Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- CiteFactor
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2020) Volume 11, Issue 5
Impact of Fusarium graminearum on Seed Germination and Seedling Blight in Hard Red Spring Cultivars in South Dakota
Navjot Kaur, Sunish K. Sehgal, Karl D Glover, Emmanuel Byamukam and Shaukat Ali*Received: 01-Apr-2020 Published: 11-May-2020, DOI: 10.35248/2157-7471.20.11.495
Abstract
Fusarium graminearum causes crown rot in wheat affecting seed germination, seedling establishment hence impacting crop productivity. Integrated disease management approaches includes cultural practices, use of good quality seed, resistant varieties, seed treatment. In this study, we surveyed wheat plant samples from South Dakota to determinethe prevalence and distribution of root rot pathogens. F. graminearum and Bipolaris sorokiniana were recovered from 100% and 48% of the root samples (n=39) collected, respectively. Further, we studied the effect of F. graminearum on the seed germination and seedling blight on spring wheat cultivars under greenhouse and field conditions. F. graminearum significantly affected the seed germination and seedling blight in 11 spring wheat cultivars, ranging from 75-96% and 0-9%, respectively in infested seed as compared to uninfested seed (control). The cultivars ‘Forefront’ and ‘Select’ were least impacted by the pathogen; whereas, ‘Russ’ and ‘Oxen’ showed a significant reduction in germination and higher seedling blight. Field experiments were conducted to determine the impact of source of inoculum, fungicide seed treatment and varieties on seed germination and seedling survival at two locations in 2015 and 2016. Cultivars Russ and Oxen showed poor seed germination and high seedling blight; whereas, Forefront, Selectand Briggs had higher germination and seedling survival. Fungicide treatment significantly increased seed germination. The source of inoculum was significant in 2015, however, it was not significant in 2016 due to less disease pressure. Our results showed that F. graminearum lowers seed germination and causes seedling blight in wheat cultivars and genetic variability exists incultivars. Hence the use of fungicide seed treatment can reduce the germination losses caused by the pathogen for fields likely to have high inoculum pressure or likely infested seed and use of tolerant cultivars like Forefront can further reduce the losses due to crown and root rot diseasent.
Keywords
Crown root rot; Fusarium graminearum; Seed germination; Seedling blight; Seed treatment
Introduction
Wheat is a priniciple staple food crop for 1/3rd of the world population and is the most widely cultivated, traded food crop, and on an average provides 21% calories and 21% protein to humans respectively [1,2]. Sustainable wheat production has contributed to the economic growth and social stabilization in many regions of the world. However, with changing weather pattern and farming practices continuously pose a threat to wheat production around the world. No-Till farming practiced with increased residue and favorable climate conditions are leading to an increase in residue and seed-borne disease [3-5]. Presence of the residue of the previous year crop can increase the primary inoculum level in the subsequent season crop along with the other cultural practices for disease management approaches [6,7]. There are many diseases that impact yield and quality of the wheat crop including Fusarium Head blight (FHB), Rust diseases and Leaf spot diseases. Along with these, there is also root rot complex which impact the seed germination and seedling blight in wheat such as Fusarium crown rot and common root rot caused by Fusarium graminearum and Bipolaris sorokiniana respectively.
Fusarium crown rot (FCR) of wheat caused by Fusarium graminearum ((Schwein.) Petch), impacts plant stand by affecting seed germination and inciting seedling blight thus ultimately decreasing crop productivity [8-10]. Yield losses of up to 24% have been reported in winter wheat plots when the seed was infested by F. graminearum in New York [11], however, the seed germination and yield loss due to F. graminearumcan be as high as 80% [12]. Further during the Fusarium head blight pressure, the seed infection with F. graminearum increased from 20% to more than 98% at maturity in both resistant and susceptible genotypes, respectively and this resulted in low germination (80%) and seed vigor in seeds inoculated with infected corn kernels [13].
The crown and root rot disease in wheat can be managed through integrated approaches, crop rotations, seed treatment, and resistant varieties. Crop rotation is one of the effective method of reducing the risk of root rot in wheat, thus wheat should be rotated with non-jost crops such as broadleaf crops such as soybean, canola, dry beans, flax, and sunflower [7]. Seed treatment with fungicides can reduce loss in seed germination and seedling blight and increased the plant emergence and number of spikes per m2 as compared to the untreated control in winter wheat varieties under field conditions [14-16]. Although seed treatment enhanced plant emergence in a seed lot infected with F. graminearum, however, it did not prevent the pathogen growth from seed to seedlings [16]. Further, the effect of fungicides as a seed treatment in improving the germination of Fusarium infected-seed lots are variable and effective (64-84% increase in germination) when seeds were treated with fungicides; Vitavax Extra RTU and Dividend as a seed treatment under controlled conditions, however, the results were not significant under field conditions [17]. Growing resistant varieties is considered the best management strategy for crown rot in wheat [18]. Significant differences in germination and yield among spring wheat and winter wheat genotypes were observed when F. pseudograminearum inoculated plots were compared with non-inoculated plots [19-21]. Resistant wheat and barley genotypes have traditionally shown reduced yield loss under field conditions as compared to susceptible cultivars [20,21].
