Indexed In
- RefSeek
- Hamdard University
- EBSCO A-Z
- Publons
- Euro Pub
- Google Scholar
- Quality Open Access Market
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2024) Volume 10, Issue 3
Incidence and Predictors of Post-Operative Hypoxemia in Pediatric Patients Undergoing General Anesthesia: A Single-Arm Prospective Multi-Center Cohort Study 2023
Semon Mengistu1*, Haile Mariam Mulugeta2, Belete Destaw2 and Simeneh Molla22Department of Anesthesiology, Dilla University College of Medicine and Health Sciences, Dilla, Ethiopia
Received: 13-Apr-2024, Manuscript No. JPMME-24-25457; Editor assigned: 16-Apr-2024, Pre QC No. JPMME-24-25457 (PQ); Reviewed: 30-Apr-2024, QC No. JPMME-24-25457; Revised: 07-May-2024, Manuscript No. JPMME-24-25457 (R); Published: 14-May-2024, DOI: 10.35248/2684-1320.24.10.275
Abstract
Background: Pediatrics use more oxygen than adults do, which has an impact on how quickly the body desaturates after apnea. Depending on age and other physiological parameters, the clinically relevant hypoxemia in pediatric patients can drop to 40% in as little as 1.5 minutes, which makes the transfer to the Post-Anesthesia Care Unit (PACU) a risky phase. Postoperative Hypoxemia (POH) happens critically postoperatively and various factors predict POH. The objective of the study is to determine the incidence and predictors of POH in pediatric patients undergoing General Anesthesia (GA).
Methodology: In 292 pediatric patients, a single-arm prospective multi-center cohort study using systematic random sampling was carried out. Data entry and analysis were performed using Statistical Package for Social Sciences (SPSS) version 25.00 software. For logistic regression, a Hosmer-Lemeshow goodness of fit test was used (variables with a p>0.05 were considered fit). Binomial logistic regression and multivariable analysis were performed. Independent variables were examined for multi collinearity, and those exhibiting a Variance Inflation Factor (VIF) >10 were removed. Additionally, the association between the variables was determined using Pearson chi-square and p<0.05 indicated statistically significant variables.
Results: The incidence of POH was 47.9%. Pediatric age (≤ 2 years), opioids, and above-abdominal surgeries are associated with POH (p ≤ 0.001) and AOR=3.263 (1.686-6.314), 5.545 (2.983-10.307), and 3.642 (1.969-6.736), respectively. Additionally, the presence of at least one comorbidity, use of muscle relaxants, prolonged anesthesia, and surgery duration are associated with POH (p=0.001, 0.003, 0.02, and 0.037) and AOR=4.259 (1.788-10.145), 29.72 (3.262-270.778), 2.153 (1.131-4.1), and 2.560 (1.058-6.196), respectively. However, anesthesia type is insignificantly associated with POH (p=0.096).
Conclusion: The incidence of POH in pediatric patients undergoing GA is high, and the presence of at least one comorbidity, pediatric age (≤ 2 years), use of opioids and muscle relaxants, above-abdominal surgeries, prolonged anesthesia, and surgery durations are significantly associated with POH. We recommend taking precautions, following them and having oxygen therapy for these high-risk pediatric patients.
Keywords
Hypoxemia; Incidence; Pediatrics; Predictors; Postoperative
Abbrevations
ARE: Adverse Respiratory Events; AOR: Adjusted Odd Ratio; CI: Confidence Interval; COR: Crude Odd Ratio; ETCO2: End-tidal Carbon Dioxide; GA: General Anesthesia; HUCSH: Hawassa University Comprehensive Specialized Hospital; OR: Odd Ratio; PACU: Post Anesthesia Care Unit; POH: Post-Operative Hypoxemia; SPO2: Peripheral Oxygen Saturation; TASH: Tikur Anbessa Specialized Hospital
Introduction
Hypoxemia is the medical term for low blood oxygen levels, which can be brought on by either insufficient arterial oxygenation or insufficient blood flow to the tissues [1]. At sea level, the typical peripheral oxygen saturation range is 97%-99%, with a lower limit of 94%. The oxygen therapy threshold is if the oxygen saturation is <90%, since even a tiny decrease from 90% could cause a severe drop in the partial pressure of oxygen [2]. The ideal oxygen saturation range for anesthesia is between 95% and 100%. If this range is not met, the patient becomes hypoxemic and needs to be treated right away, or if it is less than 90%, a clinical emergency occurs [3].
Oxygen carried by hemoglobin is compromised by hypoventilation, which reduces the amount of oxygen in the alveoli due to obstruction of the airway, a rise in Partial Pressure of Carbon Dioxide (PaCO2), or a condition when blood flow and lung ventilation are not balanced (ventilation-perfusion mismatch). Differential diagnoses for hypoxia include the inability of the lungs to oxygenate blood (hypoxemic hypoxia); low hemoglobin results in inadequate oxygen delivery (anemic hypoxia); pump failure, in which the heart cannot pump enough blood, resulting in impaired oxygen delivery (circulatory hypoxia); and even though there is enough oxygen, the cells are unable to utilize it (histotoxic hypoxia) [4].
The lung's resting volume is determined by the equilibrium of the chest and lung recoil pressures. Compared to older children or adults, younger children have a lower Functional Residual Capacity (FRC). Due to their greater propensity for airway collapse (higher compliance), children have fewer oxygen reserves, which causes a drop in FRC and increases their risk of hypoxemia [5].
Pediatric patients may be more susceptible to hypoxemia due to a variety of physiological issues. An infant's oxygen consumption is comparatively higher than an adult's; some publications report discrepancies between the two at rest, 6 mL/kg/min against 3 mL/kg/min. In addition, there is an increase in CO2 production, roughly 100–150 mL/kg/min as opposed to an adult's 60 mL/ kg/min. This can cause fast desaturation during apnea, especially when paired with a slightly reduced functional residual capacity. Children's respiratory rates are higher to meet the need for higher minute ventilation to expel CO2 because their tidal volume is generally consistent with an adult's [6].
Hypoxemia is thought to be most likely to develop in the early postoperative period. Following various surgeries, this frequency is common in the PACU. POH developed as a result of compromised gas exchange during anesthesia due to the chest wall muscles' lower tone and continues into the recovery stage [7].
Pulse oximetry gives clinicians a non-invasive, affordable, precise, and quick way to measure oxygen saturation. Patients without pulse oximetry monitoring had a statistically significant increase in the number of hypoxic episodes and the duration of hypoxemia, regardless of the level of clinical experience [8]. The pulse oximeter is a non-invasive, painless, and cost-effective device used for monitoring peripheral oxygen saturation to detect hypoxemia (which can be detected before clinical signs such as cyanosis appear) [9].
In the operating room, Adverse Respiratory Events (AREs) are still one of the leading causes of morbidity and death. The incidence of postoperative respiratory events ranges from 5% to 80%, contingent on patients and diagnostic standards, and 1.3% to 6.9% of these events occur after admission to a PACU. Regardless of the cause, hypoxemia results in significant morbidity and mortality [10].
