Indexed In
- Open J Gate
- JournalTOCs
- The Global Impact Factor (GIF)
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2024) Volume 12, Issue 4
Impact of Switch to Non-Prescription Status on the Safety Profile of Sildenafil and Tadalafil: Assessment of Pharmacovigilance Databases
Mark Waring and Amritdeep Gill*Received: 07-Nov-2024, Manuscript No. JP-24-27482; Editor assigned: 11-Nov-2024, Pre QC No. JP-24-27482(PQ); Reviewed: 25-Nov-2024, QC No. JP-24-27482; Revised: 02-Dec-2024, Manuscript No. JP-24-27482(R); Published: 09-Dec-2024, DOI: 10.35841/2329-6887.24.12.497
Keywords
Non-Rx; OTC switch; Sildenafil; Tadalafil; Pharmacovigilance; Global database; Consumer Healthcare; Consumer healthcare; PDE5I; Erectile dysfunction
Abbreviation
AE: Adverse event; CHC: Consumer Healthcare; FAERS: Food and Drug Administration Adverse Event Reporting System; EVDAS: EudraVigilance Data Analysis System; Non-Rx: Non-Prescription; OTC: Overthe- Counter; RR: Reporting Rate; PDE5Is: Phosphodiesterase Type 5 Inhibitors; ED: Erectile Dysfunction; EV: EudraVigilance
Introduction
Phosphodiesterase Type 5 Inhibitors (PDE5Is) including sildenafil and tadalafil are vasodilating medicines that work by inhibiting the degradative action of Phosphodiesterase Type 5 (PDE5) on Cyclic Guanosine Monophosphate (cGMP) in vascular smooth muscle cells
Sildenafil and tadalafil are approved as first line treatment of Erectile Dysfunction (ED). Other PDE5Is approved in the United States (US) and European Union (EU) as first line therapy for ED include vardenafil and avanafil. Further PDE5Is (udenafil, lodenafil and mirodenafil) are available for treatment of ED in other countries. Sildenafil and tadalafil are also approved for the treatment of Pulmonary Arterial Hypertension (PAH). In the US, tadalafil is also approved for the treatment of symptoms associated with benign prostatic hyperplasia [1,2].
PDE5Is have a well-established safety profile and are generally well-tolerated in patients with ED [2-4]. Class-specific undesirable effects are related to inhibition of PDE5 in the smooth muscle vascular and digestive systems and inhibition of PDE6 in the retina and include headache, flushing, dyspepsia, nasal congestion, dizziness, abnormal vision, back pain and myalgia [2]. Theoretical concerns about an increased risk of cardiovascular events due to vasodilation-induced hypotension in patients with underlying cardiovascular disease have not been confirmed in randomized trials and retrospective analyses [4]. Consistent with their known effects on the nitric oxide/cGMP pathway, PDE5Is potentiate the hypotensive effects of nitrates and their co-administration with nitric oxide donors or nitrates is therefore contraindicated [2-4].
Sildenafil and tadalafil were first approved for treatment of ED in 1998 and 2002, respectively. In recent years, both medicines have increasingly been made available as Non-Prescription (non- Rx) or Over-The-Counter (OTC) medication; sildenafil is available via pharmacists without prescription for treatment of ED in New Zealand since October, 2014 and as a non-prescription medication in Poland since May 2016, in the United Kingdom (UK) since March, 2018 via pharmacy, in Norway since August, 2019 and in Ireland since January, 2021. Tadalafil is available as OTC medication for treatment of ED in Poland since March, 2022 and as non-Rx in the UK since June, 2023. In this context, Sanofi is considering applications in further countries for changing the classification for the supply of their tadalafil products from “medicinal product subject to medical prescription” to “medicinal product not subject to medical prescription”.
In the context of the evaluation of the suitability for classification of sildenafil and tadalafil products as non-prescription medicines, the purpose of this analysis was to assess postmarketing pharmacovigilance data from open access databases for any evidence that the recent switches of sildenafil and tadalafil from prescription-only to non-prescription status in the above-mentioned countries had an impact on the safety profile of these medicines or resulted in new safety concerns.
Specifically, the first objective of this analysis was to describe trends in the frequency of Individual Case Safety Reports (ICSRs) over time (i.e., pre and post non-Rx or OTC switch) in those countries where sildenafil or tadalafil have already undergone through non- Rx or OTC switch. The second objective was to explore whether these switches from prescription to non-prescription status were associated with any changes to the characteristics of the ICSRs, such as the types, seriousness or outcomes of reported Adverse Drug Reactions (ADRs), or evidence of incorrect use such as concomitant use of contra-indicated drugs.
Materials and Methods
Sources of postmarketing pharmacovigilance data
The European Medicines Agency’s (EMA) EudraVigilance (EV) database, the United States FAERS and the SMARS database of New Zealand’s Centre for Adverse Reactions Monitoring (CARM) were queried for ICSRs concerning sildenafil or tadalafil.
Of note, regulatory requirements may require marketing authorization holders to report ICSRs (in particular those describing serious ADRs) into multiple databases. Consequently, significant overlaps exist for these three databases.
EudraVigilance (EV)
EV is the system for managing and analyzing information on suspected ADRs to medicines which have been authorized or are being studied in clinical trials in the European Economic Area (EEA). The system is operated by EMA on behalf of the European medicines regulatory network. As a crude approximation, the database includes serious and non-serious ICSRs reported from an EEA country, whereas only ICSRs from non-EEA countries are generally captured only if they describe serious ADRs.
For this analysis, a Level 1 query of the EVDAS was performed. This query provides case level outputs of the ICSR receipt date, patient demographics (age group and sex), tradename, active ingredient, indication, treatment duration, dose and route of administration of suspect and interacting medicinal product(s), concomitant drugs, as well as Medical Dictionary for Regulatory Activities (MedDRA) Preferred Terms (PT), seriousness and outcome of the reported ADRs (Appendix 1). Country of reporting is not displayed in Level 1 retrievals but can generally be inferred from the Worldwide Unique Case Identification number. Case narratives or information on patient medical history, comorbidities, reporter causality assessments or the mode of dispensing of the suspect drug (prescription or non-prescription/OTC) are not available.
EVDAS is updated daily. For this analysis, data were retrieved with a cut-off date of 30 April, 2024.
FDA Adverse Event Reporting System (FAERS)
The FAERS is a database that contains ICSRs reporting suspected ADRs, medication errors and product quality complaints resulting in ADRs that were submitted to FDA by marketing authorization holders, healthcare professionals and consumers. The database includes serious and non-serious ICSRs reported from the US, whereas ICSRs from ex-US are generally captured only if they describe serious ADRs.
Limited public access to FAERS is possible through the FAERS Public Dashboard, which allows for case level access to ADR reports. Case information includes originating country and receipt date of the report, patient age and sex, tradename, active ingredient and indication of the suspect medicinal product(s), concomitant drugs, MedDRA PTs of reported ADRs, as well as ADR onset date, seriousness and outcomes (Appendix 1). Case narratives or information on patient medical history, comorbidities, reporter causality assessments or the mode of dispensing are not available.