Several reports suggest an increasing trend in the incidence of root rot diseases in different parts of the United States [4,8,9,11,22,23]. However, there is no information available on the effects of these root rot pathogens on spring wheat cultivars grown in South Dakota and northern Great Plains region and their impact on seed germination and seedling blight. It is therefore vital to know the prevalence of root rot pathogens in the state and their effects on seed germination and seedling blight in wheat cultivars grown in the region and devise disease management strategies for root rot diseases. The specific objectives of this study were 1) to determine the prevalence and distribution of root rot pathogens of wheat across in South Dakota and 2) determine the effects of F. graminearum on seed germination and seedling blight in hard red spring wheat cultivars grown in the region.
Materials and Methods
Roots samples collection
Thirty-nine samples were collected from randomly chosen winter wheat and spring wheat fields from 17 counties (Aurora, Brule, Buffalo, Brown, Edmunds, Hand, Hughes, Hyde, Jerauld, Kingsbury, Lincoln, Minor, Potter, Sanborn, Spink, Tripp, and Walworth) during the growing season of 2014 and 2015. From each field, 8-10 non-symptomatic plants root samples were randomly collected at flowering to early milk stage (Feekes 10.5 -11.1) without damaging the roots. The root portions were separated by cutting the plants above the crown and samples were kept in the paper bags. The collected samples were kept in a cooler during the sampling and later kept in the refrigerator until processed.
Sample preparation for pathogens isolation
The roots samples were individually thoroughly washed under running tap water to remove the soil and then were dried overnight by placing them on 5-6 layers of towel paper. From the dried roots samples, crown roots were excised, cut into about 1 cm long segments and then each sample was divided into three subsamples. Two subsamples were surface disinfected with 5% sodium hypochlorite for 60 sec and then rinsed thrice with distilled sterile water for 60 seconds/per wash for isolating Fusarium species and B. sorokiniana. For isolating Gaeumannomyces graminis var. tritici, one of the three subsamples was disinfected by washing the root segments with 1% silver nitrate for 10 sec and then washed with distilled sterile water for 60 sec followed by removal of excess moisture before plating on the specific medium modified SM-GGT7 [24]. For Fusarium species, 40-50 root segments were plated on ½ strength potato dextrose agar prepared from raw potatoes (150 g of potatoes were boiled in 350 ml of water, followed by autoclaving with 150 ml of potato broth was added along with 22 g of agar, 20 g of dextrose and volume was made up to 1L in a conical flask). The plates were placed under 12 hours’ light and 12 hours’ dark cycle at room temperature for the fungal recovery. The fungal colonies produced by the segments were marked based on their colony color [25], and colonies with similar colors were counted separately and transferred individually onto fresh ½ strength PDA plates. The samples were incubated for seven days followed by examination under the compound microscope for the identification of Fusarium species based on the fungal morphological characteristics [26]. For B. sorokiniana, root segments were plated on water agar media, and the plates were kept under 12 hours’ light and dark cycle at room temperature. They were examined after four days under a stereoscope for the identification of the B. sorokiniana. 10-15 single conidia of B. sorokiniana were picked-off from the samples with the fungus and placed them individually onto V8PDA (150 ml of V8 juice, 10 g of agar, 10 g of PDA and 3 g of calcium carbonate) [27]. The plates were incubated under alternative 12 hours light and dark cycle for five days. The identity of thecultures were confirmed based on their morphological characters as described in Shoemaker [28]. For Gaeumannomyces graminis var. tritici, root segments were plated on a specific medium modified SM-GGT7 [24] under dark for 12 days. After 12 days, the dark black shining colonies if present were transferred to the wheat leaf agar medium (100 g of green wheat leaves were boiled in 1L of distilled water for 10 min; then the liquid was poured through two layers of cheesecloth and volume was adjusted to one liter with distilled water. Finally, 20 g of agar was added and autoclaved for 30 minutes) for the fungal perithecial development [29].
Effect of Fusarium graminearum on seed germination and seedling blight Seed preparation for F. graminearum inoculation
The seed of 11 spring wheat genotypes that include nine cultivars, Advance, Brick, Briggs, Forefront, Oxen, Prevail, Russ, Select, Traverse and two advanced lines, SD 4189, and SD 4125 were obtained from SDSU Hard Red Spring Wheat Breeding Program. All cultivar seeds were tested for their germination using towel paper method before conducting the experiments [30]. The seed was surface disinfected with 95% alcohol before infesting with F. graminearum. Each of the 11 wheat genotypes seed were infested individually with the spore suspension of F. graminearum isolate SD Fg41. For infesting the seed, fresh cultures of F. graminearum on ½ strength PDA was prepared and spore concentration was adjusted to 100K spores/ml. The seed of each genotype (500 g) was placed in a plastic tray (Figure 1) and sprayed with F. graminearum using a hand-held sprayer (Preval, Coal City, IL) and was dried overnight by placing it on the paper towel on a lab bench. The uninfested seed was sprayed with distilled sterile water and served as a check. To determine if the seed was 100% infested with the fungus, 100 F. graminearum infested seed of each cultivar was plated on ½ strength PDA. The plates were kept under 12 hours’ light and 12 hours’ dark cycle at room temperature for four days. After four days, carmine red color colonies of F. graminearum was observed. Further, slides from 10 randomly selected colonies of F. graminearum from the plates were prepared and examined under a microscope to confirm the presence of the fungus. The data was recorded on the number of seeds infested with the fungus.