Pediatrics typically experience perioperative ARE, which is one of the most common adverse events observed in the PACU. In about 5% of children in a PACU, an example of hypoxia has been documented. These occurrences have the potential for serious repercussions and are responsible for 44% of pediatric cardiac arrests in the PACU [11]. Although postoperative episodic hypoxemia in surgical patients is poorly understood, it might be useful in determining the likelihood of early postoperative respiratory problems. Intermittent hypoxia is linked to worse outcomes after serious medical emergencies like myocardial infarction [12].
Both acute and chronic hypoxia can manifest symptoms; dyspnea and tachypnea are possible in the acute form. Tachycardia can be caused by severe hypoxia, which gives the tissues enough oxygen. The most common symptom of the chronic presentation, which is usually less severe, is dyspnea upon exertion. A severe impediment to oxygen delivery will begin to impair organ function. Headaches, restlessness, and confusion are a few of the neurologic symptoms associated with moderate hypoxia. In severe circumstances, if coma and altered mental status are not treated immediately, there may be a fatal outcome [4].
Patients who are transferred from the operating room to the PACU are believed to have a higher risk of developing hypoxemia. Hypoxemia most likely happens in the first 20 minutes after extubation. Tissue hypoxia mostly affects the cardiovascular and central nervous systems. It contributes to surgical morbidity primarily through its effects on the heart (ischemic or rhythmic) and the nervous system (an acute confusional condition that may result in postoperative delirium) [13].
Hypoxemia could result in arrhythmias and abnormal blood pressure, which could have serious impacts. Even mild hypoxia might impair the central nervous system, and very severe hypoxia might result in an abrupt loss of consciousness. Anesthetists better identify hypoxemia and hypoxia-related episodes by monitoring SPO2, but overall hypoxemia incidence seems somewhat unchanged [14].
Early postoperative desaturation was somewhat associated with postoperative acute kidney injury and myocardial infarction. It might have had a duration-dependent relationship with poor discharge, post-procedure complications, and ICU admission [15]. Undiagnosed postoperative oxygen hypoxemia could result in pulmonary hypertension, hyperkalemia, and brain ischemia, respiratory, cardiac, and multiorgan failure [16].
In addition to impairing wound healing, POH might be linked to serious problems such as cognitive dysfunction, dysrhythmias, and myocardial ischemia. These complications could be frequently seen in the first week following surgery. POH, particularly in individuals with multiple risk factors, might be linked to higher mortality, longer hospital stays, and higher expenses. Patients at risk may experience prolonged respiratory assistance and re-intubation, which could have resulted in poor overall outcomes [17].
Despite recent attempts, child mortality rates remain high worldwide, particularly in developing countries. Hypoxemia is a frequent consequence of curable, deadly children’s illnesses [18]. There is an elevated likelihood of death from hypoxemia, especially in Low and Middle-Income Countries (LMICs). Prior research on the prevalence of hypoxemia has been overly limited to a small number of illnesses or particular patients [19]. The aim of the study is to determine the incidence and predictors of POH in pediatric patients undergoing General Anesthesia (GA).
Methodology
Study area
The study was conducted at HUCSH, which is situated 273 kilometers away from Addis Ababa in Hawassa City, Sidama Regional State. HUCSH is a teaching hospital and offers a variety of services, including pediatrics. With 400 beds in total, it receives about 5,000 outpatient and emergency visits every month. Both inpatient and outpatient services are offered by the pediatrics department [20].
The study also took place at TASH, which is a teaching center and where 3,70,000-4,00,000 patients visit per year, while the precise number is unknown [21]. A wide range of specialties provide services for patients, including pediatrics. Patients who are referred by other hospitals can receive care from the pediatrics department [22]. TASH has 800 beds and provides services for the most critical patients. Around 13,300 presentations per year are seen at pediatric emergency departments [23].
Emperor Haile Selassie established Saint Peter Specialized Hospital in 1953. It is the first tuberculosis referral hospital in the country. Saint Peter Specialized Hospital has been the sole hospital in the country treating tuberculosis for more than 40 years. It has recently changed to become a multi-service specialty hospital with more than 14 different specialties and an excellence center, including services for pediatrics [24].
Study design and period
A single-arm prospective multi-center cohort study was conducted, and data collection was carried out from May 1, 2023, to July 30, 2023, at selected hospitals.
Population
The population consists of pediatric patients aged 16 years or younger who are undergoing either elective or emergency surgery with general anesthesia and are subsequently transferred to the Post-Anesthesia Care Unit (PACU).
Source of population: Pediatric patients undergoing surgery with GA.
Study population: Pediatric patients (≤ 16 years) undergoing surgery with GA (both elective and emergency) were transferred to the PACU.
Eligibility criteria
The eligibility criteria include pediatric patients (≤ 16 years) undergoing elective or emergency surgery with an Endo-Tracheal Tube (ETT) or Laryngeal Mask Airway (LMA), transferred to the PACU between May 1, 2023, and July 30, 2023, excluding those with preoperative peripheral oxygen saturation below 95%, receiving oxygen therapy upon entering the operating room, requiring intraoperative methylene blue, experiencing problems with reading SPO2, or remaining intubated and transferred to the ICU.
Inclusion criteria: Pediatric patients (≤ 16 years) undergoing surgery (both elective and emergency) with the Endo-Tracheal Tube (ETT) or Laryngeal Mask Airway (LMA) were transferred to the PACU from May 1, 2023, to July 30, 2023.
Exclusion criteria: Preoperative peripheral oxygen saturation (<95%), patients entering the operation room with oxygen therapy, intraoperative use of methylene blue, problems with reading SPO2, and patients remained intubated and transferred to the ICU.
Study variables
The study variables include independent variables such as pediatric age, co-existing diseases, anesthesia type, surgery type, anesthesia drugs (opioids and muscle relaxants), duration of anesthesia, and duration of surgery, while the dependent variable is the incidence of Postoperative Hypoxemia (POH).
Independent variables: After pediatric age, co-existing diseases, anesthesia type, surgery type, anesthesia drugs (opioids and muscle relaxants), duration of anesthesia, and duration of surgery.
Dependent variable: Incidence of POH.
Operational definition
The operational definitions include postoperative hypoxemia as peripheral oxygen saturation below 95% for at least one minute after extubation, pediatrics as patients aged 16 years or younger, general anesthesia (GA) as surgery with GA using either an Endo- Tracheal Tube (ETT) or Laryngeal Mask Airway (LMA), prolonged anesthesia time as anesthesia lasting more than one hour, and prolonged surgery time as surgery lasting more than one hour.
Postoperative hypoxemia: Peripheral oxygen saturation <95% in the blood for at least a minute exists after extubation.
Pediatrics: Patients with an age of ≤ 16 years [25].
GA: Pediatrics undergoing surgery either with GA+ETT or GA+LMA.
Prolonged anesthesia time: Anesthesia that lasts >1 hour [26].
Prolonged surgery time: Surgery that lasts >1 hour [26].
