Indexed In
- Academic Keys
- ResearchBible
- CiteFactor
- Access to Global Online Research in Agriculture (AGORA)
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2021) Volume 10, Issue 1
Immunogenetic Mechanisms of Black Water Fever: Article Review
Joseph M. Bodi1*, Celestin N. Nsibu1 and Kenji Hirayama22Department of Immunogenetics, University of Nagasaki, Nagasaki, Japan
Received: 28-Dec-2020 Published: 18-Feb-2021, DOI: 10.35248/2329-6682.21.10.160
Abstract
The authors systematically reviewed the literature on mechanisms of Black Water Fever ‘BWF) on immunology and genetics point of view to determine whether immunity and genetics are involved in the occurrence of Acute Intravascular haemolysis in malaria, leading to BWF.. All original reports of BWF were retrieved from Embase, Medline from 1935 to December 2018. Information extracted from each article included study design, definitions of BWF, pathogeny and risk factors of the disease. Descriptive, Prospective cohort, cross sectional, and case-control studies were assessed. Malaria IgG1 antibodies were measured. MBL2 gene were amplified and sequenced. BWF was associated to high level of malaria IgG1 antibodies. The geometric mean of antibodies in patients with BWF was high [1,95mg/l (IC95% :1,55-2,44) compared to [ 1,19 mg/l (IC95%: 0,98–1,43) ] in children with uncomplicated malaria. High malaria IgG1 were statistically significantly associated with increased risk to develop BWF Children with MBL2 gene variants were less susceptible to develop BWF compared to children with normal MBL2 gene. Genotypes MBL2 AB&AC (AO) were more frequent in the control children group compared to the BWF cases: [OR: 0,21 (0,06-0,78) avec p=0,019] suggesting a protection conferred by gene mutations. Because of high level of MBL protein in MBL2AA genotypes patients; complement activation do to this protein can increase risk to acute intravascular hemolysis, the major mechanism of BWF Malaria .High level IgG1 and the MBL2 AA genotypes seem to the risk factors incriminated in the occurrence of BWF.
Keywords
Blackwaterfever; Malaria; IgG1; MBL2gene polymorphism
Introduction
According to WHO 2018, there were an estimated 228 million cases compared to 231 million in 2017.The global incidence rate of malaria (number of cases per 1000 population) fell from 71 in 2010 to 57 in 2014 and remained at similar levels through. Estimated deaths due to malaria fell globally from 585 000 in 2010 to 405 000 in 2018. However, the rate of reduction of malaria mortality was slower in the period 2016–2018 than in the period 2010–2015. Children under the age of 5 years accounted for two thirds (67%) of global malaria deaths in 2018.. More than half of all cases were in six countries: Nigeria (25% of cases); Democratic Republic of the Congo (12%); Uganda (5%); Côte d’Ivoire, Mozambique and Niger (4% each). About 3.4% of all malaria cases were in the WHO South-East Asia Region and 2% in the WHO Eastern Mediterranean Region [1].
In Democratic Republic of Congo; Falciparum specie is the most frequent [2]. Black Water Fever is one of the severe threatening malaria clinical form described in non-immune Europeans expatriates who stayed in malaria endemic area and used irregularly auto medication to Quinine to prevent malaria [3-9]. First of all; BWF was considered as non- immune Europeans expatriates disease until description of cases was done in autochthone population supposed to have malaria protection immunity [5,10-17].
Many factors seem to be involved in the occurrence of bwf
• Falciparum. plasmodium: It causing severe hemolysis by the invasion of Red Blood cells, the enzymatic activity deviated by plasmodium and oxidative stress caused by malaria infection.
• Malaria immunity: Malaria immunity should be involved because BWF was described firstly in none-immune population of European expatriates. Autochthone populations who develop BWF have probably malaria immune deficiency exposing them to develop massive intravascular hemolysis as expatriate individuals. Measure of malaria IgG is necessary to explore this issue.
• Genetics: All European expatriates who used Quinine in prevention and treatment of malaria did not develop BWF. Genetic factor should be involved. MBL gene can be analyzed because many studies showed the involvement of this gene in malaria occurrence.
• Quinine: This drug was incriminated in the occurrence of BFW since the first case described whether in none immune expatriate population than in autochthone inhabitants.
Immunology Pathogenesis Of Bwf
Pathogenesis of BWF is complex. Malaria immune deficiency observed in expatriate population and the use of Quinine are incriminated [4,6,8,9].
Action of P. Falciparum On Red Blood Cells (Rbc)
Basically, Red Blood Cells are protected by many mechanisms like NADPH [18,19]. Those protective mechanisms based on enzymatic system as glutathione reductase, peroxidase, dismutase and catalase, can be deviated by plasmodium parasites profit. Study in vitro performed on the activity reduction of glutathione, glutathione peroxidase and the quantity of peroxidation products of RBC membrane lipids showed moderate increasing of glutathione only mature schizontes compared to other stage of RBC invaded by Plasmodium where Glutathione activation was low. The products of RBC membrane lipid peroxidation as malonic aldehyde were four times increased in schizontes than none parasite RBC. This parasite aggression leads to alteration of RBC membrane and his destruction [18,19]. It is clear that parasite plays an important role in the occurrence of BWF [20]. Interaction between parasite, RBC. Immune activated cells and products of activation can cause important damages on RBC even none parasite RBC which become more susceptible to hemolysis showing the toxic effect of parasite products [18,19].
Action of Quinine On Red Blood Cells (Rbc)
Quinine salts highly concentrated causes hemolytic action on Red blood cells. The association of Quinine administration and the occurrence of BWF was reported in many studies. The frequency of BWF decrease when Quinine was replaced by other malaria drugs in the treatment of malaria [10,19,21-30].
The action of Quinine and parasite can be combined. In fact, the contact of RBC with plasmodium and antimalarial metabolite produce a neo-antigen. The contact of neo-antigen and the immune system leads to the synthesis of an auto-antibodies named hemolysin. This contact with the fraction C1q of complement cause hemolysis [3, 31]. The liver is the site of hemolysin production [22].
Combined action of P. falciparum and quinine on the immune system
The auto-antibodies will react against quinine and Plasmodium falciparum during new episodes of the disease causing acute intravscularis hemolysis [11, 13-16, 29-31]
Pathogenesis of acute intravascular hemolysis in BWF
Pathogenesis of acute massive intravascular hemolysis in Blackwater Fever (BWF) is recognized to be very complex.