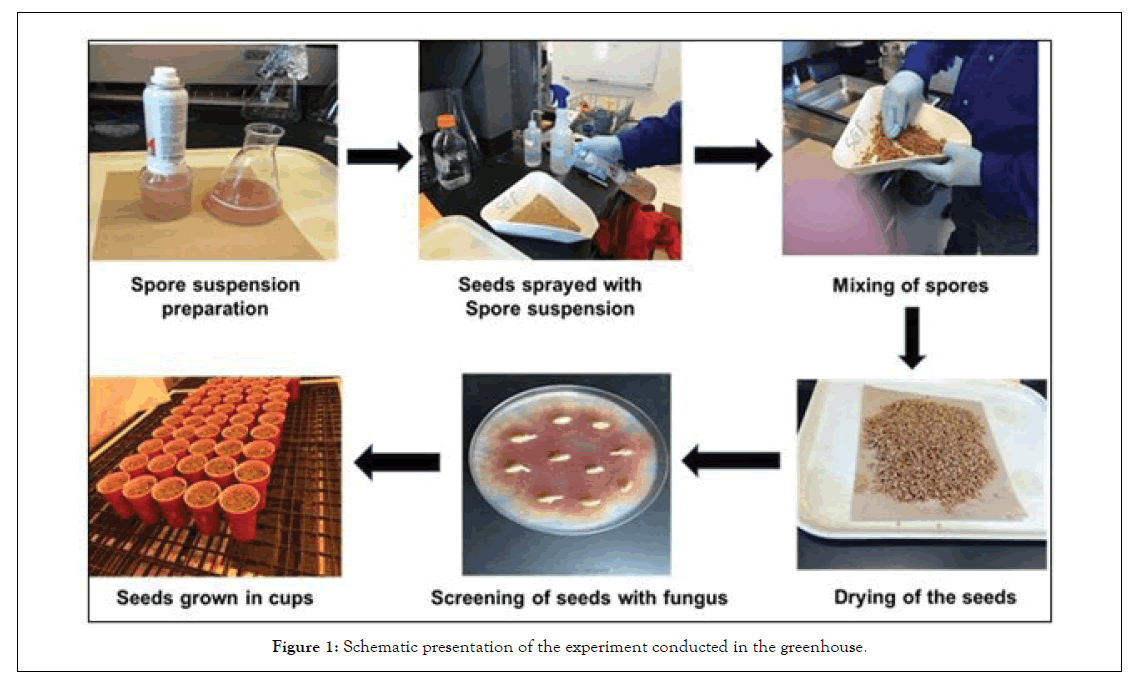
Figure 1: Schematic presentation of the experiment conducted in the greenhouse.
Greenhouse experiments
One hundred seed infested with F. graminearum and the un-infested seed of each cultivar (Control) were planted in paper cups (10 seed/cup) in a completely randomized design along with the uninfested 100 seeds as a control. The experiment was conducted in the greenhouse in fall 2014 and fall 2015. The plants were watered daily and fertilized as needed. The germination and the seedling survival data were recorded after 10 and 20 days of planting, respectively. Seedling blight data was determined by deducting the number of seedlings that died from the total germinated seedlings. Germination and seedling survival notes were discontinued when no increase in germination in un-infested treatment was observed (10 days), and no increase in seedling blight was observed in the infested seed treatments (20 days), respectively.
Field experiment
In 2015, seven cultivars Advance, Briggs, Forefront, Oxen, Russ, Prevail and one advanced breeding line SD 4215 were evaluated for F. graminearum impact on seed germination under field conditions. In 2016, eleven cultivars, (Advance, Briggs, Forefront, Oxen, Russ, Prevail, Traverse, Select, Brick, SD 4215 and SD 4189) were evaluated. In all cultivars, the seed was infested with F. graminearum as described in the greenhouse experiment. All cultivar seeds for both greenhouse and field experiments were used from the same seed lot. The field experiment was planted by hand in a split-split plot randomized block design where the main plot was a source of inoculum (wheat seed infested with F. graminearum and oat kernels infested with F. graminearum mixed with the wheat seed at planting). The sub-plot included varieties, and the sub-subplot included four treatments; un-infested seed without fungicide treatment (T1); uninfested seed treated with the fungicide Raxil (T2); infested seed with F. graminearum and treated with fungicide (T3); and infested seed with F. graminearum and no fungicide treatment (T4). The oats kernels were autoclaved under wet cycle for 60 minutes to eliminate any fungal infection before infesting them with F. graminearum. Each plot was planted manually with 100 seeds in a 1.6 m row length. Five grams of infested oat kernels were spread across the row. The trials were planted on May 8, 2015, May 10, 2016, at Volga and May 10, 2015, May 15, 2016, at Brookings. The germination data was recorded when the plants started to emerge. The stand count data were taken for three consecutive weeks’ post-planting for both locations and years.
In this study, we considered germination is complete when no further seed germination was observed in the control plots. The number of seedlings observed at the final date of germination data recording was considered as % seedling survival (number of seedlings at the last time of data collection). Observations were discontinued when no further seedling died or wilted in the infested seed plots.
Data Analysis
Analysis of variance was performed to determine the treatment effects on the percent seed germinated and percent seedling survival in the greenhouse experiment. As the data was in percentage, the arcsine and square root transformations were applied to normalize the data prior to analysis. Tukey’s HSD was conducted to compare the cultivars if they were different for treatments. The field experiment was analyzed using a split-split plot design in R software. Tukey’s HSD test was done for the mean comparisons among the source of inoculum, varieties, and treatments. Experiments were analyzed separately for both years and locations.