The FAERS Public Dashboard is updated on a quarterly basis. At the time of data retrieval for this analysis, data were current through 31 March, 2024.
Suspected Medicine Adverse Reaction Search (SMARS)
SMARS is a publicly available query tool of New Zealand’s national pharmacovigilance database (Appendix 5). SMARS provides limited case level information on serious and non-serious ICSRs reported from New Zealand, including receipt date, patient age and sex, active ingredient of suspect and concomitant medicine(s) and the MedDRA PTs of reported ADRs (Appendix 1). Case narratives or information on drug indications, mode of dispensing, ADR seriousness or outcomes, patient medical history or comorbidities or reporter causality assessments are not available. Reports from other countries are not included.
SMARS is updated on a monthly basis. At the time of data retrieval for this analysis, data were current through 01 May 2024.
Sanofi global pharmacovigilance database
Sanofi Consumer Healthcare’s (CHC) pharmacovigilance database holds full case information for all ICSRs reported for Sanofi medicinal products from worldwide sources. ICSRs concerning sildenafil are not systematically collected in this database because Sanofi does not hold any marketing authorizations for sildenafil products. Currently, UK is the only country where Sanofi is marketing authorization holder and has launched a tadalafil product. In accordance with applicable regulatory requirements, ICSRs are entered into this database not only if they concern a Sanofi CHC product, but also if a Sanofi CHC product cannot be ruled out based on the reported information. Therefore, cases potentially concerning non-Sanofi CHC or not belonging to nonprescription tadalafil products might be stored in this database.
The cut-off date for this database was 30 June, 2024.
Data retrievals and selection of relevant ICSRs
FAERS, EVDAS and SMARS were queried for ICSRs where sildenafil was reported as a suspect or interacting drug and that had been initially entered into the respective database on or after 01 Jan, 2010. This date was chosen to allow for retrieval of ICSRs occurring with prescribed medicine that could serve as a baseline for comparison with ICSRs reported after the non-Rx/OTC switch. FAERS, EVDAS were also queried for ICSRs concerning tadalafil based on the same criteria. Sanofi CHC’s global pharmacovigilance database was queried for any ICSRs concerning tadalafil as suspect drug.
Data from FAERS, EVDAS and Sanofi CHC’s global pharmacovigilance database were downloaded as Microsoft Excel® files (ICSR line listings). The ISCR listing resulting from the SMARS website query was also transferred into Excel®. Excel® was used to organize, classify, tabulate and visualize retrieved ICSRs.
In line with the research questions, retrieved cases concerning sildenafil were included for further analysis only if they originated from Ireland, New Zealand, Norway, Poland or the UK. Cases concerning tadalafil were included for further analysis only if they originated from Poland or the UK. ICSRs concerning females, patients younger than 18 years of age, or describing use of sildenafil or tadalafil in patients with PAH (as defined in Appendix 2) were excluded from all analyses.
ICSR duplicates
Within the FAERS dataset, almost half of the retrieved, relevant ICSRs were suggestive for duplicates (i.e., ICSRs reporting the same country of origin, patient age and sex, suspect and concomitant drugs, ADRs and/or ADR onset dates, but received from different marketing authorization holders and with different Worldwide Unique Case Identification Numbers).
Because full case information was not accessible through the FAERS Public Dashboard, definitive identification of case duplicates was not possible. Therefore, suspected duplicates were not categorically excluded from the FAERS dataset. However, probable duplicates were flagged and the effect of excluding these cases on reporting patterns was explored in the country-specific analyses.
In the EVDAS Level 1 retrievals, exact patient age and ADR onset dates are not provided at all, thereby prohibiting reliable identification of potential duplicates.
There was no indication of the presence of duplicates in Sanofi CHC’s global pharmacovigilance database or, from the limited information available, in the SMARS dataset.
Number of ICSRs and reporting rates over time
The mode of dispensing of the suspect drug is not reported in the outputs of any of the queried databases and could also not be reliably established from the trade name of the suspect PDE5i, which was missing in approximately 52.5% of the relevant ICSRs of tadalafil retrieved from Sanofi CHC’s company database. Unknown delays between dispense, administration, ADR onset and ADR reporting also prohibited reliable classification of ICSRs as “prescription cases” vs. “non-Rx/OTC cases” based on ADR onset date or report date.
Therefore, the annual number of ICSRs over time (i.e., pre and post non-Rx/OTC switch) was displayed and assessed per country, acknowledging that ICSRs reported after the non-Rx/OTC switch date would comprise an unknown mix of ADRs occurring in prescription and non-prescription settings.
To crudely adjust for changes in patient exposure over time, country-specific, annual RR (number of ICSRs per million sold tablets) were calculated for Poland, UK, Norway and Ireland for 2014-2024 on the basis of IQVIA sales data. For New Zealand, sales data were only available for 2016-2024 (Appendix 4).
These analyses were stratified by case seriousness (EVDAS and Sanofi CHC’s global pharmacovigilance database only) and duplicate classification (probable duplicate yes/no; FAERS only).
Case characteristics
ADR summary tabulations on MedDRA PT level were produced from the downloaded case listings and evaluated for any noteworthy trends in the distribution of ADRs over time that could indicate towards potential changes in the characteristics of the ICSRs received before and after the non-Rx/OTC switch.
As possible indicators of change of patient characteristics or drug utilization over time, ICSRs were evaluated for changes of average patient age (FAERS only) and for evidence of concomitant use of contraindicated drugs (EVDAS, FAERS, SMARS and Sanofi CHC’s pharmacovigilance database; Appendix 3).
Results
Overview of Sildenafil
Sildenafil is a medication primarily used to treat erectile dysfunction and pulmonary arterial hypertension. Sildenafil works by relaxing the muscles in the blood vessels of the penis, allowing increased blood flow during sexual stimulation, leading to an erection.
EudraVigilance: The query of EVDAS resulted in a total of 29,752 ICSRs concerning sildenafil with an EV Gateway receipt date ≥01 Jan 2010, of which 1,472 originated from one of the countries of interest. Of these, 392 cases, concerning children, females and/or treatment of PAH, were excluded. The remaining 1,080 cases were included in the analysis (Figure 1).
Figure 1: Flowchart of ICSRs retrieved from EVDAS.