Deficiency in malaria immunity often observed in the European expatriate population, Quinine treatment and Plasmodium falciparum parasite are the most commonly incriminated factors [4,8,12]. Malaria Antibodies increase gradually with age in autochthone population living in malaria endemic areas due to sustained contacts with the vector and malaria parasite [32-47]. This malaria antibodies elevation is a complex process .It is divided in 3steps.
Children aged below 6 months, children between 6 months and 5 years and children more than 5 years Children under 6 months are under protection provided by maternally antibodies transmitted, fetal hemoglobin, the low concentration of Para Amino Benzoic Acid contained in maternal breast milk, and the use of the Insecticide Treated Net (ITN). Later, children between 6 months and 5 years lose most of the protective factors and become exposed to malaria this vulnerability is confirmed by the occurrence of about 90% of malaria gross mortality during this time window [48]. Finally after 5 years of age, children develop acquired protective malaria immunity and clinical malaria episodes decrease both in frequency and severity [48-50].Regarding the specific aspects of this malaria immunity, IgG1 directed against Apical Membrane 1 (AMA1) and the carboxyl-terminal region of the Merozoite Surface Protein 1(MSP1-19), are predominant in malaria immunity protection during the first 2 years of life [45,51].From the 3rd year of the life, malaria IgG3, directed against MSP2, increase [45,51]. Basically, the role of these Antibodies is to protect against malaria episodes. Surprisingly, the majority of children with BWF are above 5 years old, an age where children are expected to have already acquired protective immunity against malaria in the stable endemic area [15, 52-54]. To date, Results of study of immunity during BWF episode shows that IgG1 were highly elevated in BWF patients compared to children with uncomplicated malaria episode [55].These data suggest that patients with BWF did not realize a good immune transition. In fact non-immunes malaria travelers coming back in France after a stay in malaria endemic area have exclusively malaria sub-class IgG1 antibodies against MSP2 antigen and not IgG3 [56] According to this results, we can assume that autochthone children with BWF have the same immune profile like non- immunes Europeans expatriates. These observations were noted in prospective longitudinal studies about susceptibility to develop malaria in children between 3 and 8 years. Studies show a high prevalence of IgG3 in malaria asymptomatic patients whereas patients with symptomatic malaria have a high elevated level of IgG1 [57,58].
During some studies, malaria IgG1 directed against AMA1 and MSP1-19 antigen were predominant in young age and present all other age, whereas malaria IgG3 to MSP2 depended strongly to the age. IgG1 antibodies were observed in the young children, but IgG3 were observed in old children and adults. We can conclude that the premonition is mostly linked to IgG3 directed to MSP- 2 and not IgG1 directed to AMA1 and MSP-19 which are more antiparasitemia [59,60] These results are supported by those of Taylor [51-61].
Children with high malaria IgG1 develop BWF. This result suggests the role played by these antibodies in the occurrence of the disease. BWF shows realty the role played by malaria IgG1 in the occurrence of acute massive intravascular hemolysis leading to disease. There is evidence that acute massive intravascular hemolysis which occurs in expatriates population arriving recently in malaria endemic area is due to low concentration of malaria antibodies [4-9]. Autochthone children more than 5 years old who develop BWF have high concentration of malaria antibodies [55]. These facts depend to immunology mechanisms. It should be the same mechanism between expatriates and autochthones. It is probably a hyper sensibility reaction type III according to Gel and Coombs due to toxicity of antigen-antibodies complex. In expatriate population, this reaction occurs in the presence of high malaria antigen (Plasmodium falciparum) whereas in malaria autochthone children population the same reaction occurs in the presence of high concentration of malaria antibodies (IgG1) [55,62].
Hyper sensibility reaction type II due to reaction of quinine antibodies directed to quinine metabolite fixed on RBC is possible also. Unfortunately, we did not yet measure quinine antibodies.
Genetics and BWF
Host genetics influence the malaria outcome especially in children. [63-69]. Host genetic factors can determine the occurrence, the clinic features and the evolution of the disease [70]. Among the gene which influence malaria outcome, we have the MCH (Major Complex Histocompatibility), the gene of Complement Receptor-1: CR1), the genes of sickle cell disease, the Tumor Necrosis (TNF-α), the G6PD, Interleukin-10 and 12, thalassemia and mannose binding lectin 2 [68,71]. We focus our research on MBL2 gene because we fund opportunity to amplify and sequence this gene in immuno-genetic laboratory of Nagasaki University in Japan.
Description of Mannose Binding Lectin 2 gene (MBL2)
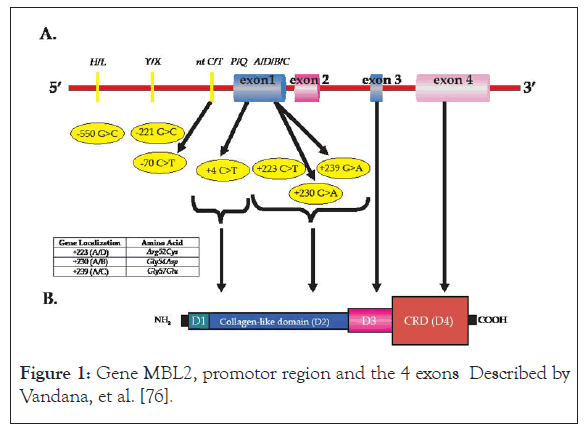
MBL2 gene has 969 base pairs with 4 exons and code for the synthesis of Mannose Binding Lectin protein (MBL protein), which belongs to C-lectin, family playing a capital role in innate immunity. The gene is located at chromosome 10, [10,11,2-21]. Each exon has a function in the synthesis of MBL protein. Exon 1 codes for the synthesis of N-terminal protein part of the protein and a portion of " collagen-like ",part, exon 2 codes for the synthesis of the other portion of "collagen-like" of the protein, exon 3 codes for the alpha-helical of the protein and exon 4 codes for C-terminal part with the domain of recognition: Carboxylate Recognition Domain (CRD). The region of promotor of the gene of Mannose Binding Lectin 2 (MBL2)
Carries the sites bind of transcription factors involved in the response to the acute phase of malaria infection or any other infection [72-77].
The next figure shows MBL2 gene with the region of promotor and the 4 exons .It presents polymorphism positions in the region of promotor in positions -550, -221, -70 and at exon 1. At exon 1, there are different polymorphism sites, +4, +223, +230, +239. The figure shows also the alleles at different positions (Figure 1).