Results
Survey of wheat fields
In the both 2014 and 2015 survey, two fungal species, Fusarium graminearum and Bipolaris sorokiniana were the predominant species associated with root samples across the South Dakota. However, the % recovery of fungal species varied between the years (Table 1). In 2014, F. graminearum was recovered from all 31 (100%) roots samples analyzed; whereas, 16 (51.6%) of the samples harbored with B. sorokiniana. In 2015, F. graminearum was recovered from all eight (100%) samples while B. sorokiniana was isolated from three (37.5%) of the samples. In total, 163 isolates of F. graminearum and 70 isolates of B. sorokiniana were recovered from the samples and stored at -20°C for future studies on genotypic variationin the isolates (Table 1). G. graminis var tritici that causes ‘Take-all’ in wheat was not recovered from any of the collected root samples in both years. Other Fusarium species such as F. equiseti, F. verticillioides, F. acuminatum, F. oxysporum, F. semitectum, F. dimerum and F. avenaceum were recovered with a very low frequency from some samples (data not shown).
| Year | Samples | Fungal Species | Isolates Recovered | % Samples Infected |
|---|---|---|---|---|
| 2014 | 31 | F. graminearum | 125 | 100.0 |
| B. sorokiniana | 62 | 51.6 | ||
| 2015 | 8 | F. graminearum | 38 | 100.0 |
| B. sorokiniana | 8 | 37.5 |
Table 1: Recovery of crown and root rot associated pathogens from root samples collected from commercial wheat fields in South Dakota during the 2014 and 2015 growing seasons.
Effect of F. graminearum on seed germination and seedling survival under greenhouse conditions
In greenhouse experiments, F. graminearum significantly impacted seed germination (P < 0.05) in the eleven wheat cultivars evaluated. The germination ranged from 75-97% in seed infested with F. graminearum as compared to 100% in un-infested seed (control) (Figure 2). Wheat cultivars Russ (75%), SD 4189 (84%), and Oxen (83%) had the lowest germination due to F. graminearum as compared to Forefront (94%) and Select (97%) and Prevail (96%). Further, the evaluated cultivars were significantly (P < 0.05) different in the reaction for seedling blight. The seedling blight in all the eleven cultivars ranged from 1to 9%. The cultivars Advance (5.5%), Briggs (4%), SD4189 (5.3%), and Traverse (9.3%) had the highest seedling blight; whereas, Forefront (3.5%), Oxen (2.1%) and Select (0.7%) had the lowest seedling blight (Figure 3).
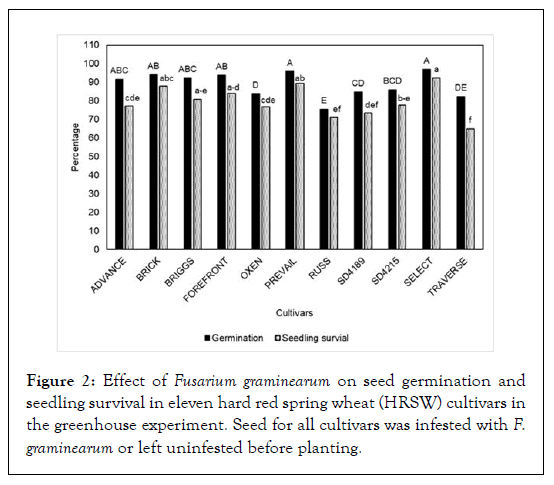
Figure 2: Effect of Fusarium graminearum on seed germination and seedling survival in eleven hard red spring wheat (HRSW) cultivars in the greenhouse experiment. Seed for all cultivars was infested with F. graminearum or left uninfested before planting.
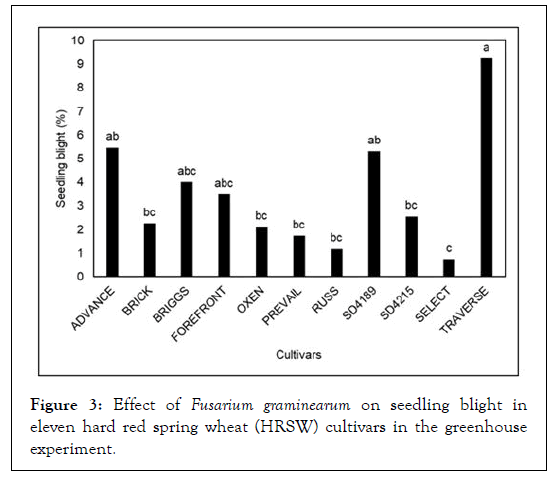
Figure 3: Effect of Fusarium graminearum on seedling blight in eleven hard red spring wheat (HRSW) cultivars in the greenhouse experiment.