Consistent with high exposure estimates for sildenafil in the UK (Section 3.1.2.3) as well as traditionally high spontaneous ADR reporting rates in the UK, 763 (71%) of the 1,080 cases included in the analysis were reported from the UK [5]. Further 153 cases (14%) were from Norway, 76 cases (7%) from Poland, 45 cases (4%) from Ireland and 43 cases (4%) from New Zealand. The proportion of consumer cases ranged from 14% for Norway to 74% for New Zealand. 487 cases (45%) were serious, including 43 (4%) fatal cases. The proportion of serious cases ranged from 16% for Norway to 52% for UK (Table 1 and 2).
| Country | Healthcare professionals | Consumers | Total | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| New Zealand | 11 | 25.60% | 32 | 74.40% | 43 | 100.00% |
| Poland | 21 | 27.60% | 55 | 72.40% | 76 | 100.00% |
| UK | 395 | 51.80% | 369 | 48.40% | 763 | 100.00% |
| Norway | 132 | 86.30% | 21 | 13.70% | 153 | 100.00% |
| Ireland | 18 | 40.00% | 27 | 60.00% | 45 | 100.00% |
| Total | 577 | 53.40% | 505 | 46.70% | 1080 | 100.00% |
Table 1: Sildenafil/EVDAS: ICSRs by reporter type and country.
| Country | Fatal | Other serious | Non-serious | Total | ||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |
| New Zealand | 1 | 2.30% | 14 | 32.60% | 28 | 65.10% | 43 | 100.00% |
| Poland | 9 | 11.80% | 24 | 31.60% | 43 | 56.60% | 76 | 100.00% |
| UK | 27 | 3.50% | 371 | 48.60% | 366 | 48.00% | 763 | 100.00% |
| Norway | 2 | 1.30% | 22 | 14.40% | 129 | 84.30% | 153 | 100.00% |
| Ireland | 4 | 8.90% | 13 | 28.90% | 28 | 62.20% | 45 | 100.00% |
| Total | 43 | 4.00% | 444 | 41.10% | 594 | 54.20% | 1095 | 100.00% |
Table 2: Sildenafil/EVDAS: ICSRs by seriousness and country.
The distribution of the 1,080 included cases by country and calendar year is shown in Table 3. Approximately one third of the cases (324/1,095 cases) were reported in 2011 alone. Further investigation of this cluster revealed that 239 of these 324 cases (27 serious, 212 non-serious) were reported into EV by a single marketing authorization holder over a 2-day period. No such cluster in 2011 was apparent in FAERS. A small subset of the serious cases could also be identified in FAERS, where they all had event onset dates and/or FDA receipt dates in 2009-2010. Together, these observations suggest that the case cluster seen in EVDAS for 2011 is due to delayed reporting of a batch of cases from earlier years (Table 3).
| EV Gateway Receipt Date | New Zealand | Poland | UK | Norway | Ireland | Total |
|---|---|---|---|---|---|---|
| 2010 | 7 | 2 | 78 | 2 | 7 | 96 |
| 2011 | 18 | 7 | 170 | 114 | 15 | 324 |
| 2012 | 7 | 5 | 28 | 2 | 1 | 43 |
| 2013 | 5 | 4 | 48 | 4 | 1 | 62 |
| 2014 | 2 | 1 | 16 | 0 | 0 | 19 |
| 2015 | 2 | 2 | 16 | 3 | 0 | 23 |
Table 3: Sildenafil, EVDAS: ICSRs by country and calendar year. Shaded cells indicate the year of switch of prescription status in the respective country.
Because relevant variables for identification of case duplicates such as exact patient age or ADR onset date are not available in the EVDAS Level 1 retrievals, reliable identification of duplicate ICSRs was not possible within this dataset. ICSR review did however reveal case clusters suggestive for possible duplicates.
FAERS: The query of FAERS resulted in a total of 47,238 ICSRs with FDA receipt date ≥01 Jan 2010, of which 1,414 originated from one of the countries of interest. Of these, 624 cases, concerning children, females and/or treatment of PAH, were excluded. The remaining 790 cases were included in the analysis (Figure 2).
Figure 2: Flowchart of ICSRs retrieved from FAERS.
Of the 790 relevant cases, 686 (87%) were from the UK, 54 (7%) from Poland, 20 (3%) from Norway, 18 (2%) from New Zealand and 12 (2%) from Ireland. Consistent with the regulatory reporting requirements for foreign ICSRs into FAERS, overall case numbers per country were lower than in EVDAS and all cases but one (99.9%) were serious. The proportion of consumer cases ranged from 15% for Norway to 72% for New Zealand (Table 4).
| Country | Healthcare professionals | Consumers | Total | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| New Zealand | 5 | 27.80% | 13 | 72.20% | 18 | 100.00% |
| Poland | 40 | 74.10% | 14 | 25.90% | 54 | 100.00% |
| UK | 426 | 62.10% | 259 | 37.80% | 686 | 100.00% |
| Norway | 15 | 75.00% | 3 | 15.00% | 20 | 100.00% |
| Ireland | 8 | 66.70% | 4 | 33.30% | 12 | 100.00% |
| Total | 494 | 62.50% | 293 | 37.10% | 790 | 100.00% |
Table 4: Sildenafil, FAERS: ICSRs by reporter type and country.
In the FAERS case set, a high proportion of probable case duplicates (i.e., ICSRs reporting the same country of origin, patient age, suspect and concomitant drugs, ADRs and ADR onset dates but received from different marketing authorization holders and with different Worldwide Unique Case Identification numbers) was apparent: overall, 391 of the 790 relevant cases (49%) were categorized as probable duplicates of other cases included in the case set. In some instances, more than 10 retrieved ICSRs were found to probably refer to the same case. This phenomenon started in 2014, likely as a consequence of the launch of generic sildenafil products, which led to the same ICSRs (e.g., those detected in the scientific literature) being reported multiple times by different marketing authorization holders. The distribution of the 790 cases across country and calendar year is shown in Table 6 (Table 5 and 6).
| Country | Categorized as probable duplicate | Not categorized as probable duplicate | Total | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| New Zealand | 0 | 0.00% | 18 | 100.00% | 18 | 100.00% |
| Poland | 30 | 55.60% | 24 | 44.40% | 54 | 100.00% |
| UK | 352 | 51.30% | 334 | 48.70% | 686 | 100.00% |
| Norway | 5 | 25.00% | 15 | 75.00% | 20 | 100.00% |
| Ireland | 4 | 33.30% | 8 | 66.70% | 12 | 100.00% |
| Total | 391 | 49.50% | 399 | 50.50% | 790 | 100.00% |
Table 5: Sildenafil, FAERS: ICSRs by duplicate categorization and country.
| Initial FDA Received Date | New Zealand | Poland | UK | Norway | Ireland | Total |
|---|---|---|---|---|---|---|
| 2010 | 1 | 1 | 14 | 3 | 2 | 21 |
| 2011 | 0 | 0 | 14 | 1 | 1 | 16 |
| 2012 | 0 | 0 | 13 | 0 | 0 | 13 |
| 2013 | 5 | 1 | 32 | 1 | 1 | 40 |
| 2014 | 0 | 0 | 34 | 0 | 0 | 34 |
| 2015 | 2 | 0 | 47 | 3 | 0 | 52 |
| 2016 | 0 | 0 | 35 | 4 | 0 | 39 |
| 2017 | 0 | 2 | 85 | 0 | 2 | 89 |
| 2018 | 0 | 0 | 67 | 1 | 0 | 68 |
| 2019 | 1 | 4 | 56 | 1 | 0 | 62 |
| 2020 | 1 | 9 | 34 | 2 | 0 | 46 |
| 2021 | 0 | 11 | 99 | 4 | 6 | 120 |
| 2022 | 0 | 22 | 34 | 0 | 0 | 56 |
| 2023 | 8 | 4 | 99 | 0 | 0 | 111 |
| £31 Mar 2024 | 0 | 0 | 23 | 0 | 0 | 23 |
| Total | 18 | 54 | 686 | 20 | 12 | 790 |
Table 6: Sildenafil, FAERS: ICSRs by country and calendar year. Shaded cells indicate the year of switch of prescription status in the respective country.