Figure 1:Gene MBL2, promotor region and the 4 exons Described by Vandana, et al. [76].
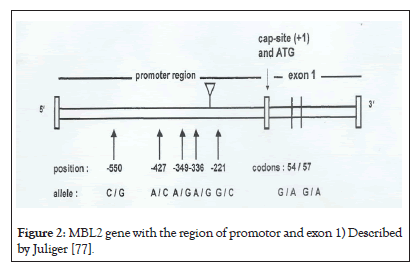
The follow figure is illustration of MBL2 gene according to Juliger S with the polymorphic sites at region of promotor (-550, -427, -349, -338 et-221) and codons 54, 57 of exon1. Position +4 and codon 52 are not represented in this scheme (Figure 2).
Figure 2: MBL2 gene with the region of promotor and exon 1) Described by Juliger [77].
The three polymorphic sites at exon 1, identified at codons [52,54,57] of MBL2 Gene by Sum iya et al., Lipsombe et al and Madsen et al. [78-80] are conventionally represented by the letters D, B, and C respectively whereas the normal gene is represented by A [78,81-82]. Codons [52,54,57] can also be designed by g (+223, +230, +239). When variants MBL2 Gene structure is described, many authors adopt the nomenclature A/A for homozygotes individuals for the normal allele, A/O for heterozygotes and O/O for homozygotes in variants [56]. Other variants couples made by Single Nucleotide Polymorphism (SNP), are also described and represented by conventional symbols either at promoter region of gene (H/ L at position -550 and Y/X at position-221) or at position +4(P/Q). Other diverse combinations of different variants are described on whole gene, Seven haplotypes were first of all described: HYPA, LYPA, LYQA, LXPA, HYPD, LYPB, LYQC), which are accompanied with decreasing concentration of MBL protein in circulation, with a consequence on the alteration of complement activation pathway [82-85]. The variants (H, L, X, and Y) located at promoter region have drastic effects in the MBL protein concentration. Different frequencies of variants reflect inter-racial differences in the serum concentration of MBL protein [86-88]. Initially; three haplotypes were investigated (HY, LY, LX) [86]. However, many other haplotypes are possible if we consider polymorphisms at position +4 (P/Q) and those of exon 1 [89].
World distribution of MBL haplotypes
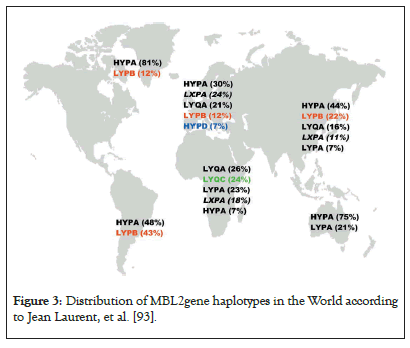
Studies were performed worldwide [90] about the distribution of some haplotypes of MBL2 gene. Those studies reported haplotypes distribution in different autochthone populations from Africa autochthones [82-83], Europe [71,83], Asia [91,92], Australia [93], Greenland [82-83] South America [83].
Haplotypes presented in below have frequency more than 5%. Haplotypes who carry mutations at exon 1 (B, C, or D) are presented with color and deficient functional halophytes (LXPA) is in italic. Haplotype LYQC is typically observed in Sub-Saharan Africa, HYPD is frequently met in Caucasian population and LYPB is spreader in Europe until American natives via Asian populations following population’s historic migrations. The three haplotypes miss in Australian population [92]. (Figure 3).
Figure 3:Distribution of MBL2gene haplotypes in the World according to Jean Laurent, et al. [93].
Role of MBL2 Gene In Malaria
The results about the role of MBL2gene in malaria is somewhat contradictory [78,94] Bellamy et al. studied polymorphism of MBL2 gene in Gambian children with severe malaria, uncomplicated malaria and health children considered as group control. Authors did not observe significant differences in different groups [94]. Garred et al. did not observe differences in Ghanaians children [85]
In opposite, other studies reported significant differences between children in different groups’. Luty et al. [72] observe that mutations at the region of promoter of MBL2 gene influence the clinic evolution of the disease. Other studies showed the evidence of association between the evolutions of malaria in regard of concentration [77]. Studies performed in Gabon revealed 14 new haplotypes and authors have found significant association between a new mutation (g797 C>A) and severe malaria [75].
Protein Mbl
Definition: Mannose Binding Protein called also mannan binding lectin protein is a protein of C-lectin family protein. The protein is produced in the liver and is linked to residue of Carbone hydrate like mannose, glucosamine in the presence of calcium. His molecular weight is 96 KDa by monomeric unit.
Functions of MBL protein: MBL protein plays important role in immunity:
• MBL protein is linked by his collagen- like part to collecting receptor of phagocytes cells [92] ;
• MBL Protein recognize residues of Carbone hydrates (mannose and N-acetyl glucosamine) at the surface of bacteria, champignons, virus, and parasites like plasmodium [95-103].
• MBL protein plays an important role during opsonisation process of pathogen micro-organisms [102,104-110].
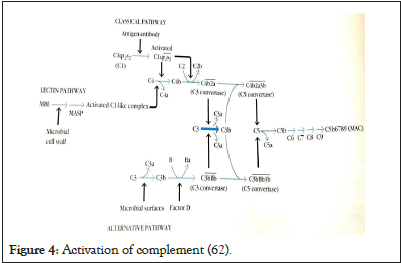
• MBL protein is associated to serines proteases 1, 2 and 3. His function in the activation of complement with serines proteases 1 et 2 acting on the fractions C1 et C3 of complement (105-110). MBL protein is the 3e pathway of complement activation, an independent pathway of antigen-antibodies presence (Ag-Ab), but which is near by the activation of C4 and C2 complement fractions [62,72,81,82,111,112]. Deficiency in MBL protein is not rare and seems to predispose to severe infections [113] (Figure 4).
Figure 4: Activation of complement [62].
MBL protein is a protein of acute phase of inflammation [78]. The level of his synthesis is linked to the normal allele and haplotypes of MBL2 gene MBL2 and also the evolution of the disease.In case of normal gene; the concentration of the protein is 50-5000ng/L according to many authors. In the allelic forms and haplotypes of gene, diminution of MBL protein concentration was reported [114- 118].
The diminution of MBL protein concentration was linked to the occurrence of sepsis and severe features of many bacteria and parasite infections because this diminution increases susceptibility to infection especially during childhood period [74,83,115-120].