Effect of F. graminearum on seed germination and seedling survival under field conditions
The fungus F. graminearum significantly (P < 0.05) affected the seed germination and seedling survival in several cultivars at both Brookings and Volga locations in 2015 and 2016 (Tables 2 and 3). Cultivar ‘Russ’was found to be most susceptible to F. graminearum, whereas, other genotypes including Advance (87.4%), Forefront (87.9%), and Prevail (88.4%) are showed good level of resistance to F. graminearum (Table 2). The percent seed germination varied in different genotypes both greenhouse and multiyear multi-location field trials (Tables 2 and 3). The source of inoculum had significant impact on seed germination and seedling survivalin 2015 where infested oat seed when used as the inoculum source had low impact on seed germination and survival when compared to infested seed itself (Tables 2 and 3). However, in 2016 the effect inoculum source was not significant.
| Variables | 2015y | 2016z | ||
|---|---|---|---|---|
| Germination | Germination | |||
| Source | Brookings | Volga | Brookings | Volga |
| Source of Inoc. | ||||
| i) Seed | 83.3 b | 75.6 b | 89.5 a | 88.2 a |
| ii) Oats | 93.1 a | 85.6 a | 81.6 a | 87.6 a |
| p value | 0.001 | 0.001 | 0.072 | 0.12 |
| Varieties | ||||
| Briggs | 93.3 a | 84.3 a | 85.6 abc | 89.5 ab |
| Prevail | 91.8 a | 87.4 a | 86.1 ab | 88.4 ab |
| Advance | 91.6 a | 81.6 a | 87.3 ab | 89.3 ab |
| Forefront | 90.3 a | 84.8 a | 88.2 ab | 88.5 ab |
| SD 4215 | 80.7 a | 82.6 a | 83.3 abc | 85.0 ab |
| Oxen | 88.5 a | 80.6 a | 81.9 bc | 85.3 ab |
| Russ | 72.4 b | 61.4 b | 77.4 c | 81.6 b |
| Select | - | - | 89.8 a | 91.5 a |
| SD 4189 | - | - | 86.6 ab | 89.7 ab |
| Traverse | - | - | 85.8 abc | 88.3 ab |
| Brick | - | - | 88.8 ab | 88.2 ab |
| p value | 0.001 | 0.001 | 0.0001 | 0.04 |
| Treatments | ||||
| T1 (UT+UINF) | 89.5 b | 83.5 b | 86.1 a | 86.5 b |
| T2 (T+UINF) | 93.5 a | 88.5 a | 88.3 a | 91.3 a |
| T3 [T+INFT(Fg)] | 89.5 b | 85.6 ab | 86.0 a | 90.5 a |
| T4 [UT+INFT(Fg)] | 80.8 c | 62.5 c | 82.3 b | 82.3 c |
| p value | 0.001 | 0.001 | 0.001 | 0.001 |
| Treatment × Source of Inoc. | 0.001 | 0.001 | 0.202 | 0.001 |
| Treatment × Varieties | 0.050 | 0.143 | 0.167 | 0.999 |
| Treatment × Source of Inoc. × Varieties | 0.080 | 0.066 | 0.107 | 0.989 |
ySeven spring wheat cultivars were included in the experiment in 2015
zEleven Spring wheat cultivars were included in the experiment in 2016T1= Untreated and uninfested seed (Control), T2 = treated seed with fungicide, T3 = treated seed with fungicide and infested with F. graminearum, T4 = untreated seed and infested with F. graminearum Where Inf= infested; UT= untreated, Fg = F. graminearum
Means in a column followed by the same letter(s) are not significantly different according to Tukey’s HSD test. Percentage data were arcsine and square root transformed prior to statistical analysis.
Table 2: Effect of Fusarium graminearum on seed germination of hard red spring wheat (HRSW) cultivars in Brookings and Volga in 2015 and 2016. Seed of the cultivars was either directed infested with F. graminearum or inoculated with autoclaved infested oats.
| Variables | 2015 | 2016 | ||
|---|---|---|---|---|
| Survival (%) | Survival (%) | |||
| Source | Brookings | Volga | Brookings | Volga |
| Source of Inoc. | ||||
| i) Seed | 76.6 b | 69.3 b | 87.5 a | 85.1 a |
| ii) Oats | 88.8 a | 80 a | 79.4 a | 84.6 a |
| p value | 0.039 | 0.013 | 0.053 | 0.588 |
| Varieties | ||||
| Briggs | 93.3 a | 78.1 a | 83.6 ab | 86.6 ab |
| Prevail | 91.8 a | 80.3 a | 83.2 abc | 85.8 ab |
| Advance | 91.6 a | 75.4 a | 84.7 ab | 85.9 ab |
| Forefront | 90.3 a | 78.8 a | 86.3 a | 84.8 ab |
| SD 4215 | 88.7 a | 76.6 a | 81.7 abc | 82.5 ab |
| Oxen | 88.6 a | 74.5 a | 80.2 bc | 83.0 ab |
| Russ | 72.5 b | 58.3 b | 75.4 c | 78.9 b |
| Select | - | - | 88.2 a | 88.9 a |
| SD 4189 | - | - | 85.4 ab | 86.2 ab |
| Traverse | - | - | 84.5 ab | 84.4 ab |
| Brick | - | - | 85.7 ab | 85.3 ab |
| p value | 0.001 | 0.001 | 0.001 | 0.077 |
| Treatments | ||||
| T1 (UT+UINF) | 88.7 a | 78.6 ab | 83.9 a | 84.2 b |
| T2 (T+UINF) | 86.3 ab | 82.2 a | 85.8 a | 88.1 a |
| T3 [T+INFT(Fg)] | 85.1 b | 78.3 b | 84.6 a | 87.2 ab |
| T4 [UT+INFT(Fg)] | 77. c | 58.4 c | 80.0 b | 79.3 c |
| p value | 0.001 | 0.001 | .001 | .077 |
| Treatment × Source of Inoc | 0.001 | 0.001 | 0.202 | 0.004 |
| Treatment × Varieties | 0.066 | 0.090 | 0.167 | 0.999 |
| Treatment × Source of Inoc × Varieties |
0.065 | 0.165 | 0.107 | 0.976 |
ySeven spring wheat cultivars were included in the experiment in 2015
zEleven Spring wheat cultivars were included in the experiment in 2016
T1= Untreated and uninfested (Control), T2 = treated with fungicide T5 = treated with fungicide and infested with F. graminearum, T6 = untreated and infested with F. graminearum, Where Inf = infested; UT= untreated, Bs = B. sorokiniana; Fg = F. graminearum
Means in a column followed by the same letter(s) are not significantly different according to Tukey’s HSD test. Percentage data were arcsine and square root transformed prior to statistical analysis.