SMARS: The query of the New Zealand SMARS database revealed 43 cases, 37 of which were deemed relevant for this analysis. No obvious case duplicates were identified. Further information on data retrieved from SMARS is presented in below section (Figure 3).
Figure 3: Flowchart of ICSRs retrieved from SMARS.
Individual countries
In the following subsections, trends of specific countries over time are further described. Countries are presented in chronological order of the switch of sildenafil to non-prescription status. In the following analyses, the annual number of ICSRs and county-specific annual reporting rate is presented for the cases retrieved from EVDAS and FAERS. EVDAS data are stratified by seriousness and FAERS data are stratified by duplicate classification. In addition, potential changes in case characteristics over time are presented. For New Zealand, ICSRs retrieved from SMARS are additionally presented.
New Zealand
Since October, 2014 in New Zealand, pharmacists are supplying Sildenafil for the treatment of ED without prescription.
Number of ICSRs and reporting rates over time: The annual number of relevant ICSRs retrieved from EVDAS and FAERS, stratified by seriousness, are shown in Tables 7 and 8, respectively. There was no indication of a significant increase in the number of reports following the non-Rx/OTC switch in 2014. Because most cases in EVDAS and FAERS were reported before 2016 whereas annual sales data were available only from 2016 onwards, calculation of meaningful reporting rates was not possible (Table 7 and 8).
| Year | Fatal | Other serious | Non-serious | Total |
|---|---|---|---|---|
| 2010 | 0 | 2 | 5 | 7 |
| 2011 | 0 | 1 | 17 | 18 |
| 2012 | 0 | 1 | 6 | 7 |
| 2013 | 1 | 4 | 0 | 5 |
| 2014 | 0 | 2 | 0 | 2 |
| 2015 | 0 | 2 | 0 | 2 |
| 2016 | 0 | 0 | 0 | 0 |
| 2017 | 0 | 0 | 0 | 0 |
| 2018 | 0 | 0 | 0 | 0 |
| 2019 | 0 | 0 | 0 | 0 |
| 2020 | 0 | 0 | 0 | 0 |
| 2021 | 0 | 0 | 0 | 0 |
| 2022 | 0 | 0 | 0 | 0 |
| 2023 | 0 | 2 | 0 | 2 |
| ≤ 01 May, 2024 | 0 | 0 | 0 | 0 |
| Total | 1 | 14 | 28 | 43 |
Table 7: EVDAS, Sildenafil, New Zealand: Number of relevant ICSRs retrieved, by seriousness. Shaded row indicates the year of switch of prescription status.
| Year | Fatal | Other serious | Non-serious | Total |
|---|---|---|---|---|
| 2010 | 0 | 1 | 0 | 1 |
| 2011 | 0 | 0 | 0 | 0 |
| 2012 | 0 | 0 | 0 | 0 |
| 2013 | 1 | 4 | 0 | 5 |
| 2014 | 0 | 0 | 0 | 0 |
| 2015 | 0 | 2 | 0 | 2 |
| 2016 | 0 | 0 | 0 | 0 |
| 2017 | 0 | 0 | 0 | 0 |
| 2018 | 0 | 0 | 0 | 0 |
| 2019 | 0 | 1 | 0 | 1 |
| 2020 | 0 | 1 | 0 | 1 |
| 2021 | 0 | 0 | 0 | 0 |
| 2022 | 0 | 0 | 0 | 0 |
| 2023 | 0 | 8 | 0 | 8 |
| ≤ 31 Mar, 2024 | 0 | 0 | 0 | 0 |
| Total | 1 | 17 | 0 | 18 |
Table 8: FAERS, Sildenafil, New Zealand: Number of relevant ICSRs retrieved, by seriousness. Shaded row indicates the year of switch of prescription status.
There was no indication of a trend towards a greater proportion of serious or fatal cases in either database after the switch to nonprescription status. A cluster of cases in FAERS (and SMARS) but not in EVDAS in 2023 mostly comprised reports of perceived drug ineffectiveness in the ED indication. It is unclear why these cases were classified as serious in FAERS.
The number of relevant ICSRs retrieved from SMARS and the corresponding reporting rates are presented in Table 9. Because sales data were only available for 2016-2024, annual reporting rates could only be calculated for the post non-Rx/OTC switch period. These rates were consistently <1 per 1 million sold tablets until 2019 and <2 per 1 million sold tablets in 2020 and 2023.
| Year | Number of ICSRs | Exposure2 (million tablets sold) | Reporting rate(Number of ICSRs per million sold tablets) | ||
|---|---|---|---|---|---|
| Serious1 | Total | Serious | Total | ||
| 2010 | 0 | 3 | NA | 0 | NA |
| 2011 | 0 | 3 | NA | 0 | NA |
| 2012 | 1 | 4 | NA | NA | NA |
| 2013 | 0 | 2 | NA | 0 | NA |
| 2014 | 3 | 5 | NA | NA | NA |
| 2015 | 0 | 3 | NA | 0 | NA |
| 2016 | 0 | 2 | 2.21 | 0 | 0.9 |
| 2017 | 0 | 1 | 2.43 | 0 | 0.41 |
| 2018 | 0 | 1 | 2.72 | 0 | 0 |
| 2019 | 0 | 2 | 2.95 | 0 | 0.34 |
| 2020 | 0 | 3 | 2.05 | 0 | 1.46 |
| 2021 | 0 | 1 | 3.19 | 0 | 0.31 |
| 2022 | 0 | 0 | 3.34 | 0 | 0 |
| 2023 | 1 | 6 | 3.49 | 0.28 | 1.72 |
| 20243 | 0 | 0 | 11.5 | 0 | 0 |
| Total | 5 | 37 | - | - | - |
Table 9: Sildenafil, New Zealand: Number of relevant ICSRs retrieved from SMARS and corresponding reporting rates. Shaded row indicates the year of switch of prescription status.
The majority of the ICSRs were reported in the year 2023 in which most frequently reported PT was Drug ineffective (n=5). No specific trend in adverse event reporting was observed.
Case characteristics: The ADRs included in the ICSRs retrieved from EVDAS, FAERS and SMARS respectively. From the limited number of ICSRs reported, no noteworthy ADR clusters or trends over time were observed.