Punctual mutations at codon 52 (Arg → cys), 54 (gly → Asp) et 57(gly → glu) at exon 1 play an importatn role in the synthesis of MBL protein [78-80,85].
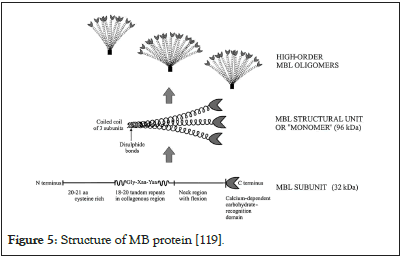
Structure of MBL protein: MBL protein is a macromolecular complex containing 6 structural molecules united by disulfures bonds. Each structural molecule is monomer molecule and the molecular weight is 96 KDa and is composed of 3 polypeptide chains or sub-units of 32 KDa. Each chain has 228 amino acid and 4 domains which reflect the structure of MBL2 gene [121]:
• The rich-cystein domain located at N- terminal extremity and has 20 amino acids;
• The collagen-like domain containing tandems in repetitions of 18 to 20 amino acids;
• The domain of alpha helice or flexible domain named neck
• The recognition domain of Carbone hydrate on the surface of Figure 3: Distribution of MBL2gene haplotypes in the World according to Jean Laurent, et al. [93]. Figure 4: Activation of complement (62). pathogen agents at C-terminal extremity (Figure 5).
Figure 5: Structure of MB protein [119].
In a case- control study carried out at Kinshasa, capital town of RDC about exploring association between MBL2 gene polymorphisms and the occurrence of clinical Blackwater fever in Congolese children, authors observed that heterozygotes genotypes MBL2 A/B&A/C (A/O) were more frequent in the control group with uncomplicated malaria whereas most of children with clinical Blackwater fever have the normal genotypes A/ A (72%) (1122).
Previous studies reported that carriers of variant allele and genotypes * B, *C and *D represented by O (O/O) for the homozygotes and A/O for the heterozygotes, are more susceptible to develop infections especially malaria. Individual’s carrier of normal allele A (A/A) were resistant to develop malaria [78,114-115-117].
Surprisingly for Blackwater fever, patients with genotype MBL2 A/B or A/C who are heterozygotes A/O with genotype MBL2 A/B or A/C were protected to develop BWF compared genotype homozygote MBL2 A/A [122]
In genetic point of view of BWF pathogenesis, MBL protein should be involved in the occurrence of acute massive intravascular hemolysis, the major mechanism of BWF by Complement activation pathway by MBL protein .In fact, heterozygotes for MBL2 gene have a reduced concentration of MBL protein. Decreasing concentration of MBL protein is known to hamper phagocytosis of intracellular bacteria and parasites [83,115,123,124-126].
However, this diminution of MBL protein concentration reduce also excessive complement activation which is deleterious for the host. This mechanism can explain the protection of heterozygotes and variants against acute massive intravascular hemolysis. Mutations at exon 1 offer protection to develop acute massive intravascular hemolysis the major mechanism of BWF. [79,123,126].
As known, MBL protein is the 3e pathway of complement activation, an independent pathway of antigen-antibodies presence (Ag-Ab), but which is near by the activation of C4 and C2 complement fractions [78,81-82,112,119]. Deficiency MBL protein is not rare and seems to predispose to severe infections [113].
Majority of children with normal genotypes A/A(72%) develop BWF [122]. We assume that excessive complement activation by normal concentration of MBL protein is the main mechanism causing acute massive hemolysis and BWF in those patients with normal genotypes known to have high MBL protein.
Pathogenesis of BWF is really complex. Immune activation and genetics mechanisms are really involved in the occurrence of BWF especially malaria immune complex antigen-antibody, MBL protein and excessive complement activation. Future studies are needed to explore those mechanisms to get more evidence by the measure of IgG3 antibodies, MBL protein; complement activity and quinine antibodies.
Conclusion
Pathogenesis of BWF is really complex. Immune activation and genetics mechanisms are really involved in the occurrence of BWF especially malaria immune complex antigen-antibody, MBL protein and excessive complement activation. Future studies are needed to explore those mechanisms to get more evidence by the measure of IgG3 antibodies, MBL protein; complement activity and quinine antibodies.
Limitations of Study
We did not measure quinine anti-bodies, malaria IgG3, MBL protein and complement activation.
REFERENCES
- WHO malaria report. 2019.
- Ministry of Planning and Monitoring of the Implementation of the Revolution and Modernity, Ministry of Public Health. Demographic and Health Survey. Democratic Republic of the Congo. 2013
- James G. Autoantigens and autoantibodies in the pathogenesis of disease with special reference to Blackwater fever. Trans R Soc Trop Med Hyg. 1946;39 (4):301- 314.
- Ende JVD, Coppens G, Verstraeten T, Haeghenborgh TV, Depraetere K, Gompel VA, et al. Recurrence of blackwater fever: triggering of relapses by different antimalarials. Trop Med Int Health. 1998;3:632-639.
- Assi LE, Brouth Y, Sissoko J, Amonkou AA, VilsacoB, Gnionsahe A, et al. La fièvre bilieuse hémoglobinurique (FBH); Aspects cliniques; paracliniques, thérapeutiques et écologiques, à propos de 13 cas observés à Abidjan. Méd Afr Noire. 1999;46(10):451-454.
- Bisoffi Z, Marocco S, Montero G, Marsiaj M. Acute intravascular haemolysis (blackwater fever) after malarial treatment. Trop Med Int Health. 1999;4:72-73.
- Bruneel F, Gachot B, Wolff M, Regnier B, Danis M, Vachon . Resurgence of black water fever in long- term European expatriates in Africa: Report of 21 cases and review. Clin Infect Dis. 2001;32(8):1133-1140.
- Bruneel F, Gachot B, Wolff M, Bedos JP, Regnier B, Danis M, et al. Blackwater fever. Presse Méd. 2002;32(8):1329-1334.
- Olga PS, Correa DF. Black water fever: A case-report. Rev Cubana Med Trop. 2003;55(1):50-53.
- WHO. 1990.
- Tran TH, Day NP, Ly VC, Nguyen TH, Pham PL, Nguyen HP, et al. Blackwater fever in southern Vietnam: A prospective descriptive study of 50 cases. Clin Infect Dis. 1996;23:1274-1281.