Table 3: Effect of Fusarium graminearum on seedling survival of HRSW cultivars in Brookings and Volga in 2015 and 2016.
We further looked at the effect of fungicide treatment on germination for infested seed and observed improvement in seed germination ranging from 9-23% (2015) and 4-8% (2016) (Tables 2 and 3). On average there was increase (4.5%) in germination when theseed was treated with fungicide as compared to the untreated seed (control) in 2015 at both the locations. However, in 2016, fungicide treatment increased seed germination only at Volga (4.5%), whereas, no improvement was observed at Brookings.
Discussion
Survey results of this study showed that F. graminearum was the major pathogen responsible for crown rot in wheat followed by B. sorokiniana causing common root rot in South Dakota. While some other Fusarium spp. were also recovered from the collected samples, their frequency was minuscule. These species included F. equiseti, F. verticillioides, F. acuminatum, F. oxysporum, F. semitectum, F. dimerum and F. avenaceum. The recovery of F. graminearum (100%) and B. sorokiniana (44.5%) from most of the collected samples indicates the potential occurrence of crown rot and common root rot in wheat in the region if the planted cultivars are susceptible and the conducive conditions prevailed for disease development. Several independent studies wheat roots disease pathogens surveys conducted in Chile [31], Canada [32-44], Australia [45,46], Asia [20,47], Europe [48] and South America [49] recovered B. sorokiniana and F. graminearum/F. culmorumfrom wheat roots. In the US, survey have been reported in several states like New York [11] where F. graminearum was most abundant followed by B. sorokiniana; in Minnesota [33] though F. graminearum was isolated from most wheat root samples F. culmorum and F. avenaceum was also recovered and in California [32]. C. sativus, F. graminearum, and G. graminis var. tritici and Calonectria graminicola were most common pathogens infecting the sub crown internode, crown, and basal culm tissue thus further developed brown discoloration and caused root damage.
Though we did not recover G. graminis f. sp. tritici from the collected root samples, this does not eliminate the possibility of the presence of this pathogen in the region as it had been reported previously mostly in winter wheat in South Dakota and the Pacific Northwest [4]. There could be two primary reasons of not observing or recovering G. graminis; i) take-all disease usually occurs in patches, and the random sampling might have missed these patchy infested areas; ii) the soil conditions may not have been favorable for the fungus for plants roots colonization. Though most of the previous studies examined the collected root samples for any discoloration prior to isolations, the current study did not examine the roots for any symptoms prior to the isolations. G. graminis f. sptritici from root samples were only recovered when the plants exhibited takeall disease symptoms. Our results confirm previous independent studies that wheat is more prone to common root rot and crown rot as compared to take-all in the region.
Evaluation of wheat varieties both under greenhouse and field conditions suggested significant differences in response to seed germination and seedling blight in the cultivars when the seed was infested with F. graminearum (Figures 2 and 3). The fungus significantly reduced the germination and caused seedling blight in the susceptible cultivars as compared to resistant cultivars. Our results suggest that wheat varities available in South Dakota region posses variable level of resistance to F. graminearum causing loss in seed germination and seedling blight. Similar level of variability in wheat genotypes have been reported in different countries/regions [12,50-53]. Further, fungicide as seed treatment improved the seed germination in the range of 9-23% and suggests a promising method to improve seed germination and seedling survival caused by F. graminearum. Prevoiusly Jones [14] reported use of the seed surface sterilization enhanced the seed germination up to 32%. Thus fungicide seed treatmentcan significantly reduce seedling blight and improved seed germination in F. graminearum infested seed.
The source of inoculum was significant in the first year (2015) when the disease pressure was higher due to frequent rain events when compared to the year 2016. The germination was significantly reduced in the treatments (2015) where the infested seed was used as a source of inoculum. Our results suggests that when the seed is carrying the pathogen or when the seed is infested, the germination losses will be higher as compared to when the pathogen is already present in the soil (Oats inoculum). This may be due to fact that the pathogen requires sometime to reach and colonize the seed before its germination. Experiments conducted at two different locations over the two years resulted in a similar trend of reduction in seed germination, however, there was lower germination observed in Brookings as compared to Volga in 2016. The lower soil moisture content at Brookings as compared to Volga, may have resulted inlower germination at Brookings as compared Volga.
Conclusion
Our results suggest that continuous monitoring of root rot pathogens is necessary for the management of root rot diseases in the region. Insights into distribution and incidence of the root rot pathogens obtained in this study will help in developing diseases management strategies including identification of sources of resistance to these pathogens. Further, careful selection of resistant cultivars can reduce loss due to poor stand. In addition, use of fungicide as a seed treatment can also reduce the plant stand losses caused by F. graminearum if the producer anticipates risk from poor quality of seed and higher pathogen prevalence in crop residue.