Sample size and sampling technique
Sample size: The sample size was determined by using the incidence of hypoxemia (P=22.2%), which is taken from a prospective multicenter cohort study done in Nigeria due to the fact that there is no such multicenter cohort study done in Ethiopia. The study included 23,926 pediatric patients, was studied within 2 years, was conducted in 12 study areas, and had similar study participants to our study area (HUCSH, TASH, and St. Peter Specialized Hospital) [27].
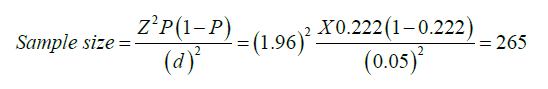
Where Z=1.96 for a 95% confidence interval
P=incidence of hypoxemia
d=margin error (5%)
By adding 10% non-response rate=292.
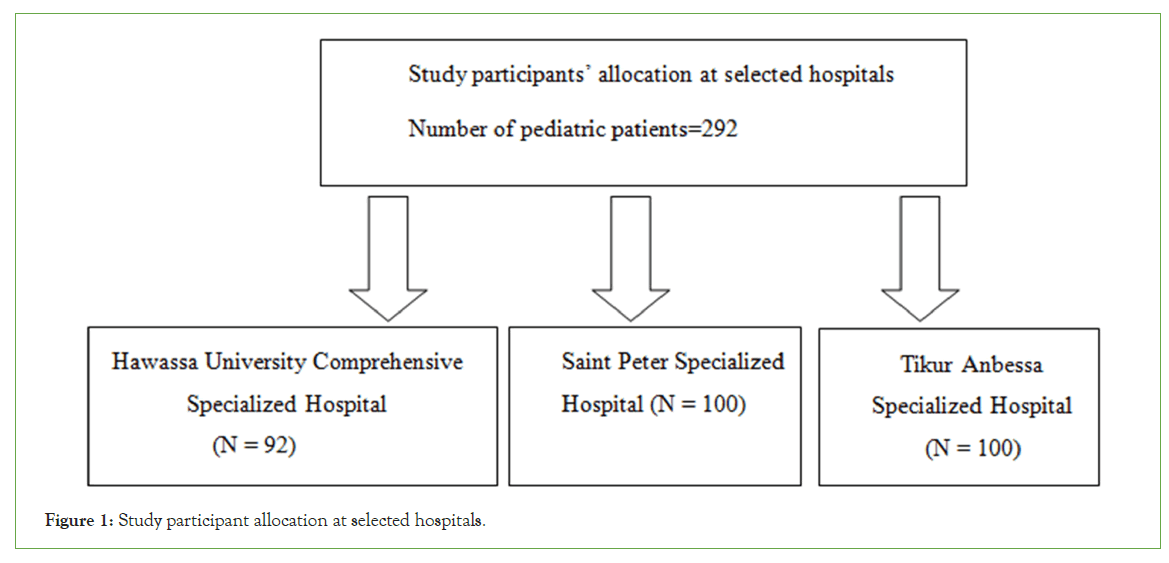
Sampling technique: A systematic random sampling technique was applied (Figure 1).
Figure 1: Study participant allocation at selected hospitals.
These three hospitals were selected randomly. Situational analysis was done, and information was taken from a recorded logbook. 189, 201, and 209 pediatric cases were done at HUCSH, Saint Peter Specialized Hospital, and TASH in two months (February 1-March 30).
The patient sampling interval was conducted by: k=N/n; (N=209, n=292); and k=209/292=1. The first patient was selected randomly.
Where k=sample interval size
N=Number of pediatric patients
n=Sample size
Data collection procedure
After a Support letter and ethical clearance were obtained from the Institutional Review Board (IRB) of Dilla University College of medicine and health science with Protocol Unique No: duirb/034/23-05 data was gathered at three selected hospitals (HUCSH, TASH, and Saint Peter Specialized Hospital) using a structured questionnaire from May 1, 2023, to July 30, 2023. Study participants and parents of study participants (forest parents) were informed about the collection of data, including objectives, benefits, harms, and other issues of the study, in Amharic and other local languages, with translation provided. Data collection was commenced with pediatric patients (≤16 years) who had undergone GA and satisfied the inclusion criteria; after the procedure was completed and the patient was extubated, these pediatric patients were followed throughout their stay in the PACU, and pediatric patients with peripheral oxygen saturation <95% for at least a minute were considered hypoxemic pediatric patients. A questionnaire was developed from patient, anesthesia, and surgery-related information. The data was collected with three nurses assigned to PACU in each selected hospital. The study was registered on the research registry and has got a research registration Unique Identifying Number (UIN) of research registry 9825 https://www.researchregistry.com/browse-the-registry#home/
Data processing and analysis
The statistical software SPSS version 25.00 was used to enter the data. Variables with a p>0.05 were deemed fit for logistic regression, according to the Hosmer-Lemeshow goodness of fit test. Following binary logistic regression analyses, multivariable analysis was carried out once variables that match the final model were included using a p-value<0.25. Independent variables were examined for multicollinearity, and those exhibiting a Variance Inflation Factor (VIF) greater than 10 were removed.
Additionally, the association between variables was determined with Pearson chi-square. A p-value of less than 0.05 indicated that a variable was statistically significant. Frequency tables, pie charts, and graphs were used to summarize and illustrate the data that had been gathered. Finally, using the data, the results were compared and discussed, and then conclusions were forwarded.
Data quality assurance
Two weeks before data collection started in the selected study area, two days of training on the objectives, data procedure, benefits, harms, relevance of the study, and brief explanations of the data collection tool (questionnaire) of the study were provided to assure the quality of the data. A pre-test data collection tool (a questionnaire) was performed on 5% of patients who satisfied the inclusion criteria but were not included in the study during data collection at selected hospitals. The investigator verified the completeness, accuracy, consistency, and clarity of the gathered data by cross-referencing them with the questionnaire. If there were any questions or misunderstandings among participants, they were reported.
Results
Pediatric patients related status
In total, 292 pediatric patients underwent surgery with GA. The majority of pediatric patients were male (63.7%) and aged 3-7 years. 47 (16.1%) of pediatrics had at least one comorbidity (Table 1).
| Variables | Categories | Frequency of POH, n (%) | Total, n (%) | |
|---|---|---|---|---|
| Yes, n (%) | Yes, n (%) | |||
| Age | ≤ 2 years | 66 (61.7%) | 41(38.3%) | 107 (100%) |
| 3-7 years | 53 (48.2%) | 57 (51.8%) | 110 (100%) | |
| 8-16 years | 21 (28%) | 54 (72%) | 75 (100%) | |
| Sex | Male | 83 (44.6%) | 103 (55.4%) | 186 (100%) |
| Female | 57 (53.8%) | 49 (46.2%) | 106 (100%) | |
| Neurologic | 4 (66.7%) | 2 (33.3%) | 6 (100%) | |
| Co-morbidities | Cardiac | 2 (66.7%) | 1 (33.3%) | 3 (100%) |
| Respiratory | 29 (80.6%) | 7 (19.4%) | 36 (100%) | |
| Other | 1 (50%) | 1 (50%) | 2 (100%) | |
Table 1: Pediatric patients undergoing GA characteristics from May 1, 2023, to July 30, 2023.