- Colette CDL, Taelman H, Wery M. An etiologic study of hemoglobinuria and Blackwater fever in Kivu Mountains, Zaïre. Ann Soc Bel.1995;75:51
- 63
- Rogier C, Imbert P, Tall A, Sokhna C, Spiegel A, Trape JF. Epidemiological and clinical aspects of blackwater fever among African children suffering frequent malaria attacks. Trans R Soc Trop Med Hyg. 2003;97(2):193-197.
- Gobbi F, Audagnotto S, Trentini L, Nkurunziza I, Corachan M, Di Perri G. Blackwater fever in children in Burundi. Emer Infect Dis. 2005;7:1118-1120.
- Khandelwal V, Udawat H, Kumhar, Goval RK. Blackwater fever treated with artmether. J Assoc Phys India. 2001;49:1191-1192.
- Bouldouyre MA, Dia D, Carmol T, Carmoi T, Fall KB, Chevalier B, et al. Une fièvre bilieuse hémoglobinurique de gravité moyenne (A mild blackwater fever). Méd et Mal Infect. 2006;36:343-345.
- Mohan K, Ganguly NK, Dubey ML, Mahajan RC. Oxidative damage of erythrocytes infected with Plasmodium falciparum: An in vitro study. Ann Hematol. 1992;65:131-134.
- Rogier C, Imbert P, Tall A, Sokhna C, Spiegel A, Trape JF. Epidemiological and clinical aspects of blackwater fever among African children suffering frequent malaria attacks. Trans R Soc Trop Med Hyg. 2003;97(2):193-197.
- Aloni NM, Nsibu NC, Bodi MJ, Ngiyulu MRM, Lumbala KP. Acute renal failure in Congolese children: a tertiary institution experience. Acta Tropica. 2012; 101(11):e514-8.
- Klabunde J, Uhlemann AI, Tebo AE. Recognotion of Plasmodium falciparum protein by mannan binding lectin : A component of the human innate immune system. Parasitol Res. 2002;80(2):13-17.
- Winkel JGJVD, Capel PJA. Human IgG Fc receptor heterogeneity: molecular aspects and clinical implications. Immunol Today.1993;14:215-221.
- Foy H, Kondi AK. Researches on Blackwater Fever in Greece:.Introduction and History. Ann Trop Med Parasit. 1935;29:383-393.
- Salama A, Göttsche B, Schleiffer T, Mueller-Eckhardt C. Immune complex mediated intravascular hemolysis due to IgM cephalosporin-dependant antibody. Transmission. 2003;27(6):460-463.
- Foy Henry, Athena K. Spleen fundings in black water fever. Trans R Soc Trop Med Hyg. 1938;32(3):347-354.
- Maegraith BG . The role of quinine in haemolysis. Trop Dis Bull. 1946;65:181-196.
- Beutler B, Grau G. Tumor necrosis factor in the pathogenesis of infectious diseases. Crit Care Med. 1993;21:S423-S435.
- Dausset J et Contu L: Drugs induced hemolysis . Ann Rev of Medecine. 1967;18:55-70.
- Salama A et Mueller E. On the mechanisms of sensitization and attachment of antibodies to Red Blood Cell in drug induced immune hemolytic anemia. Blood. 1987;69:1006-1010.
- Wauter JL, Rouger P. Drugs induced hemolytic anemia. Transfus Clin Biol. 2001;8:377-380.
- Bradley CG, Fredberg RC. Autoimmune hemolytic anemia. Expert Opinio Drug Safety. 2002;8(1):71-79.
- Howard MA, Hibbard A, Terreli D. Quinine allergy causing acute severe systemic illness. Report of 4 patients manifesting multiple hematologic, renal and hepatic abnormalities. BUMC proceedings 2003;16:21-26.
- Foy H, Kondi A. Lyso-lecithin fragility in blackwater fever and hemolytic jaundice. Roy Trop Med Hyg. 1943;37(1):1-18
- Polley SD, Mwangi T, Kocken CH, Thomas AW, Dutta S, Lanar DE, et al. Human antibodies to recombinant protein constructs of Plasmodium falciparum Apical Membrane Antigen 1 (AMA1) and their associations with protection from malaria. Vaccine. 2004;23:718-728.
- Cavanagh DR, Dodoo D, Hviid L, Kurtzhals JAL, Theander TG, Akanmori BD, et al. Antibodies to the N-terminal block 2 of Plasmodium falciparum merozoite surface protein 1 are associated with protection against clinical malaria. Infect Immun. 2004;72:6492-6502.
- Lusingu J, Vestergaard L, Minja D, Gesase S, Mmbando B, Malecela EA, et al. PlasmodiumIgG level to vary a conserved Plasmodium falciparum erythrocytes membrane protein-1, predict protection against anemia and febrile malaria {Abstract}. Acta tropica. 2005;95:157.
- Dodoo D, Aikins A, Kusi KA, Lamptey H, Remarque E, Milligan P, et al. Cohort study of the association of antibody levels to AMA1, MSP119, MSP3 and GLURP with protection from clinical malaria in Ghanaian children. Malar J. 2008;7:142.
- Aubouy A, Migot-Nabias F, Deloron P. Correlations between treatment outcome and both anti-MSP119 antibody response and erythrocyte-related genetic factors in Plasmodium falciparum malaria. Infect Genet Evol. 2007;7:147-154.
- Bousema T, Kreuels B, Gosling R. Adjusting for heterogeneity of malaria transmission in longitudinal studies. J Infect Dis. 2011;204(1):1-3
- Iriemenam NC, Khirelsied AH, Nasr A, ElGhazali G, Giha HA, A-Elgadir TME, et al. Antibody responses to a panel of Plasmodium falciparum malaria blood stage antigens in relation to clinical disease outcome in Sudan. Vaccine. 2009;27:62-71
- Fowkes FJ, Richards JS, Simpson JA, Beeson JG. The relationship between anti-merozoite antibodies and incidence of Plasmodium falciparum malaria: A systematic review and meta-analysis. PLoS Med. 2010;7(1):1-20.
- Nebie I, Diarra A, Ouedraogo A, Soulama I, Bougouma EC, Tiono AB, et al. Humoral responses to Plasmodium falciparum blood-stage antigens and association with incidence of clinical malaria in children living in an area of seasonal malaria transmission in Burkina Faso. West Africa. Infect Immun. 2008;76:759-766.
- Osier FH, Fegan G, Polley SD, Murungi L, Verra F, Tetteh KK, et al. Breadth and magnitude of antibody responses to multiple Plasmodium falciparum merozoite antigens are associated with protection from clinical malaria. Infect Immun. 2008;76:2240-2248.