Patient age, reported in 26 cases retrieved from SMARS, ranged from 39 to 81 years (median, 63.5 years). No time trend in patient age was apparent.
No contraindicated medicines were reported as concomitant or cosuspect drugs in any of the ED cases from New Zealand retrieved from any of the three databases.
Poland
Sildenafil is available as non-prescription medicine for treatment of ED in Poland since May, 2016.
Number of ICSRs and reporting rates over time: The annual number of relevant cases and corresponding annual reporting rates for ICSRs retrieved from EVDAS (stratified by seriousness) and from FAERS (stratified by duplicate classification) for 2014 to 2024, are shown in Figures 4 and 5, respectively. The trend towards increasing annual ICSR numbers coincided with an almost 5-fold increase in exposure between 2014 and 2023 (full year data is not available for year 2024, hence data of 2023 was taken for comparison). Consequently, exposure-adjusted reporting rates did not increase after the non-Rx/OTC switch in 2016. Increasing case numbers also need to be seen against the backdrop of a general increase in spontaneous ADR reporting observed in Europe. Moreover, for FAERS, more than half of the serious cases reported from Poland in recent years were determined to be probable case duplicates (Figures 4 and 5).
Figure 4: EVDAS/Sildenafil/Poland – Number of ICSRs and reporting rates, by seriousness.
Figure 5: FAERS/Sildenafil/Poland – Number of ICSRs and reporting rates, by duplicate classification.
The annual ICSR reporting rates for Poland were notably lower than for the other countries included in this analysis. This observation is consistent with the traditionally low rate of spontaneous ADR reporting from Poland [5].
Case characteristics: The ADRs included in the ICSRs retrieved from EVDAS and FAERS, respectively. A cluster of the ADRs ‘Death’ (N=7) in 2022 was identified. ICSR review revealed 7 spontaneous cases each describing ‘Intentional product use issue’ and ‘Death’ and reported into EV/FAERS by one marketing authorization holder on the same day. The cases provide only minimal information, prohibiting further assessment. Otherwise, no noteworthy ADR clusters or trends over time were observed.
Patient age was reported in 32 of the 54 cases retrieved for Poland from FAERS and ranged from 30 to 71 years (median, 64 years). No time trend in patient age was apparent.
No contraindicated medicines were reported as concomitant or cosuspect drugs in any of the ED cases from Poland retrieved from either database.
United Kingdom
Sildenafil is available as non-prescription medicine for treatment of ED in the UK since March, 2018.
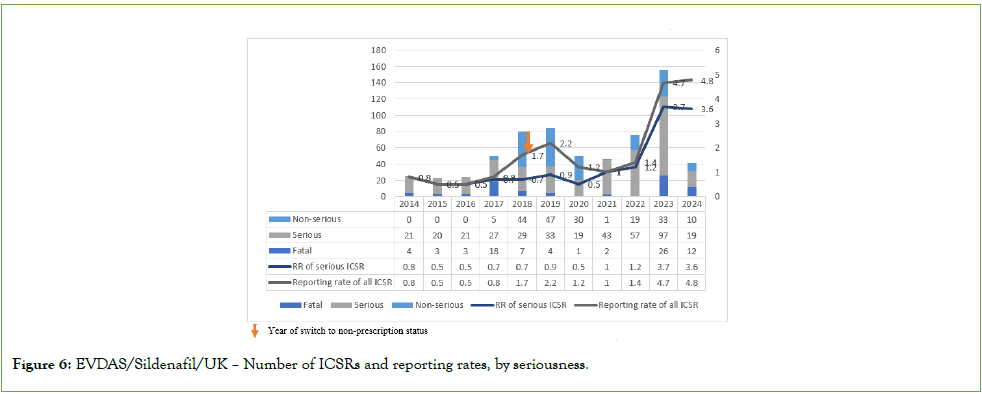
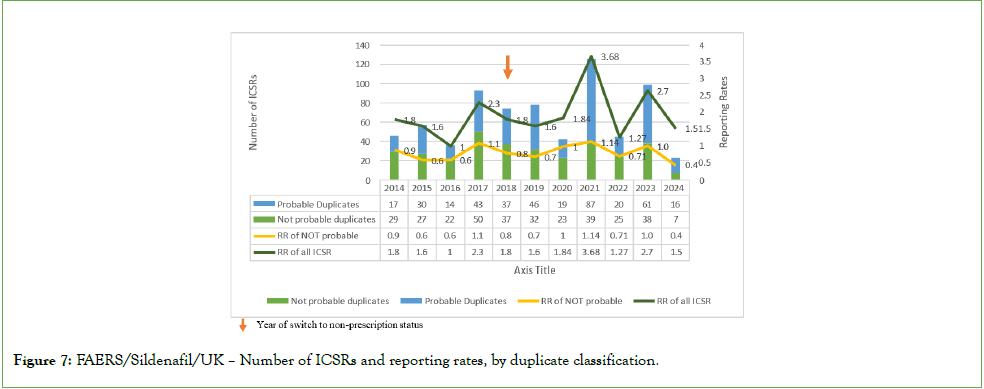
Number of ICSRs and reporting rates over time: Consistent with high exposure estimates for sildenafil in the UK as well as a traditionally high spontaneous ADR reporting rate, the majority of the ICSRs selected from EVDAS and FAERS (71.93% and 87.30%, respectively) were received from the UK (5). An increase in ICSR numbers over time was observed. This trend coincided with an approximately 75% increase in exposure between 2014 and 2022 and also needs to be seen against the backdrop of a general increase in spontaneous ADR reporting observed in Europe in recent years. Annual exposure-adjusted ICSR reporting rates were consistently very low (<4 to 5 ICSRs per million sold tablets per year) but showed a transient increase for non-serious cases in 2018- 2019 and increase for serious cases in 2023 (Figure 6). In FAERs, this increase was found to predate the non-Rx/OTC switch and to be mostly explained by reporting of probable duplicates (Figure 7). There was no indication of a trend towards a greater proportion of fatal cases after the switch to non-prescription status in either database.
Figure 6: EVDAS/Sildenafil/UK – Number of ICSRs and reporting rates, by seriousness.
Figure 7: FAERS/Sildenafil/UK – Number of ICSRs and reporting rates, by duplicate classification.
Case characteristics: The ADRs included in the ICSRs retrieved from EVDAS and FAERS, respectively. Possible clusters of individual ADRs (MedDRA PTs) after 2018 were consistently found to be artefacts, mostly due to probable case duplicates:
A possible trend towards higher numbers of ADRs from the SOC “Nervous system disorders” was observed in both databases which was primarily attributed to a higher incidence of specific adverse events such as ‘Headache’, ‘Loss of consciousness’, ‘Syncope’, ‘Dizziness’ and ‘Subarachnoid haemorrhage’. Review of the corresponding cases in FAERS revealed that most of the cases reported in or after 2018 were duplicates (16 of the 29 cases reporting Headache were duplicates, 17 of the 23 cases reporting Syncope were duplicates, 16 of the 17 cases reporting Loss of consciousness were duplicates and 12 of the 21 cases reporting dizziness were duplicates). This high proportion of duplicate reports suggests that the actual number of unique adverse events may be considerably lower than initially indicated by the raw data. Two of the 8 cases reporting ‘Subarachnoid haemorrhage’ were identified as probable duplicates of a case initially reported in 2009 and further 5 cases were probable duplicates of a single case occurring in 2019.