239 (81.85%) pediatric patients used intraoperative muscle relaxants, and 136 (56.9%) were desaturated; 53 (18.15%) pediatric patients didn’t receive intraoperative muscle relaxants, and 16 (30.2%) became hypoxemic. 174 (59.6%) of pediatrics use opioids as analgesia, and 108 (62.1%) became hypoxemic among pediatrics who use opioids as analgesia (Table 2).
| Variables | Categories | Frequency of POH, n (%) | Total, n (%) | |
|---|---|---|---|---|
| Yes, n (% | Yes, n (%) | |||
| Analgesia | Opioid use | 108 (62.1%) | 66 (37.9%) | 174 (100%) |
| Opioid sparing use | 32 (27.1%) | 86 (72.9%) | 118 (100%) | |
| Muscle relaxant use | Yes | 136 (56.9%) | 103 (43.1%) | 239 (100%) |
| No | 16 (30.2%) | 37 (69.8%) | 53 (100%) | |
Table 2: Characteristics of anesthesia drugs in pediatric patients undergoing GA from May 1, 2023, to July 30, 2023.
Figure 2 showed that 247 (84.6%) pediatric patients undergo surgery with GA+ETT and 45 (15.4%) pediatric patients undergo surgery with GA+LMA. 134 (54.3%) and 18 (40%) were desaturated from pediatric patients undergoing GA+ETT and GA+LMA, respectively. 240 (82.19%) were undergoing prolonged anesthesia time, and 137 (57.1%) were desaturated, compared to 15 (28.846%) who were desaturated with anesthesia duration less than or equal to one hour.
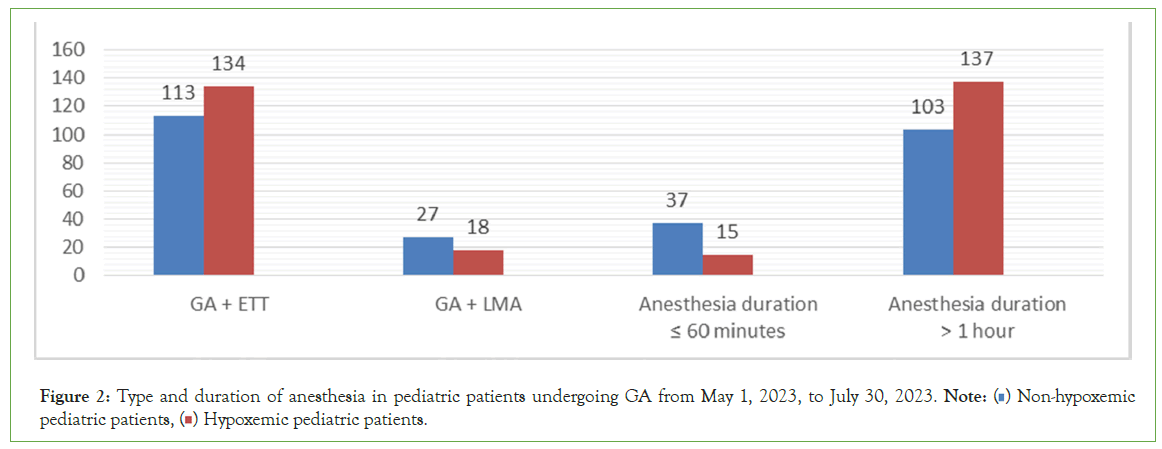
Figure 2: Type and duration of anesthesia in pediatric patients undergoing GA from May 1, 2023, to July 30, 2023. Note: ( ) Non-hypoxemic pediatric patients, (
) Non-hypoxemic pediatric patients, ( ) Hypoxemic pediatric patients.
) Hypoxemic pediatric patients.
Figure 3 showed, 132 (45.2%) of pediatric patients underwent above-abdominal surgeries. 77 (58.34%) of pediatric patients who had undergone above-abdominal surgeries were desaturated, compared to 63 (39.4%) desaturated pediatric patients who had undergone other surgeries. 155 (53.08%) pediatric patients underwent prolonged surgery (>1 hour). 93 (60%) desaturated pediatric patients underwent prolonged surgery (>1 hour) as compared to 59 (43.1%) who became desaturated when pediatric patients underwent surgery less than or equal to one hour.
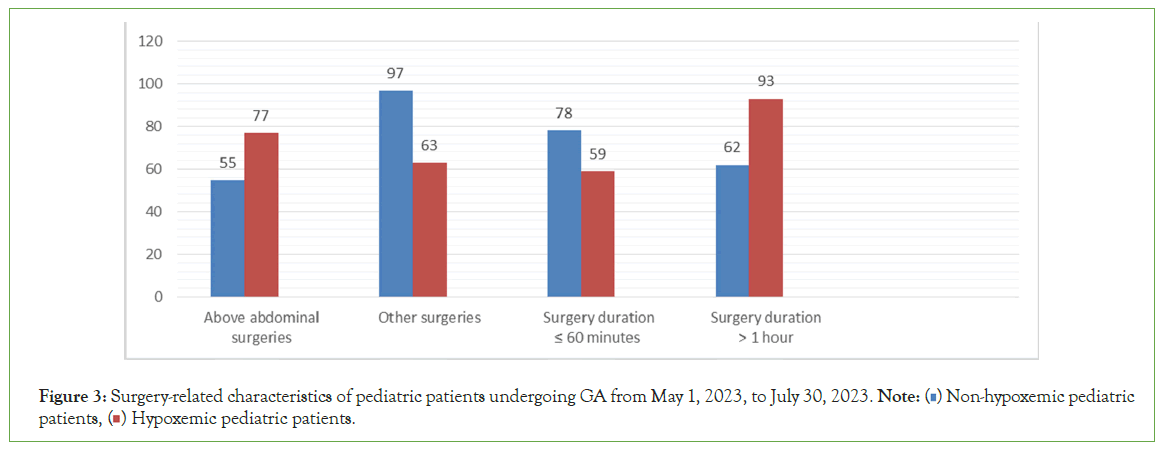
Figure 3: Surgery-related characteristics of pediatric patients undergoing GA from May 1, 2023, to July 30, 2023. Note: (  ) Non-hypoxemic pediatric patients, (
) Non-hypoxemic pediatric patients, ( ) Hypoxemic pediatric patients.
) Hypoxemic pediatric patients.
Figure 4 pie chart illustrates the overall incidence of POH, which was 47.9% among the 292 pediatric patients who underwent surgery with GA between May 1, 2023, and July 30, 2023.