- Greenhouse B, Ho B, Hubbard A, Njama-Meya D, Narum DL, Lanar DE, et al. Antibodies to Plasmodium falciparum antigens predict a higher risk of malaria but protection from symptoms once parasitemic. J Infect Dis. 2011;204(1):19-26.
- Murungi LM, Kamuyu G, Lowe B, Bejon P, Theisen M, Kinyanjui SM, et al. A threshold concentration of anti-merozoite antibodies is required for protection from clinical episodes of malaria. Vaccine 2013;31(37):3936-3942.
- Roussilhon C, Oeuvray C, Muller-Graf C, Tall A, Rogier C, Trape JF, et al. Long-Term Clinical Protection from Falciparum Malaria Is Strongly Associated with IgG3 Antibodies to Merozoite Surface Protein 3 Plos med. 2007;4:1791-1801.
- Tongren JE, Drakeley CJ, McDonald SL, Reyburn HG, Manjurano A, Nkya WM, et al. Target antigen, age, duration of antigen47. Tongren JE, Drakeley CJ, McDonald SL, Reyburn HG, Manjurano A, Nkya WM, et al. Target antigen, age, duration of antigen exposure independently regulate immunoglobulin G subclass switching in malaria. Infect Immun. 2006;74:257-264.
- Stanisic DI, FowkesFJL, Koinari M, Javati S, Lin E, Kiniboro B, et al. Acquisition of antibodies against Plasmodium falciparum merozoites and malaria immunity in young children: influence of age, force of infection, and magnitude of response. Infect Immun. 2014;4(11):1-50.
- Stanisic DI, Richards JS, McCallum FJ, Michon P, King CL, Schoepflin S, et al. Immunoglobulin G subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infect Immun. 2009;77(3):1165-1174.
- Marsh K, Kinyanjui S. Immune effector mechanisms in malaria. Parasite Immunol. 2006;28:51-60.
- Alles HK, Mendis KN, Carter R. Malaria mortality rates in South Asia and in Africa: Implications for malaria control. Parasitol Today. 1998;14:369-75.
- WHO: World Malaria Report. Geneva: World Health Organization. 2011
- Taylor RR, Allen SJ, Greenwood BM, Riley EM. IgG3 antibodies to Plasmodium falciparum merozoite surface protein 2 (MSP2): Increasing prevalence with age and association with clinical immunity to malaria. Am J Trop Med Hyg. 1998;58:406-413
- Bodi MJ, Nsibu NC, Longenge LR, Aloni NM, Akilimali PZ, Thibassu MP, et al . Blackwater fever in Congolese children: A report of clinical, laboratory features and risk factors. Malar J. 2013;12:205-211.
- Bodi J, Nsibu C, Aloni M, Lukute G, Kunuanuna T, Tshibassu P, et al. Black water fever associated with acute renal failure among Congolese children. Saudi J Kidney Dis Transplant. 2014;25(6):1352-1358.
- Eisen DP, Wang L, Jouin H, Murhandarwati EE, Black CG, Mercereau-Puijalon O, et al. Antibodies elicited in adults by a primary Plasmodium falciparum blood stage infection recognize different epitopes compared with immune individuals. Malar J. 2007;6:86.
- Kunuanunua ST, Nsibu NC, Gini EJL, Bodi MJ, Ekulu P, Situakibanza NH, et al. Insuffisance rénale aiguë dans les formes graves du paludisme chez les enfants vivant à Kinshasa. Nephrologie et thérapeutique. 2013;9(3):160-165
- Bodi J, Nsibu C, Longenge R, Aloni M, Akilimali P, TshibassuP, et al. HighIgG1 malaria antibodies level in children is a possible risk factor of blackwater fever: A case-control study. Pediatr Health Res. 2018;3:9
- Clark IA, Alyaman FM, Jacobson LS. The biological basis of malarial disease. Int J Parasitol. 1997;27:1237-1249.
- Stanisic DI, Richards JS, McCallum FJ, Michon P, King CL, Schoepflin S, et al. Immunoglobulin G subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infect Immun. 2009;77(3):1165-1174.
- Ramasamy R, Jones GL, Lord R. Characterisation of an inhibitory monoclonal antibody-defined epitope on a malarial vaccine candidate antigen. Immunol Lett.1990;23:305-310.
- Kuby, Kindt T, Coldsy R, Osborne B. The complement activation p 168-170 and Hyprsesibility reaction p:392 In Immunology, sixth edition, 2007 with Freeman and Company, New York.
- Taylor RR, Allen SJ, Greenwood BM, Riley EM. IgG3 Antibodies to Plasmodium falciparum Merozoite Surface Protein 2(MSP2): Increasing prevalence with age and association with clinical immunity malaria. Am J Trop Med Hyg. 1998;58(4):406-413.
- Stanisic DI, Richards JS, McCallum FJ, Michon P, King CL, Schoepflin S, et al. Immunoglobulin G subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infect Immun. 2009;77(3):1165-1174
- Taylor A, Verhagen J, Blaser K, Akdis M, Akdis CA. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-ß: The role of T regulatory cells. Immunology. 2009;117:433-442.
- Hill AV, Allsopp CE, Kwiatkowski D, Anstey NM, Twumasi P, Rowe PA, et al. Common west African HLA antigens are associated with protection from severe malaria. Nature. 1991;352:595-600.
- Williams TN, Mwangi TW, Wambua S, Peto TEA, Weatherall DJ, Gupta S, et al. Negativ epitasis between the malaria protective effects of alpha +-thalassemia and the sickle cell trait. Nature Genet. 2005;37(11):1253-1257.
- Rihet P, Abel L, Traoré Y, Traoré-Leroux T, Aucan C, Fumoux F. Human malaria: segregationan lysis of blood levels in A suburban area and rural Area In Burkina faso. Genet Epidemiol. 1998;15:436-450.
- Mackinnon MJ, Mwangi TW, Snow RW, Marsh K, Williams TN. Heritability of malaria in Africa. Plos Med. 2005;2(12):1253-1259.
- Wellems TE, Fairhust RM. Malaria-protective traits at odds in Africa?. Nature Genet. 2005;37(11):1660-1662.
- Mockenhaupt FP, Ehrhardt S, Burkhardt J, Bosomtwe SY, Laryea S, Anemana SD, et al. Manifestation and outcome of severe malaria in children in northern Ghana. Am J Trop Med Hyg. 2004;71:167-172.