Acknowledgments
We would like to acknowledge Rick Geppert for helping in greenhouse and field experiments and root samples collection. Also, we would like to recognize the South Dakota Wheat Commission, Minnesota Wheat Research and Promotion Council, and South Dakota Agriculture Experiment Station for funding this project.
REFERENCES
- Braun HJ, Atlin G, Payne T. Multi-location testing as a tool to identify plant response to global climate change. Climate change and crop production. 2010;1:115-138.
- FAO. Cereals and other starch‐based staples: are consumption patterns changing? 2004.
- Shaver TM, Peterson GA, Ahuja LR, Westfall DG. Soil sorptivity enhancement with crop residue accumulation in semiarid dryland no-till agroecosystems. Geoderma. 2013;192:254-258.
- Paulitz TC, Smiley RW, Cook RJ. Insights into the prevalence and management of soilborne cereal pathogens under direct seeding in the Pacific Northwest, USA. Can J Plant Pathol. 2002;24:416-428.
- Sharma-Poudyal D, Schlatter D, Yin C, Hulbert S, Paulitz T. Long-term no-till: a major driver of fungal communities in dryland wheat cropping systems. PLoS One. 2017;12:1-9.
- Mehra LK, Cowger C, Weisz R, Ojiambo PS. Quantifying the effects of wheat residue on severity of Stagonosporanodorum blotch and yield in winter wheat. Phytopathology. 2015;105:1417-1426.
- Bockus WW, Bowden RL, Hunger RM, Murray TD, Smiley RW. Compendium of wheat diseases and pests (3rd edn), American Phytopathological Society, APS Press, USA. 2010.
- Cook RJ. Fusarium diseases of wheat and other small grains in North America. In: Fusarium: Diseases, Biology, and Taxonomy P. E. Nelson, T. A. Toussoun R. J. Cook (Eds). Pennsylvania State University Press, University Park. 1981; 39-52.
- Smiley RW, Patterson LM. Pathogenic fungi associated with Fusarium foot rot of winter wheat in the semiarid Pacific Northwest. Plant Dis. 1996;80:944-949.
- Smiley RW, Gourlie JA, Easley SA, Patterson LM, Whittaker RG. Crop damage estimates for crown rot of wheat and barley in the Pacific Northwest. Plant Dis 89:595-604.
- Kane RT, Smiley RW, Sorrells ME. Relative pathogenicity of selected Fusarium species and Microdochium bolleyi to winter wheat in New York. Plant Dis. 1987;71:177-181.
- Wong L, Tekauz A, Leisle D, Abramson DA, McKenzie R. Prevalence, distribution, and importance of Fusarium head blight in wheat in Manitoba. Can J Plant Pathol. 1992;14:233-238.
- Argyris J, Van Sanford D, TeKrony D. Fusarium graminearum infection during wheat seed development and its effect on seed quality. Crop Sci. 2003;43:1782-1788.
- Jones RK. Seedling blight development and control in spring wheat damaged by Fusarium graminearum group 2. Plant Dis. 1999;83:1013-1018.
- Schaafsma A,Tamburic-Ilincic L. Effect of seeding rate and seed treatment fungicides on agronomic performance, Fusarium head blight symptoms, and DON accumulation in two winter wheats. Plant Dis. 2005;89:1109-1113.
- Fernandez M, Turkington T, May W. Effectiveness of fungicide seed treatments for preventing seed-to-seedling transmission of Fusarium graminearum under controlled-environment conditions. Can J Plant Sci. 2009;89:811-821.
- Wiersma JJ, Kandel HJ. The response of Fusarium graminearum infected seed of hard red spring wheat to Vitavax Extra RTU and Dividend XL seed treatments. Plant Health Prog. 2014;5:1.
- Wildermuth G, Purss G. Further sources of field resistance to crown rot Gibberellazeae of cereals in Queensland. Aust J Exp Agric Ani. 1971;11:455-459.
- Smiley RW, Yan H. Variability of Fusarium crown rot tolerances among cultivars of spring and winter wheat. Plant Dis. 2009;93:954-961.
- Liu Y, Ma J, Yan W, Yan G, Zhou M, Wei Y, et al. Different tolerance in bread wheat, durum wheat and barley to Fusarium crown rot disease caused by Fusarium pseudograminearum. J Phytopathol. 2012;160:412-417.
- Tinline RD, Ledingham R J. Yield losses in wheat and barley cultivars from common root rot in field tests. Can J Plant Sci. 1979;59:313-320.
- Draper MA, Stymiest C, Jin Y. Common Root and Crown Rot Diseases of Wheat in South Dakota. Extension Extra. 2000;341.
- Smiley RW, Gourlie JA, Easley SA, Patterson LM. Pathogenicity of fungi associated with the wheat crown rot complex in Oregon and Washington. Plant Dis. 2005;89:949-957.
- Juhnke ME, Mathre DE, Sands DC. A selective medium for Gaeumannomycesgraminis var. tritici. Plant Dis. 1984;68:233-236.
- Komada H. Development of a selective medium for quantitative isolation of Fusarium oxysporum from natural soil. Rev Plant Prot Res. 1975;8:114-125.
- Leslie JF,Summerell BA. The Fusarium Laboratory Manual. Blackwell Publish Ltd, UK 2006 ; 388.
- Lamari L, Bernier C. Evaluation of wheat lines and cultivars to tan spot Pyrenophoratritici-repentis based on lesion type. Can J Plant Pathol. 1989;11:49-56.