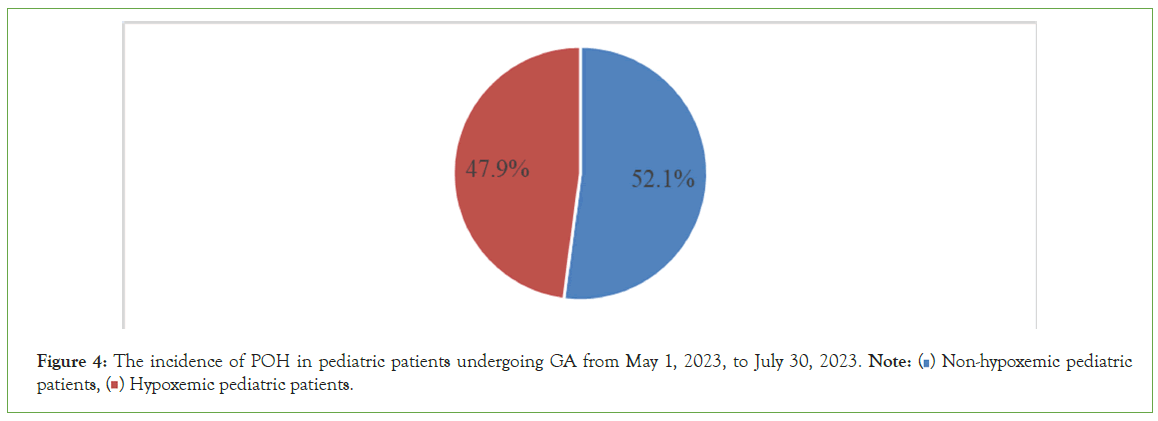
Figure 4: The incidence of POH in pediatric patients undergoing GA from May 1, 2023, to July 30, 2023. Note: ( ) Non-hypoxemic pediatric patients, (
) Non-hypoxemic pediatric patients, ( ) Hypoxemic pediatric patients.
) Hypoxemic pediatric patients.
Predictors of POH
The presence of at least one comorbidity, pediatric age (≤ 2 years), opioid use, and prolonged anesthesia duration (>1 hour) are associated with POH (p<0.001) and COR=4.437 (2.157-9.126), 2.415 (1.482-3.935), 4.398 (2.645-7.312), and 3.281 (1.709-6.298), respectively.
Muscle relaxant use, type of surgery, and prolonged surgery duration (>1 hour) are associated with POH (p=0.001, 0.001, and 0.004) and COR=3.053 (1.610-5.790), 2.156 (1.348-3.447), and 1.983 (1.244- 3.162), respectively. However, anesthesia type is associated with POH (p=0.081) (Table 3).
| Variables | Categories | Frequency of POH, n (%) | Crude Odd Ratio (COR) at 95% CI | p-value | |
|---|---|---|---|---|---|
| Yes, n (%) | No, n (%) | ||||
| Co-morbidities | Yes, n (%) | 36 (76.1%) | 11(23.9%) | 4.437 (2.157-9.126) | <0.001 |
| No, n (%) | 104 (42.4%) | 141 (57.6%) | 1 | ||
| Pediatrics age | ≤ 2 years | 66 (61.68%) | 41 (38.32%) | 2.415 (1.482-3.935) | <0.001 |
| >2 years | 74 (40%) | 111 (60%) | 1 | ||
| Analgesics | Opioids | 108 (62.1%) | 66 (37.9%) | 4.398 (2.645-7.312) | <0.001 |
| Opioid sparing | 32 (27.1%) | 86 (72.9%) | 1 | ||
| Muscle relaxants | Yes, n (%) | 136 (56.9%) | 103 (43.1%) | 3.053 (1.610-5.790) | 0.001 |
| No, n (%) | 16 (30.2%) | 37 (69.8%) | 1 | ||
| Anesthesia type | GA with ETT | 134 (54.3%) | 113 (45.7%) | 0.562 (0.294-1.073) | 0.081 |
| GA with LMA | 18 (40%) | 27 (60%) | 1 | ||
| Surgery type | Above abdominal surgeries | 77 (58.3%) | 55 (41.7%) | 2.156 (1.348-3.447) | 0.001 |
| Other surgeries | 63 (39.4%) | 97 (60.6%) | 1 | ||
| Anesthesia duration | >1hour | 137 (57.1%) | 103 (42.9%) | 3.281 (1.709-6.298) | <0.001 |
| ≤ 60 minutes | 15 (28.85%) | 37 (71.15%) | 1 | ||
| Surgery duration | >1 hour | 93 (60%) | 62 (40%), | 1.983 (1.244-3.162) | 0.004 |
| ≤ 60 minutes | 78 (59.34%) | 59 (40.66%) | 1 | ||
Note: 1 stands for reference group.
Table 3: Bivariate analysis of predictors of POH in pediatric patients undergoing GA from May 1, 2023, to July 30, 2023.
For a binary logistic regression analysis of p<0.25, multivariable logistic regression was used and use of opioids, pediatric age (≤ 2 years), and above-abdominal surgeries were associated with POH (p<0.001) AOR=5.545 (2.983-10.307), 3.263 (1.686-6.314), and 3.642 (1.969-6.736), respectively. Additionally, the presence of at least one comorbidity, use of muscle relaxants, prolonged (<1 hour) anesthesia, and surgery durations are significantly associated with POH (p=0.001, 0.003, 0.02, and 0.037) and AOR=4.259 (1.788- 10.145), 29.72 (3.262-270.778), 2.153 (1.131-4.1), and 2.560 (1.058- 6.16), respectively. However, anesthesia type is insignificantly associated with POH (p=0.096) (Table 4).
| Variables | Categories | Frequency of POH, n (%) | Adjusted Odd Ratio (AOR) at 95% CI | p-value | |
|---|---|---|---|---|---|
| Yes, n (%) | No, n (%) | ||||
| Co-morbidities | Yes, n (%) | 36 (76.1%) | 11 (23.9%) | 4.259 (1.788-10.145) | 0.001 |
| No, n (%) | 104 (42.4%) | 141 (57.6%) | 1 | ||
| Pediatrics age | ≤ 2 years | 66 (61.68%) | 41 (38.32%) | 3.263 (1.686-6.314) | <0.001 |
| >2 years | 74 (40%) | 111 (60%) | 1 | ||
| Analgesics | Opioids | 108 (62.1%) | 66 (37.9%) | 5.545 (2.983-10.307) | <0.001 |
| Opioid sparing | 32 (27.1%) | 86 (72.9%) | 1 | ||
| Muscle relaxants | Yes, n (%) | 136 (56.9%) | 103 (43.1%) | 29.72 (3.262-270.778) | 0.003 |
| No, n (%) | 16 (30.2%) | 37 (69.8%) | 1 | ||
| Anesthesia type | GA with ETT | 134 (54.3%) | 113 (45.7%) | 6.620 (0.717-61.102) | 0.096 |
| GA with LMA | 18 (40%) | 27 (60%) | 1 | ||
| Type of surgery | Above abdominal surgeries | 77 (58.3%) | 55 (41.7%) | 3.642 (1.969-6.736) | <0.001 |
| Other surgeries | 63 (39.4%) | 97 (60.6%) | 1 | ||
| Anesthesia duration | >1 hour | 103 (42.9%) | 137 (57.1%) | 2.153 (1.131-4.1) | 0.02 |
| ≤ 60 minutes | 37 (71.15%) | 15 (28.85%) | 1 | ||
| Surgery duration | >1 hour | 62 (40%) | 93 (60%) | 2.560 (1.058-6.196) | 0.037 |
| ≤ 60 minutes | 78 (59.34%) | 59 (40.66%) | 1 | ||
Note: 1 stands for reference group.