- Evans JATC, König IR, Kleensang A, Rüschendorf F, Lenzen J, Sievertsen J, et al. Genome-wide linkage analysis of malaria infection intensity and mild disease. Plos Genet. 2007;3(3):393-400.
- Gundula Min-oo, Fortin A, Pitari G, Tam M, Stevenson MM, Gros P. Complex genetic control of susceptibility to malaria: positional cloning of the char 9 locus. The J of Exper Med. 2007;204(3):511-525.
- Allen SJ, O'Donnell A, Alexander ND, Alpers MP, Peto TE, Clegg JB, et al. Alpha+-Thalassemia protects children against disease caused by other infections as well as malaria. Proc Natl Acad Sci USA. 1997;94:14736-14741.
- Luty AJ, Kun FJ, Kremsner PG. Mannose-binding lectin plasma levels and gene polymorphisms in Plasmodium falciparum malaria. J Infect Dis. 1998;178:1221-1224.
- Juliger S, luckner D, Mordmuller B, May Jurgen, Welirich A, Lell B, et al. Promoter variants of human Mannose-Binding Lectin Gene Show different Binding. Biochem and Biophys Res Commu. 2000;275:617-622.
- Steffensen R, Thiel S, Varming K, Jersild C, Jensenius JC. Detection of structural gene mutations and promoter polymorphisms in the mannan-binding lectin (mbl2) gene by polymerase chain reaction with the sequence-specific primers. JIM. 2000;241:33-42.
- Boldt ABW, Luty AA, Grobusch MP, Dietz K, Dzeing A, Kombila M, et al. Association of a new mannose-binding lectin variant with severe malaria in Gabonese children. Genes Immun. 2006;7:393-400.
- Pradhan V, Surve P, Ghosh K. Mannose Binding Lectin (MBL) in Autoimmunity and its Role in Systemic Lupus Erythematosus (SLE). Ind Council of Med Res. 2010;58.
- Juliger S, luckner D, Mordmuller B, Jurgen M, Welirich A, Lell B, et al. Promoter variants of human Mannose-Binding Lectin Gene Show different Binding. Biochem and Biophys Res Commu. 2000;275:617-622.
- Sumiya M, Super M, Tabona P, Levinsky RJ, Arai T, Turner MW, et al. Molecular basis of opsonic defect in immunodeficient children. Lancet. 1991;337:1569-1570.
- Lipscombe RJ, Sumiya, MHill AV, Lau YL, Levinsky RJ, Summerfield JA, et al. High frequencies in African and non-African populations of independent mutations in the mannose binding protein gene. Hum Mol Genet. 1992;1:709-715.
- Madsen HO, Garred P, Kurtzhals JAL, Lamm LU, Ryder LP, Thiel S, et al. A new frequent allele is the missing link in the structural polymorphism of the human mannan-binding protein. Immuno Genet. 1994;40:37-44.
- Garred P, Madsen HO, Hofmann B, Svejgaard A. Increased frequency of homozygosity of abnormal mannan-binding protein alleles in patients with suspected immunodeficiency. Lancet. 1995;346:941-943.
- Madsen HO, Garred P, Thiel S, Kurtzhals JAL, Lamm LU, Ryder LP, et al. Interplay between promoter and structural gene variants control basal serum level of mannan-binding protein. J Immunol. 1995;155:3013-3020.
- Madsen HO, Satz ML, Hogh B, Svejgaard A, Garred P. Different molecular events result in low protein levels of mannam-binding lectin in populations from southeast Africa and South America. The J of Immunol. 1998;161:3169-75.
- Wallis R. Structural and fonctionnal aspects of complement activation by mannose binding protein. Immunology. 2002;205(4-5):433-435.
- Garred P, Nielsen AM, Kurthazhals JAL, Rajneesh M, Madsein OH, GOka BQ, et al. Manose Binding Lectin Is a Modifier in Clinical Malaria and May Function as Opsonin for Plasmodium falciparum infected Erythrocyte. Infection and Immunity Am Soc for microbiol. 2003(7):5245-5253.
- IP WK, Lau YL, Chan SY, Mok CC, Chan D, Tong KK, et al. Mannose–Binding Lectin and rheumatoid arthritis in southern chinese. Arthitis & Rheumatism. 2000;8:1679-1687.
- Lee SG, Yum JS, Moon HM, Kim HJ, Yang YJ, Kim HL, et al. Analysis of mannose-binding lectin 2 (mbl2) genotype and the serum protein levels in Korean population. Mol immunol. 2005;42:969-977.
- Holmberg V, Schuster F, Dietz E, Sagarriga VJC, Anemana SD, Bienzle U, et al. Mannose –binding lectin variant associated with severe malaria in young African children. Microb Infect. 2008;10(4):342-348.
- Koturoglu G, Onay H, Midilli R, Pehlivan S, Eren E, Itirli G, et al. Evidence of an association between mannose binding lectin codon 54 polymorphism and adenoidectomy and/or tonsillectomy in children. Int J Pediatr Otorhinolaryngol. 2007;71(8):1157-1161
- Casanova JL, Abel L. Human Mannose-binding Lectin in Immunity: Friend, Foe,or Both?. J Exper Med. 2004;199(10):1295-1299.
- Matsushita M, Miyakawa H, Tanaka A, Hijikata M, Kikuchi K, Fujikawa H, et al. Single nucleotide polymorphisms of the mannose- binding lectin are associated with susceptibility to primary biliary cirrhosis. J Autoimmun. 2001;17:251-257.
- Turner MW, Dinan L, Heatley S, Jack DL, Boettcher B, Lester S, et al. Restricted polymorphism of the mannose-binding lectin gene of indigenous Australians. Hum Mol Genet. 2000;9:1481-1486.
- Casanova JL, Abel L. The human model: a genetic dissection of immunity to infection in natural conditions. Nat Rev Immunol. 2004;4:55-66.
- Bellamy R, Ruwende C, Mcadam KPWJ, Thursz M, Sumiya M, Summerfield J, et al. Mannose binding protein deficiency is not associated with malaria, hepatitis B carriage nor tuberculosis in Africans. Q J Med. 1998;91(1):13-8.
- Cross CE, Bancroft GJ. Ingestion of a capsular Cryptococcus neoformans occurs via mannose and b-glucan receptors, resulting in cytokine production and increased phagocytosis of the encapsulated form. Infect Immun. 1995;63:2604-2611.