Since 2018, there has been a notable increase in Adverse Drug Reactions (ADRs) reported within the System Organ Class (SOC) of Vascular disorders from FAERS. This rise was attributed to reports of known undesirable effects, ‘Flushing’, ‘Orthostatic hypotension’, ‘Hypotension’ and ‘Hot flush’.
In FAERS, 32 cases reported AE of Flushing, 15 cases reported orthostatic hypotension, 13 cases reported hypotension and 12 cases reported for hot flush. More than half of these above mentioned cases were probable duplicates. (22 out of 32 cases reporting Flushing were duplicates, 12 out of 15 cases reporting Orthostatic hypotension were duplicates, 9 out of 13 cases reporting Hypotension were duplicates and 9 out of 12 cases reporting Hot flush were duplicates).
Review of a cluster of reports of ‘Cardiac arrest’ (14 ADRs in FAERS in 2021) revealed that 11 of these 14 cases are probable duplicates referring to the same patient. The case describes a lifethreatening suspected drug-drug interaction with an alpha receptor agonist and a beta blocker, which are listed drug interactions where particular caution is advised. Further information on this case is not available. Of the other 3 ICSRs, one is a literature report where exposure to sildenafil predated the non-Rx/OTC switch and one is a published report from the Netherlands describing death primarily attributed to illicit use of 3-methylmethcathinone; both cases are thus unrelated to the non-Rx switch in the UK. In the remaining third article, radiological and clinical findings of HIV associated pneumonia and patient being a heavy smoker are risk factor for cardiac arrest leading to fatality.
Patient age was reported for 541 of the 770 cases retrieved from FAERS and ranged from 22 to 91 years (median, 59 years). No time trend in patient age was apparent.
No contraindicated medicines were reported as concomitant or cosuspect drugs in any of the ED cases from the UK retrieved from either database.
Norway
Sildenafil is available as non-prescription medicine for treatment of ED in Norway since August, 2019.
Number of ICSRs and reporting rates over time: ICSR numbers and reporting rates over time for cases retrieved from EVDAS and FAERS are shown in Figures 8 and 9, respectively. Based on small case numbers, a possible trend towards higher in case numbers in EVDAS is apparent; however, this trend started several years before the non-Rx switch in Norway and was mostly due to non-serious cases.
Figure 8: EVDAS/Sildenafil/Norway – Number of ICSRs and reporting rates, by seriousness.
Figure 9: FAERS/Sildenafil/Norway – Number of ICSRs and reporting rates, by duplicate classification.
Increasing case numbers also need to be seen against the backdrop of a general increase in spontaneous ADR reporting observed in Europe.
No fatal cases were retrieved for Norway from either database since non-Rx switch (August, 2019).
Case characteristics: The ADRs included in the ICSRs retrieved from EVDAS and FAERS. No noteworthy ADR clusters or trends over time were observed.
Patient age was reported for 17 of the 22 cases retrieved from FAERS and ranged from 31 to 81 years (median, 63 years). No time trend in patient age was apparent.
No contraindicated medicines were reported as concomitant or cosuspect drugs in any of the ED cases from Norway retrieved from either database.
Ireland
Sildenafil is available as non-prescription medicine for treatment of ED in Ireland since January, 2021.
Number of ICSRs and reporting rates over time: The annual number of relevant cases and corresponding annual reporting rates for ICSRs retrieved from EVDAS, stratified by seriousness, are shown in Figure 10. There was no increase in case numbers or reporting rates during the limited period since non-Rx switch in Ireland.
Figure 10: EVDAS/Sildenafil/Ireland – Number of ICSRs and reporting rates, by seriousness.
Of the 17 cases retrieved for Ireland from FAERS for Ireland (Table 6), 4 cases were classified as probable duplicates. In view of the low case numbers, ICSR reporting rates for cases retrieved from FAERS for Ireland were not calculated.
Case characteristics: The ADRs included in the ICSRs retrieved from EVDAS and FAERS respectively. From the limited number of ICSRs reported in EVDAS and FAERS, no noteworthy ADR clusters or trends over time were observed.
Patient age was reported for 18 of the 20 cases retrieved from FAERS and ranged from 28 to 69 years (median, 59 years). No time trend in patient age was apparent.
No contraindicated medicines were reported as concomitant or cosuspect drugs in any of the ED cases from Ireland retrieved from either database.
Overview of Tadalafil
Poland
Tadalafil is available as OTC medicine for treatment of ED in Poland since March, 2022.
The query of EVDAS for cases concerning tadalafil resulted in a total of 13 946 ICSRs with an EV Gateway receipt date ≥ 01 Jan, 2010 of which 15 ICSRs originated from Poland (Figure 11). The query of FAERS yielded 27,569 ICSRs concerning tadalafil with FDA receipt date ≥ 01 Jan, 2010 of which 23 ICSRs originated from Poland. All cases retrieved from EVDAS and FAERS for Poland treated for ED or unknown indications and were included in the analysis.
Figure 11: EVDAS, Tadalafil, Poland: Number of ICSRs and Reporting rate.
Number of ICSRs and reporting rates over time: Almost all cases in both databases were reported in 2022. Case review in FAERS suggested that 17 of the 23 cases were probable duplicates, of which 13 probable duplicates created from a single literature report that was published in February 2022 (prior to tadalafil becoming available as non-prescription medicine). Thus, there was no increase in the number of reports after the tadalafil OTC switch in Poland (Tables 10 and 11).
| Year | Fatal | Other serious | Non-serious | Total |
|---|---|---|---|---|
| 2010 | 0 | 0 | 0 | 0 |
| 2011 | 0 | 0 | 0 | 0 |
| 2012 | 0 | 0 | 1 | 1 |
| 2013 | 0 | 0 | 0 | 0 |
| 2014 | 0 | 0 | 0 | 0 |
| 2015 | 0 | 0 | 0 | 0 |
| 2016 | 0 | 0 | 0 | 0 |
| 2017 | 0 | 0 | 0 | 0 |
| 2018 | 0 | 0 | 0 | 0 |
| 2019 | 0 | 0 | 0 | 0 |
| 2020 | 0 | 0 | 0 | 0 |
| 2021 | 0 | 1 | 2 | 3 |
| 2022 | 0 | 8 | 1 | 9 |
| 2023 | 0 | 1 | 0 | 1 |
| ≤ 30 April, 2024 | 0 | 0 | 1 | 1 |
| Total | 0 | 10 | 5 | 15 |
Table 10: EVDAS, Tadalafil, Poland: Number of relevant ICSRs retrieved, by seriousness. Shaded row indicates the year of switch of prescription status.