- Shoemaker R. Nomenclature of Drechslera and Bipolaris, grass parasites segregated from ‘Helminthosporium’. Can J Bot. 1959;37:879-887.
- Mathre D. Take all disease on wheat, barley and oats. Online Plant Health Progress. 2000.
- Rao NK, Hanson J, Dulloo ME, GhoshK, Nowell D, Larinde M. Handbooks for Gene banks No. 8: Manual of Seed Handling in Gene banks. Rome: Biodiversity International. 2006.
- Moya-Elizondo, E, Arismendi N, Castro MP, Doussoulin H. Distribution and prevalence of crown rot pathogens affecting wheat crops in southern Chile. Chilean Journal of Agricultural Research. 2015;75:78-84.
- Oswald JW. Etiology of cereal root and foot rots in California. Hilgardia. 1950;19:447-462.
- Warren HL, Kommedahl T. Fertilization and wheat refuse effects on Fusarium species associated with wheat roots in Minnesota. Phytopathology. 1973;63:103-108.
- Ledingham RJ, Atkinson TG, Horricks JS, Mills JT, Piening LJ, Tinline RD. Wheat losses due to common root rot in the prairie provinces of Canada, 1969-71. Can Pl Dis Surv. 1973 ;53:113-122.
- Fernandez MR, Jefferson PG. Fungal populations in roots and crowns of common and durum wheat in Saskatchewan. Can J Plant Pathol. 2004;26:325-334.
- Cook RJ. Fusarium root and foot rot of cereals in the Pacific Northwest. Phytopathology. 1968;58:127-131.
- Cook RJ. Fusarium foot rot of wheat and its control in the Pacific Northwest. Plant Dis. 1980;64:1061-1066.
- Hill JP, Fernandez JA, McShane MS. Fungi associated with common root rot of winter wheat in Colorado and Wyoming. Plant Dis. 1983;67:795–797.
- Specht LP, Rush CM. Fungi associated with root and foot rot of winter wheat and populations of Cochliobolus sativus in the Texas panhandle. Plant Dis. 1988;72:959-963.
- Dodman RL, Wildermuth GB. The effect of stubble retention and tillage practices in wheat and barley on crown rot caused by Fusarium graminearum Group 1. Plant Prot Q. 1989;4:98-99.
- Smiley RW. Diseases of wheat and barley in conservation cropping systems of the semiarid Pacific Northwest. Am J Alternative Agric. 1996;11:95-103.
- Strausbaugh CA, Bradley CA, Koehn AC, Forster RL. Survey of root diseases of wheat and barley in southeastern Idaho. Can J Plant Pathol. 2004;26:167-176.
- Cook RJ. Fusarium root, crown, and foot rots and associated seedling diseases. Compendium of wheat diseases and pests (3rd edn) In: WW Bockus, R Bowden, R Hunger, W Morrill, T Murray, and R Smiley eds The Pennsylvania State University Press, University Park, USA. 2010;37-39.
- Moya-Elizondo EA, Rew LJ, JacobsenBJ, Hogg AC, Dyer AT. Distribution and prevalence of Fusarium crown rot and common root rot pathogens of wheat in Montana. Plant Dis. 2011;95:1099-1108.
- Burgess L, Wearing A, Toussoun T. Surveys of Fusaria associated with crown rot of wheat in eastern Australia. Aust J Agric Res. 1975;26:791-799.
- Backhouse D, Abubakar A, Burgess L, Dennisc J, Hollaway G, et al. Survey of Fusarium species associated with crown rot of wheat and barley in eastern Australia. Australasian Plant Pathol. 2004;33:255-261.
- KumarJ, Schafer P, Huckelhoven R, Langen G, Baltruschat H, Stein K, et al. Bipolarissorokiniana, a cereal pathogen of global concern: cytological and molecular approaches towards better control. Mol Plant Pathol. 2002;3:185-195.
- Pettitt T, Xu X, Parry D. Association of Fusarium species in the wheat stem rot complex. Eur J Plant Pathol. 2003;109:769-774.
- Burgess LW, Backhouse D, Summerell BA, Swan LJ. Crown rot of wheat. In: Fusarium – Paul E. Nelson Memorial Symposium. BA Summerell, JF Leslie, D Backhouse, WL Bryden, and LW Burgess, eds. American Phytopathological Society, St. Paul MN. 2001; pp. 271-295.
- Grey WE, Mathre DE. Evaluation of spring barleys for reaction to Fusarium culmorum seedling blight and root rot. Can J Plant Sci. 1988;68:23-30.
- Hill J, Blunt D. Wheat seedling response to root infection by Cochliobolus sativus and Fusarium acuminatum. Plant Dis. 1994;78:1150-1152.
- Mergoum M, Hill J, Quick J. Evaluation of resistance of winter wheat to Fusarium acuminatum by inoculation of seedling roots with single, germinated macroconidia. Plant Dis. 1998;82:300-302.
- Galli JA, Fessel SA, Panizzi RC. Effect of Fusarium graminearum and infection index on germination and vigor of maize seeds. Fitopatol Bras. 2005;30:470-474.
Citation: Kaur N, Sehgal SK, Glover KD, Byamukam E, Ali S (2020) Impact of Fusarium graminearum on Seed Germination and Seedling Blight in Hard Red Spring Cultivars in South Dakota 11:495. doi: 10.35248/2157-7471.20.11.495
Copyright: © 2019 Kaur N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