Table 4: Multivariate analysis of predictors of POH in pediatric patients undergoing GA from May 1, 2023, to July 30, 2023.
Discusion
The overall finding of the study is concerned with the incidence and predictors of POH in pediatric patients (≤16 years old) undergoing GA with follow-up in the operation theater and PACU. The incidence of POH in pediatric patients (≤ 16 years old) was 47.9%, and patient, anesthesia, and surgery-related factors could predict POH.
The prevalence of hypoxemia was found to be 23.8% in a cross- sectional study conducted by Rao et al., on clinical predictors of hypoxemia in Indian children coming to the pediatric emergency room with an acute respiratory tract infection [27]. A similar cross-sectional study on determinants of hypoxemia in children associated with pneumonia by Ramawat and Sharma revealed that the prevalence of hypoxemia was 48% [28]. As compared to our study, the study by Ramawat and Sharma found that the incidence of POH is comparable, even though differences in study design exist and is higher than the study done by Rao et al. This might be due to the fact that it took into account only pediatric patients with acute respiratory tract infections.
A review by Malik et al., on predictors of hypoxemia in children (2-60 months) with lower respiratory tract infections, out of 150 children investigated, 35.3% had hypoxemia [29]. A study on the incidence of early post-anesthesia hypoxemia and related factors in the PACU done by Cifuentes et al., implied that the overall incidence of early POH was 16% [30]. A similar observational study on predictors of desaturation during patient transport to the PACU by Labaste et al., showed that the incidence of hypoxemia during transfer was 13% [13]. The incidence of POH in our study is higher than in these studies; it could be due to the fact that our study followed the incidence of POH through the patient stay in the PACU.
In the retrospective study by Tang et al., on the incidence of hypoxemia in a PACU and relevant risk factors, the incidence of POH was estimated to be 21.83% [14]. Based on Li's retrospective observational study, the incidence of hypoxemia in the PACU following general anesthesia in children was estimated to be 4.35% [31]. The incidence of POH in these retrospective studies is lower than in our study, which might be due to differences in study design.
In 4092 consecutive pediatric cases, a retrospective study by Kieran et al., on the risk factors of postoperative desaturation revealed that 27.2% of patients had desaturations [32]. The incidence of POH was found to be 14.0% in retrospective research conducted by Chen et al., on risk factors of postoperative hypoxemia following shoulder arthroscopic surgery [33]. The incidence of POH in our study is higher than these past research, which might be due to differences in study design.
A multicenter cohort study conducted in Nigeria on hypoxemia in hospitalized children and neonates by Graham et al., showed that out of 23,926 neonates and children, the incidence of neonatal hypoxemia was 22.2% (95% CI=21.2-23.2), while the incidence of hypoxemia in children was 10.2% (9.7-10.8) [26]. This multicenter cohort study has a lower incidence of hypoxemia as compared to ours, which could be due to the fact that it only took into account pediatric patients with specified admission cases.
A multicenter cohort study by McCollum et al., on the prevalence of hypoxemia and the quality of oxygen treatment for hospitalized Malawian children, 40 out of 761 (5.3%) hospitalized children under the age of 15 had SPO2 levels below 90% [18]. In a research study by Salah et al., on the prevalence of hypoxemia in under- five children with pneumonia at an emergency pediatrics hospital in Sudan, 42.7% of the patients had hypoxemia (with a pulse oximeter oxygen saturation of less than 90%) [34]. These cohort and cross-sectional studies have a lower incidence of hypoxemia as compared to ours, which could be due to the fact that they only took into account pediatric patients with specified admission cases.
An observational study on the incidence of early POH and its contributing factors by Melesse et al., stated that the incidence of POH was 26.7% [1]. A similar observational cohort study on the incidence and associated factors of POH among adult elective surgical patients by Andualem showed that the incidence of POH was 24.5% [16]. Our study’s POH incidence is higher than in these observational studies; this could be due to differences in the study participants.
A sectional research investigation by Ketema, on the magnitude and associated factors of early POH in pediatric patients implied that the magnitude of early POH was 44.5% [25]. A similar cross- sectional study by Wolde et al., on the magnitude and associated factors of immediate POH among elective surgical procedures revealed that the magnitude of immediate POH was 22.5% [35]. As compared to these cross-sectional studies, the incidence of POH in our study is high. This could be due to the fact that these research investigation took into account only elective surgical patients and early POH.
The incidence of early postoperative hypoxemia was 45.8%, in accordance with prospective cohort research by Taye et al., on predictors of hypoxemia after GA in the early postoperative period in a hospital in Ethiopia [7]. The prevalence of POH was 42.3% in a prospective observational study conducted by Berhanu et al., on the magnitude of early postoperative hypoxemia and its associated factors among adult patients undergoing emergency surgery under GA [36]. These prospective observational studies had comparable study results as compared to ours.
Pediatrics with at least one co-morbidity had a significant association with POH with multivariable analysis (p=0.001) and an AOR of 4.259 (1.788-10.145). A recent study on the risk factors for desaturation after tonsillectomy done by Kieran et al. implied that pediatrics with co-morbidities undergoing surgery had a significant association with POH (p<0.001) [32]. A similar cross- sectional study by Wolde et al., on the magnitude and associated factors of immediate POH stated that patients with respiratory co- morbidities had a significant association with POH (p=0.002) [35]. A retrospective and cross-sectional study had a comparable result to our research, even though differences in study design and study participants exist.
Pediatric age (≤ 2 years) is significantly associated with POH (p ≤ 0.001) and AOR=3.263 (1.686-6.314). In a retrospective review on whether nocturnal hypoxemia and hypercapnia are associated with desaturation immediately after adenotonsillectomy, done by Dalesio et al., it was implied that pediatric patients (≤ 2 years) had a 10.43 (95%, CI=1.89-110.9) times higher likelihood of desaturation than patients between the ages of nine and seventeen [37]. This retrospective study had comparable results to our study, which implied pediatric patients (≤ 2 years) are significantly associated with hypoxemia, even though differences in study design exist.
Opioid administration is significantly associated with POH, with multivariable analysis p<0.001, AOR=5.545 (2.983-10.307). An observational trial on postoperative opioid administration characteristics done by Urman et al., implied that postoperative opioid administration was significantly associated with POH (p<0.001) [38]. A similar study by Ishikawa and Sakamoto on postoperative desaturation and bradypnea after GA described postoperative opioid administration as significantly associated with POH and bradypnea with p=0.0019 [39]. Our study result is similar with the observational and recent study results.
There is an insignificant association between anesthesia type (either GA+ETT or GA+LMA) and POH (p=0.096). A cross-sectional study by Ketema on the magnitude and associated factors of early POH in pediatric patients implied an insignificant association between anesthesia type (either GA+ETT or GA+LMA) and POH (p=0.079) [25]. This cross-sectional study result is in line to our research result, even though differences in methodology and specified urgency in admission exist.