| Year | Fatal | Other serious | Non- serious | Total | RR of not probable | RR of all ICSR |
|---|---|---|---|---|---|---|
| 2010 | 0 | 0 | 0 | 0 | - | - |
| 2011 | 0 | 0 | 0 | 0 | - | - |
| 2012 | 0 | 0 | 0 | 0 | - | - |
| 2013 | 0 | 0 | 0 | 0 | - | - |
| 2014 | 0 | 0 | 0 | 0 | - | - |
| 2015 | 0 | 0 | 0 | 0 | - | - |
| 2016 | 0 | 0 | 0 | 0 | - | - |
| 2017 | 0 | 0 | 0 | 0 | - | - |
| 2018 | 0 | 0 | 0 | 0 | - | - |
| 2019 | 0 | 0 | 0 | 0 | - | - |
| 2020 | 0 | 0 | 0 | 0 | - | - |
| 2021 | 0 | 1 | 0 | 1 | 0.17 | 0.17 |
| 2022 | 0 | 19 | 0 | 19 | 0.18 | 0.02 |
| 2023 | 0 | 3 | 0 | 3 | 0.16 | 0.16 |
| 2024 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 0 | 23 | 0 | 23 | - | - |
Table 11: FAERS, Tadalafil, Poland: Number of relevant ICSRs retrieved, by seriousness. Shaded row indicates the year of switch of prescription status.
In view of the low total number of cases and the high number of probable duplicates, ICSR reporting rates were not calculated. Case characteristics
The ADRs included in the ICSRs retrieved from EVDAS and FAERS respectively. The listings reflect the repetitive reporting in particular of a single literature report primarily describing the occurrence of back pain in a patient with depression that was reported together with a number of additional events including tachycardia, tachypnoea and various neuropsychiatric events. No other noteworthy ADR clusters or trends over time were observed.
No time trend in patient age was apparent.
No contraindicated medicines were reported as concomitant or cosuspect drugs in any of the tadalafil cases from Poland retrieved from either database.
United Kingdom
Tadalafil is available as non-Rx medicine for treatment of ED in UK since June, 2023.
Number of ICSRs and reporting rates over time (EVDAS): The number of ICSRs reported overtime for tadalafil in UK is presented in Table 12. The higher RR from 2023-2024 was observed in serious ICSRs, of which majority accounted for the fatal outcome (N=41). In these fatal ICSRs, majority of the ICSRs (N=39; 95.1%) reported unspecified cause of death (PT-Death), with no relevant information reported. And among these 39 fatal cases, majority (N=25; 64.1%) of the cases were reported in elderly group patients (Table 12 and Figure 12).
| Year | Fatal | Other serious | Non- serious | Total | RR of not probable | RR of all ICSR |
|---|---|---|---|---|---|---|
| 2010 | 0 | 0 | 0 | 0 | - | - |
| 2011 | 0 | 0 | 0 | 0 | - | - |
| 2012 | 0 | 0 | 0 | 0 | - | - |
| 2013 | 0 | 0 | 0 | 0 | - | - |
| 2014 | 0 | 0 | 0 | 0 | - | - |
| 2015 | 0 | 0 | 0 | 0 | - | - |
| 2016 | 0 | 0 | 0 | 0 | - | - |
| 2017 | 0 | 0 | 0 | 0 | - | - |
| 2018 | 0 | 0 | 0 | 0 | - | - |
| 2019 | 0 | 0 | 0 | 0 | - | - |
| 2020 | 0 | 0 | 0 | 0 | - | - |
| 2021 | 0 | 1 | 0 | 1 | 0.17 | 0.17 |
| 2022 | 0 | 19 | 0 | 19 | 0.18 | 0.02 |
| 2023 | 0 | 3 | 0 | 3 | 0.16 | 0.16 |
| 2024 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 0 | 23 | 0 | 23 | - | - |
Table 12: Pattern of cases reported overtime from EVDAS in UK.
Figure 12: EVDAS/Tadalafil/United Kingdom – Number of ICSRs, by seriousness and reporting rate.
Other serious reported PTs were Drug ineffective (n=19), Headache (n=13), Chest pain (n=11) and Myocardial infarction (n=10). No specific trend was observed in the remaining serious events (less than 2% of the total events).
Number of ICSRs and reporting rates over time (FAERS): The number of ICSRs reported overtime for tadalafil in UK is presented in Table 13. In FAERS data, increase in RR in 2023 could be possibly due to high number of duplicates. In these ICSRs, the majority of the events reported were with PT Death (n=20) and Drug ineffective (n=6). No specific trend was noted for the remaining PTs. A total of 102 Tadalafil cases were reported in Sanofi CHC global company safety database. Of these 102 cases ,52 cases were pertaining to Cialis OTC cases which accounts for a total of 50.9% of the total tadalafil cases. In these 52 cases, only 1 case was reported with fatal outcome (unspecified cause of death with limited information) (Table 13 and Figure 13).
| Year | Not probable duplicates | Probable duplicates | Total ICSRs | RR of not probable duplicate |
RR of all ICSR |
|---|---|---|---|---|---|
| 2010 | 17 | 2 | 19 | NA | NA |
| 2011 | 21 | 0 | 21 | ||
| 2012 | 16 | 0 | 16 | ||
| 2013 | 8 | 0 | 8 | ||
| 2014 | 12 | 0 | 12 | ||
| 2015 | 9 | 1 | 10 | ||
| 2016 | 1 | 0 | 1 | ||
| 2017 | 8 | 0 | 8 | ||
| 2018 | 7 | 0 | 7 | ||
| 2019 | 9 | 13 | 22 | ||
| 2020 | 4 | 4 | 8 | 0.35 | 0.71 |
| 2021 | 11 | 24 | 35 | 0.61 | 1.95 |
| 2022 | 4 | 7 | 11 | 0.2 | 0.55 |
| 2023 | 26 | 67 | 93 | 1.1 | 3.91 |
| 2024 | 7 | 19 | 26 | 0.61 | 2.25 |
| Total | 160 | 137 | 19 | - | - |
Table 13: Pattern of cases reported overtime from FAERS in UK.
Figure 13: FAERS/Tadalafil/United Kingdom – Number of ICSRs.
The ADRs included in the ICSRs retrieved from EVDAS and FAERS.
Sanofi global company safety database: The ADRs included in the ICSRs retrieved from Sanofi CHC’s global company safety database.