- Neth O, Jack DL, Dodds AW, Holzel H, Klein NJ, Turner MW. Mannose-binding lectin binds to a range of clinically relevant microorganisms and promotes complement deposition. Infect Immun. 2000;68:688-693.
- Townsend R, Read R, Klein NJ, Turner MW, Jack DL. Differential recognition of obligate anaerobic bacteria by human mannose-binding lectin. Clin Exp Immunol. 2001;124:223-228
- Haurum JS, Thiel S, Haagsman HP, Laursen, Larsen, Jens C, et al. Studies on the carbohydrate-binding characteristics of human pulmonary surfactant-associated protein A and comparison with two other collectins:mannan-binding protein and conglutinin. Biochem J. 1993;293:873-878.
- Hartshorn KL, Sastry K, White MR, Anders EM, Super M, Ezekowitz RA, et al. Human Mannose-binding Protein Functions as an Opsonin for Influenza AViruse. J Clin Invest. 1993;91(4):1414-1420.
- Green P, Luty A, Nair S, Radford J, Raftos D. A second form of collagenous lectin from the tunicate, Styela plicata. Comparative Biochem and Physiol. 2006:144(3):1-8.
- Kelly P, Jack DL, Naeem A, Mandanda B, Pollok RCG, Klein, et al. Mannose-binding lectin is a component of innate mucosal defense against Cryptosporidium parvum in AIDS. Res Commun. 2000;119:1236-1242.
- Jack DL, Read RC, Tenner AJ, Frosch M, Turner MW, Klein NJ. Mannose-binding lectin regulates the inflammatory response of human professional phagocytes to Neisseria meningitidis serogroup B. J Infect Dis. 2001;184:1152-1162.
- Jack DL, Turner MW. Anti-microbial activities of mannose binding lectin. Biochem Soc Trans. 2003;31:753-757.
- Matsushita M, Fujita T. Activation of the classical complement pathway by mannose-binding protein in association with a novel C1s-like serine protease. J Exp Med. 1992;176:1497-1502.
- Sato T, Endo Y, Matsushita M, Fujita T .Molecular characterization of a novel serine protease involved in activation of the complement system by mannose-binding protein. Int Immunol. 1994; 6(4):665-9.
- Thiel S, Frederiksen PD, Jensenius JC. Clinical manifestations of mannan-binding lectin deficiency. Mol. Immunol.2006;43:86–96.
- Dahl MR, Thiel S, Matsushita M,2Fujita , Willis AC,Vorup-Jensen TT et al. Its Association with Distinct Complexes of the Mannan-Binding Lectin Complement Activation Pathway. Immunity. 2001;15:127-135
- Stover CM, S Thiel S,LynchNJ , Schwaeble WJ. The Rat and Mouse Homologues of MASP-2 and MAp19 components of the Lectin activation pathway of complement. J Immunol. 1999;163:6848-6859.
- Takahashi, Yuichi E, Fujita T, Matsushita M. A truncated form of mannose-binding lectin associated serine protease (MASP)-2 expressed by alternative polyadenylation is a component of the lectin complement pathway. Intern Immunol.1999;11(5):859-863.
- Gadjeva M, Thiel S, Jensenius C. The mannan-binding-lectin pathway of the innate immune response. Cur Opinio in Immunology. 2001;13(1):74-77.
- Kuhlman M, Joiner K, Ezekowitz AB. The human mannose-binfing protein functions as an opsonin. J of Exper Med. 1889;169:1733-1745.
- Rich SM, Licht MC, Hudson RR, Ayala FJ . Malaria’s Eve: evidence of a recent population bottle neck throughout the world populations of Plasmodium falciparum. Proc Natl Acad Sci USA.1998; 95: 4425-4430.
- Ayi K, Turrini F, Piga A, Arese P. Enhanced phagocytosis of ring-parasitized mutant erythrocytes: a common mechanism that may explain protection against falciparum malaria in sickle trait and beta-thalassemia trait. Blood. 2004; 104: 3364–3371.
- Garred P, Madsen HO, Halberg P,Petersen J, Kronborg G,Svejgaard A,et al . Mannose-binding lectin polymorphisms and susceptibility to infection in systemic lupus erythematosus Arthritis. Rheum. 1999.42:2145–2152
- Garred P,Madsen HO,Balslev U,Hofmann B, Pedersen C,Gerstoft J et al. Susceptibility to HIV infection and progression of AIDS in relation to variant alleles of mannose-binding lectin. Lancet. 1997;349:236-240.
- Sullivan KE, Wooten C, Goldman D, Petri M : Mannose binding protein genetic polymorphisms in black patients with systemic lupus erythematous. Arthritis Rheum. 1996;39:2046-2051.
- Summerfield JA, Ryder S, Sumiya M, Thursz M, Gorchein A, Monteil MA, et al. Mannose binding protein gene mutations associated with unusual and severe infections in adults. Lancet. 1995;345:886-889.
- Summerfield JA, Sumiya M, Levin M, Turner MW. Association of mutations in mannose binding protein gene with childhood infection in consecutive hospital series. BMJ.1997;314:1229-1232.
- Eisen DP ,Minchiton RM. Impact of mannose binding lectin on susceptibility to infectious diseases.Clin Infec Dis. 2003; 37: 1496-1505
- Garred P, Pressler T, Madsen HO, Frederiksen B, Svejgaard AN, Hoiby N, et al. Association of mannose-binding lectin gene heterogeneity with severity of lung disease and survival in cystic fibrosis. J Clin Investig. 1999;104:431-437.
- Ezekowitz RA, Kuhlman M, Groopman JE, Byrn RA. A human serum mannose-binding protein inhibits in vitro infection by the human immunodeficiency virus. J Exp Med. 1989;169:185-196.
- Bodi MJ, Nsibu NC, Longenge LR, Aloni NM, Akilimali AP, Kayembe KP, et al. Exploring association between MBL2 gene polymorphisms and the occurrence of clinical blackwater fever through a case–control study in Congolese children. Malaria Journ. 2020;19:23
- Garred P, Madsen HO, Kurtzhals JAL, Lamm LU, Thiel S, Hey AS, et al. Diallelic polymorphism may explain variations of blood Jconcentration of mannan-binding protein in Eskimos, but not in black Africans. Eur J Immunogenet. 1992;19:403-412.
Citation: Bodi JM, Nsibu CN, Hirayama K (2021) Immunogenetic Mechanisms of Black Water Fever: Article Review. Gene Technol. 10:160.
Copyright: © 2021 Bodi et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.