Pediatric patients who underwent above-abdominal surgeries were significantly associated with POH (p<0.001) and an AOR of 3.642 (1.969-6.736). A retrospective study by Tang et al., on the incidence of hypoxemia in PACU and relevant risk factors stated that surgery type (thoracic surgeries) had a significant association with POH (p<0.01) [14]. A similar retrospective study by Li et al., on risk factors for hypoxemia after GA in the PACU showed surgery type (oral surgeries) could be significantly associated with POH in the PACU (p ≤ 0.001) [31]. Even though differences in study participants and methodology exist, these studies had a consistent result compared to ours.
Prolonged anesthesia time (>1 hour) is significantly associated with POH (p=0.02) and AOR=2.153 (1.13-14.1) [40]. A cross- sectional study by Cifuentes et al., on the incidence of early post- anesthesia hypoxemia and related factors in the PACU stated that there was a significant association between prolonged anesthesia time and POH (p=0.001) [30]. This cross-sectional study finding is comparable to our study result.
Our study found that prolonged operation time (>1 hour) is significantly associated with POH (p=0.037) and AOR=2.560 (1.058-6.196). A single-center retrospective study on risk factors for POH after shoulder arthroscopy by Chen et al., described prolonged operation time as significantly associated with POH (p=0.041) [33]. A similar prospective observational study by Berhanu et al., on the magnitude of early POH and its associated factors implied that prolonged operation time is significantly associated with POH (p=0.014) [36]. Even though there is a difference in study participants and the operational definition of prolonged operation time, both studies had comparable study results to ours.
Conclusion
The incidence of POH in pediatric patients undergoing GA is high, and the presence of at least one comorbidity, pediatric age (≤ 2 years), use of opioids and muscle relaxants, above- abdominal surgeries, prolonged (>1 hour) anesthesia, and surgery durations are significantly associated with POH. However, there is an insignificant association between the type of anesthesia and POH. We recommend taking precautions, following them and having oxygen therapy for these high-risk pediatric patients. Age, comorbidities, anesthesia, and surgical factors significantly predict POH. Implementing stringent monitoring protocols and addressing these risk factors can help to reduce the hypoxemia- related complications and improve postoperative outcomes in pediatric patients.
Recommendation
We recommend taking precautions, following them and having oxygen therapy for pediatric patients with the presence of at least one comorbidity, pediatrics with an age of ≤ 2 years, use of opioids and muscle relaxants, above-abdominal surgeries, prolonged (>1 hour) anesthesia, and surgery durations. We recommend further multicenter studies with a large sample size on the outcomes of POH.
Strength of the study
The study addresses the incidence and predictors of POH in pediatric patients undergoing GA.
The study is the first multicenter cohort study on the incidence and predictors of POH in pediatric patients undergoing GA in Ethiopia.
The study is used as baseline evidence for those who want to be involved in similar studies.
Limitation of the study
J.K. Pediatric patients were followed only in the Operation Theater and PACU. Oxygen saturation of the pediatric patient’s during transfer from operating theater to PACU was not recorded.
Acknowledgment
We express our deepest gratitude to Dilla University College of Medicine and Health Sciences, Department of Anesthesiology, for giving us the opportunity to develop research on the incidence and predictors of POH in pediatric patients who undergo GA.
References
- Melesse DY, Denu ZA, Kassahun HG, Agegnehu AF. The incidence of early post-operative hypoxemia and its contributing factors among patients underwent operation under anesthesia at University of Gondar comprehensive and specialized referral hospital, Gondar, North West Ethiopia, 2018. A prospective observational study. Int J Surg Open. 2020;22:38-46.
- Sockrider M, Evey LW, Grad R, Leong A, Baker J, Gettys A. Oxygen therapy for children. Am J Respir Crit Care Med. 2019;199(3):P5-P6.
[Crossref] [Google Scholar] [PubMed]
- Arevalo m. Pulse oximetry training manual. Academiaedu. 2011.
- Dhanjal S. Treasure Island (FL): StatPearls. Publishing Copyright© 2023. 2023.
- Karcz M, Papadakos PJ. Respiratory complications in the postanesthesia care unit: A review of pathophysiological mechanisms. Can J Respir Ther. 2013;49(4):21-29.
[Google Scholar] [PubMed]
- Harless J, Ramaiah R, Bhananker SM. Pediatric airway management. Int J Crit Illn Inj Sci. 2014;4(1):65.
[Crossref] [Google Scholar] [PubMed]
- Taye MG, Molla A, Teshome D, Hunie M, Kibret S, Fentie Y, et al. Predictors of hypoxemia after general anesthesia in the early postoperative period in a hospital in Ethiopia: An observational study. Multidiscip Respir Med. 2021;16(1):782.
[Crossref] [Google Scholar] [PubMed]
- Caruso TJ, Mokhtari TE, Coughlan MJ, Wu DS, Marquez JL, Duan M, et al. Pediatric postoperative pulse oximetry monitoring during transport to the postanesthesia care unit reduces frequency of hypoxemia. Jt Comm J Qual Patient Saf. 2017;43(3):146-150.
[Crossref] [Google Scholar] [PubMed]
- Sheikh M, Ahmad H, Ibrahim R, Nisar I, Jehan F. Pulse oximetry: Why oxygen saturation is still not a part of standard pediatric guidelines in Low-And-Middle-Income Countries (LMICs). Pneumonia. 2023;15(1):3.
[Crossref] [Google Scholar] [PubMed]
- Xará D, Santos A, Abelha F. Adverse respiratory events in a post-anesthesia care unit. Arch Bronconeumol. 2015;51(2):69-75.
[Crossref] [Google Scholar] [PubMed]
- Barends CR, Yavuz P, Molenbuur B, Absalom AR. Performance of blow‐by methods in delivering oxygen to pediatric patients during transport: A laboratory study. Paediatr Anaesth. 2018;28(12):1142-1147.
[Crossref] [Google Scholar] [PubMed]
- Ramachandran SK, Thompson A, Pandit JJ, Devine S, Shanks AM. Retrospective observational evaluation of postoperative oxygen saturation levels and associated postoperative respiratory complications and hospital resource utilization. PLoS One. 2017;12(5):e0175408.
[Crossref] [Google Scholar] [PubMed]
- Labaste F, Silva S, Serin-Moulin L, Lefèvre E, Georges B, Conil JM, et al. Predictors of desaturation during patient transport to the postoperative anesthesia care unit: An observational study. J Clin Anesth. 2016;35:210-214.
[Crossref] [Google Scholar] [PubMed]
- Tang S, Zhang L, Han W, Xue Y, Tian Y, Zhang X, et al. Incidence of hypoxemia in a post-anaesthesia care unit and relevant risk factors: A retrospective study of 14604 patients with general anaesthesia. 2019.
- Rostin P, Teja BJ, Friedrich S, Shaefi S, Murugappan KR, Ramachandran SK, et al. The association of early postoperative desaturation in the operating theatre with hospital discharge to a skilled nursing or long‐term care facility. Anaesthesia. 2019;74(4):457-467.
[Crossref] [Google Scholar] [PubMed]
- Andualem AA, Yesuf KA. Incidence and associated factors of postoperative hypoxemia among adult elective surgical patients at Dessie Comprehensive Specialized Hospital: An observational study. Ann Med Surg. 2022;78:103747.