Discussion
This comprehensive safety analysis leveraged multiple large-scale pharmacovigilance databases, including EudraVigilance, FAERS and SMARS, to gather extensive real-world safety data spanning various countries. This study examined trends over an extended period from 2010 to 2024, enabling a comparison between pre- and post-OTC switch periods, with results presented as reporting rates. The analysis was multi-faceted, encompassing: Overall adverse event reporting rates, specific types of adverse events, patient demographic characteristics and potential misuse patterns. By utilizing diverse databases, covering an extended timeframe, adjusting for exposure and examining a wide range of safety parameters across multiple countries, this study provides a comprehensive and robust evaluation of real-world safety trends following the switch of these medications to over-the-counter status. A total of 820 cases pertaining to tadalafil were reported cumulatively since Jan, 2010 until July, 2024 from the Sanofi CHC global company safety database. Of these 820 cases, 183 cases were reported as serious (29 fatal) and the remaining 637 cases as nonserious.
Of these total cases, 482 cases were considered relevant as per the inclusion criteria mentioned in methodology. Among these 482 cases, 227 cases reported indication of ED and the remaining 255 cases reported unknown indication. These included 482 cases pertained to 1055 events with most commonly reported PT Drug ineffective (n=189) followed by Headache (n=52). During the FDA, EMA and other regulatory authorities provide (limited) public access to their post-marketing pharmacovigilance databases, allowing for retrieval of information about reported suspected ADRs. These databases are a valuable source to obtain real-world data on usage patterns and safety profile of medicines. However, they are primarily designed to support the post-marketing safety surveillance programs of authorities in the respective country or region and do not provide a complete picture of ADRs occurring in the post-marketing setting. The content of these databases relies on voluntary spontaneous reporting and is further determined by the reporting requirements of the respective country or region. For example, whereas ICSRs describing serious or non-serious ADRs will be included in the respective national/regional database, ICSRs containing only non-serious ADRs might not be exchanged worldwide.
The usual limitations of post-marketing pharmacovigilance systems are reflected in these databases, such as regional differences and temporal trends in reporting, heterogeneous quality and completeness of the original reports, potential for bias (e.g., notoriety bias or stimulated reporting in the context of litigation) and variable level of evidence that the reported ADR was caused by the suspect medicinal product [6,7].
For this analysis, ICSRs could not be classified as reports from prescription vs. non-prescription settings, as this information was not reported in the retrieved datasets and could also not be inferred from medicinal product tradenames, which were missing in most cases. Variable and mostly unknown latencies between product (whole) sale, dispense, use, ADR onset and ICSR reporting into the regulatory databases also prohibited use of ADR onset or report dates for robust classification of ICSRs as pre or post non- Rx/OTC reports. For example, the average latency between ADR onset (where reported) and initial receipt date in FAERS was approximately 1 year (range, 4 days to 22 years). Such latencies might have also biased the calculated reporting rates.
The higher rates of safety information from UK, could be possible due to the regulatory framework (MHRA and National Health Service), reporting culture via the Yellow card scheme and comprehensive data collection by the pharmacist before dispensing the non-Rx medication.
With the exception of the UK, annual numbers of relevant cases retrieved for the investigated countries were small, thereby impeding comparative analyses over time. Analyses were further complicated by the presence of case duplicates: almost half of the relevant cases retrieved from FAERS were identified as probable duplicates, with 10 or more unique ICSRs found to probably refer to the same patient in some instances. This phenomenon started in 2014 as a consequence of the launch of generic sildenafil products and significantly affected overall ICSR numbers, counts of individual ADRs as well as exposure-adjusted reporting frequencies.
Overall, the analyses presented in this document, in particular any comparisons over time, across regions or between products are therefore prone to potentially significant bias. All presented analyses should therefore be considered exploratory in nature and have to be used with caution to evaluate absence of risk. However, the results of the present analysis did not indicate towards any change in usage patterns of sildenafil or tadalafil or noteworthy trends in the incidence or nature of reported ADRs that could not be explained as an artefact of one or more of the above methodological limitations.
Increases over time in annual ICSR numbers were observed in most countries. These trends occurred in a context of increasing exposure in each country and against the backdrop of a general increase in spontaneous ADR reporting observed in Europe in recent years. For example, annual reporting into EV has increased by approximately 80% between 2014 and 2019 [8]. All observed trends in case numbers were ameliorated when case numbers were adjusted for patient exposure and restricted to cases unlikely to be duplicates and/or did not reasonably correlate with the date of the non-Rx/OTC switch in the respective country.
Conclusion
The ADRs included in the retrieved ICSRs were overall consistent with the known safety profile of sildenafil and tadalafil. No ADR clusters or trends over time were observed that could not be explained by one of the bias sources discussed above. There was also no indication for a change of patient characteristics or drug utilization over time. Specifically, no time trend in patient age was apparent and no cases describing the use of a contraindicated medicine were identified. In conclusion, from this analysis of realworld data from large, diverse databases, there was no indication of a change in the usage patterns of sildenafil or tadalafil for treatment of ED, of trends in the incidence or nature of reported ADRs or any other safety signal that could be reasonably associated with a switch of these medicines from prescription to non-prescription status.
Funding
This study and preparation of this article were funded by Sanofi CHC.
Availability of Data and Materials
Data are avWWailable from the corresponding author upon request.
References
- Tzoumas N, Farrah TE, Dhaun N, Webb DJ. Established and emerging therapeutic uses of PDE type 5 inhibitors in cardiovascular disease. Br J Pharmacol. 2020;177(24):5467-5488.
[Crossref] [Google Scholar] [PubMed]
- Gul M, Serefoglu EC. An update on the drug safety of treating erectile dysfunction. Expert Opin Drug Saf. 2019;18(10):965-975.
[Crossref] [Google Scholar] [PubMed]
- Yafi FA, Sharlip ID, Becher EF. Update on the safety of phosphodiesterase type 5 inhibitors for the treatment of erectile dysfunction. Sex Med Rev. 2018;6(2):242-252.
[Crossref] [Google Scholar] [PubMed]
- Reffelmann T, Kloner RA. Pharmacotherapy of erectile dysfunction: Focus on cardiovascular safety. Expert Opin Drug Saf. 2005;4(3):531-540.
[Crossref] [Google Scholar] [PubMed]
- Aagaard L, Strandell J, Melskens L, Petersen PS, Hansen EH. Global patterns of adverse drug reactions over a decade: Analyses of spontaneous reports to VigiBase™. Drug Saf. 2012;35:1171-1182.
[Crossref] [Google Scholar] [PubMed]
- ENCePP Guide on Methodological Standards in Pharmacoepidemiology. 2022
- Neha R, Subeesh V, Beulah E, Gouri N, Maheswari E. Existence of notoriety bias in FDA adverse event reporting system database and its impact on signal strength. Hosp Pharm. 2021;56(3):152-158.
[Crossref] [Google Scholar] [PubMed]
- 2014 Annual Report on EudraVigilance for the European Parliament, the Council and the Commission. 2014.
Citation: Waring M, Gill A (2024). Impact of Switch to Non-Prescription Status on the Safety Profile of Sildenafil and Tadalafil: Assessment of Pharmacovigilance Databases. J Pharmacovigil. 12:497.
Copyright: © 2024 Waring M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.