr. 2009;8:57-62.
- AOCS, Official Method Cd 8-53. American Oil Chemists Society, Champaign, IL.2003
- Rancimat Method, Application Bulletin Metrohm, Nr. 204/1e (1994). https://www.metrohm.com/en-in/applications/AB-204
- Atta S, Ahmad T, Gul S, Nagra SA, Luthfullah G, Khan M. Effect of gamma irradiation on the nutritional quality of ready to eat fast food. J Chem Soc Pak. 2008;30:879-883.
- Alimentarius C. Codex standard for named vegetable oils. Codex stan. 1999;210:1-3.
- Dhingra OD, Jham G, Napoleão IT. Ergosterol accumulation and oil quality changes in stored soybean invaded by Aspergillus ruber (A. glaucus group). Mycopathologia. 1998;143:85-91.
- Kalua CM, Bedgood Jr DR, Bishop AG, Prenzler PD. Changes in virgin olive oil quality during low-temperature fruit storage. J Agric Food Chem. 2008;56:2415-2422.
- Paul JN, Manny N, Baryan BG, Rosemary M, Peter MC, Mavis A. Plasma lipoprotein lipid and lipid changes with substitution of elaidic acid for oleic acid in the diet. Am J Clin Nutr. 1992;55:46-50.
- Huang AS, Hsieh OA, Huang CL, Chang SS. A comparison of the stability of sunflower oil and corn oil. J Am Oil Chem Soc. 1981;58:997-1001.
- Abramovič H, Abram V. Physico-chemical properties, composition and oxidative stability of Camelina sativa oil. Food Technol Biotechnol. 2005;43:63-70.
- Carelli AA, Crapiste GH, Franco IC. Efectividad de antioxidantes naturales adicionados a aceite de girasol. Grasas y aceites. 2005;56:303-310.
- Prevention of Food Adulteration Act-1954, Commercial Law Publishers Pvt. Ltd. Delhi, India, 2005.
- Khan MA, Shahidi F. Photooxidative stability of stripped and non-stripped borage and evening primrose oils and their emulsions in water. Food Chem. 2002;79:47-53.
- Khan MA, Shahidi F. Tocopherols and phospholipids enhance the oxidative stability of borage and evening primrose triacylglycerols. J Food lipids. 2000;7:143-150.
- Neff WE, Mounts TL, Rinsch WM, Konishi H, El‐Agaimy MA. Oxidative stability of purified canola oil triacylglycerols with altered fatty acid compositions as affected by triacylglycerol composition and structure. J Am Oil Chem Soc. 1994;71:1101-1109.
- Romero N, Robert P, Masson L, Ortiz J, Pavez J, Garrido C, et al. Effect of α‐tocopherol and α‐tocotrienol on the performance of Chilean hazelnut oil (Gevuina avellana Mol) at high temperature. J Sci Food Agric. 2004;84:943-948.
- Kamal‐Eldin A, Appelqvist LÅ. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids. 1996;31:671-701.
- Jung MY, Min DB. Effects of α‐, γ‐, and δ‐tocopherols on oxidative stability of soybean oil. J Food Sci. 1990;55:1464-1465.
- Bandarra NM, Campos RM, Batista I, Nunes ML, Empis JM. Antioxidant synergy of α-tocopherol and phospholipids. J Am Oil Chem Soc. 1999;76:905-913.
- Ramadan MF, Morsel JT. Proximate neutral lipid composition of niger. Czech J Food Sci. 2002;20:98-104.
- Läubli MW, Bruttel PA. Determination of the oxidative stability of fats and oils: comparison between the active oxygen method (AOCS Cd 12‐57) and the Rancimat method. J Am Oil Chem Soc. 1986;63:792-795.
- Gordon MH, Mursi E. A comparison of oil stability based on the Metrohm Rancimat with storage at 20 C. J Am Oil Chem Soc. 1994;71:649-651.
- Maszewska M. Validation of methods for determination of oxidative stability of rapeseed and sunflower refined oils (Doctoral dissertation, Dissertation for PhD thesis, Warsaw Agricultural University).
- Vidrih R, Vidakovič S, Abramovič H. Biochemical parameters and oxidative resistance to thermal treatment of refined and unrefined vegetable edible oils. Czech J Food Sci. 2010;28:376-384.
- Ramadan MF, Morsel JT. Proximate neutral lipid composition of niger. Czech J Food Sci. 2002;20:98-104.
- Morelló JR, Motilva MJ, Tovar MJ, Romero MP. Changes in commercial virgin olive oil (cv Arbequina) during storage, with special emphasis on the phenolic fraction. Food Chem. 2004;85:357-364.
- Mathäus BW. Determination of the oxidative stability of vegetable oils by Rancimat and conductivity and chemiluminescence measurements. J Am Oil Chem Soc. 1996;73:1039-1043.
Citation: Gizachew Z (2020) Influence of Storage Condition and Added Vitamin E on Oxidation Stability of Edible Sunflower and Nigger Seed Oils Produced and Sold around Bahir Dar City, Ethiopia. J Food Process Technol 11:821. doi: 10.35248/2157-7110.20.11.821
Copyright: © 2020 Gizachew Z. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