[Crossref] [Google Scholar] [PubMed]
- Liu K, Scott JB, Jing G, Li J. Management of postoperative hypoxemia. Respir Care. 2021;66(7):1136-1149.
[Crossref] [Google Scholar] [PubMed]
- McCollum ED, Bjornstad E, Preidis GA, Hosseinipour MC, Lufesi N. Multicenter study of hypoxemia prevalence and quality of oxygen treatment for hospitalized Malawian children. Trans R Soc Trop Med Hyg. 2013;107(5):285-292.
[Crossref] [Google Scholar] [PubMed]
- Lam F, Subhi R, Houdek J, Schroder K, Battu A, Graham H. The prevalence of hypoxemia among pediatric and adult patients presenting to healthcare facilities in low-and middle-income countries: Protocol for a systematic review and meta-analysis. Syst Rev. 2020;9(1):67.
[Crossref] [Google Scholar] [PubMed]
- Hussen I, Worku M, Geleta D, Mahamed AA, Abebe M, Molla W, et al. Post-operative pain and associated factors after cesarean section at Hawassa University Comprehensive Specialized Hospital, Hawassa, Ethiopia: A cross-sectional study. Ann Med Surg. 2022;81:104321.
[Crossref] [Google Scholar] [PubMed]
- Assen HE, Hassen AM, Abate A, Liyew B. Preoperative fasting time and its association with hypoglycemia during anesthesia in pediatric patients undergoing elective procedures at Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia. Biomed Res Int. 2021;2021.
[Crossref] [Google Scholar] [PubMed]
- Tsegaw H, Yimam M, Nureye D, Woldeselassie W, Hambisa S. Predictors of treatment outcomes among pediatric patients hospitalized with pneumonia in tikur anbessa specialized Hospital, Addis ababa, Ethiopia. Adv pharmacol pharm sci. 2021;2021:1-7.
[Crossref] [Google Scholar] [PubMed]
- Jofiro G, Jemal K, Beza L, Bacha Heye T. Prevalence and associated factors of pediatric emergency mortality at Tikur Anbessa specialized tertiary hospital: A 5 year retrospective case review study. BMC Pediatr. 2018;18(1):316.
[Crossref] [Google Scholar] [PubMed]
- Hailu S, Shiferaw A, Regasa T, Getahun YA, Mossie A, Besha A. Incidence of postoperative sore throat and associated factors among pediatric patients undergoing surgery under general anesthesia at Hawassa University comprehensive specialized hospital, a prospective cohort study. Int J Gen Med. 2023:589-598.
[Crossref] [Google Scholar] [PubMed]
- Ketema R. Magnitude and associated factors of ealy postoperative hypoxemia in pediatric patients undergoing elective surgery under General Anesthesia at Tikur Anbessa Specialized Hospital Ethiopia. 2020.
- Graham H, Bakare AA, Ayede AI, Oyewole OB, Gray A, Peel D, et al. Hypoxaemia in hospitalised children and neonates: A prospective cohort study in Nigerian secondary-level hospitals. EClinicalMedicine. 2019;16:51-63.
[Crossref] [Google Scholar] [PubMed]
- Rao YK, Midha T, Kumar P, Tripathi VN, Rai OP. Clinical predictors of hypoxemia in Indian children with acute respiratory tract infection presenting to pediatric emergency department. World J Pediatr. 2012;8:247-251.
[Crossref] [Google Scholar] [PubMed]
- Ramawat P, Sharma B. Determinants of hypoxemia in children associated with pneumonia. Asian J Med Sci. 2016;7(2):64-70.
- Malik S, Gohiya P, Sisodia P, Dave L. Predictors of Hypoxemia in children (2-60 months) with lower respiratory tract infection. Int J Pediatr Res. 2015;2(4):138-142.
- Quintero-Cifuentes IF, Pérez-López D, Victoria-Cuellar DF, Satizábal-Padridín N, Billefals-Vallejo ES, Castaño-Ramírez DA, et al. Incidence of early postanesthetic hypoxemia in the postanesthetic care unit and related factors. Colombian Journal of Anesthesiology. 2018;46(4):309-316.
- Li H, Zhang Y, Cai J, Wang H, Wei R. Risk factors of hypoxemia in the postanesthesia care unit after general anesthesia in children. J Perianesth Nurs. 2023;38(5):799-803.
[Crossref] [Google Scholar] [PubMed]
- Kieran S, Gorman C, Kirby A, Oyemwense N, Lander L, Schwartz M, et al. Risk factors for desaturation after tonsillectomy: Analysis of 4092 consecutive pediatric cases. Laryngoscope. 2013;123(10):2554-2559.
[Crossref] [Google Scholar] [PubMed]
- Chen W, Yu G, Ning F, Yuan Z. Risk factors of postoperative hypoxemia after shoulder arthroscopic surgery: A single-center retrospective study. 2022.
- Salah ET, Algasim SH, Mhamoud AS, Husian NE. Prevalence of hypoxemia in under-five children with pneumonia in an emergency pediatrics hospital in Sudan. Indian J Crit Care Med. 2015;19(4):203-207.
[Crossref] [Google Scholar] [PubMed]
- Wolde GD, Awol MA, Obsa MS, Wesene NG, Gemechu AD, Tadesse EN. Magnitude and associated factors of immediate postoperative hypoxemia among elective surgical procedures at Tikur Anbessa Specialized hospital, Addis Ababa, Ethiopia. J Anesth Clin Res. 2018;9(821):1-6.
- Berhanu M, Dadi N, Mengistu B, Muluken Z, Tolesa A, Tageza T, et al. Magnitude of early postoperative hypoxemia and its associated factors among adult patients who undergo emergency surgery under general anesthesia at Jimma Medical Center, Jimma, Southwest Ethiopia, 2021: A prospective observational study. Perioper Med. 2023;12(1):1.
[Crossref] [Google Scholar] [PubMed]
- Dalesio NM, McMichael DH, Benke JR, Owens S, Carson KA, Schwengel DA, et al. Are nocturnal hypoxemia and hypercapnia associated with desaturation immediately after adenotonsillectomy? Paediatr Anaesth. 2015;25(8):778-785.
[Crossref] [Google Scholar] [PubMed]
- Urman RD, Khanna AK, Bergese SD, Buhre W, Wittmann M, Le Guen M, et al. Postoperative opioid administration characteristics associated with opioid-induced respiratory depression: Results from the PRODIGY trial. J Clin Anesth. 2021;70:110167.
[Crossref] [Google Scholar] [PubMed]
- Ishikawa M, Sakamoto A. Postoperative desaturation and bradypnea after general anesthesia in non-ICU patients: A retrospective evaluation. J Clin Monit Comput. 2020;34(1):81-87.
[Crossref] [Google Scholar] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. Jama. 2013;310(20):2191-2194.
[Crossref] [Google Scholar] [PubMed]
Citation: Mengistu S, Mulugeta HM, Destaw B, Molla S (2024) Incidence and Predictors of Post-Operative Hypoxemia in Pediatric Patients Undergoing General Anesthesia: A Single-Arm Prospective Multi-Center Cohort Study 2023. J Pain Manage Med.10:275.
Copyright: © 2024 Mengistu S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.