Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2019) Volume 10, Issue 2
HPV Vaccination Concepts in the Reality of Today
Alberto Paderno1, Andrea Garolla2, Sergio Pecorelli3, Alberto Lombardi4, Carmine Pinto5, Giancarlo Icardi6, Fulvio Bonetti7, Francesco Saverio Mennini8, Michele Conversano9, Andrea Isidori10, Luciano Mariani11, Giovanni Rezza12 and Andrea Peracino4*2Dirigente Medico Presso UOC Andrologia e Medicina della Riproduzione, Azienda Ospedale, Dipartimento di Medicina, Specialista in Farmacologia Clinica, Dottore di Ricerca in Scienze Endocrine ed Ematologiche, Padova, Italy
3Giovanni Lorenzini Medical Foundation, New York, NY, USA
4Fondazione Giovanni Lorenzini Medical Science Foundation, Milano, Italy
5Direttore, Unità Operativa di Oncologia Medica, AUSL, IRCCS, Reggio Emilia, Italy
6Professore Ordinario di Igiene, Dipartimento Scienze della Salute, Università degli Studi di Genova; Direttore, UOC Igiene, Ospedale Policlinico San Martino, Genova, Italy
7Medico di Medicina Generale, ATS Brianza, Barlassina, Monza e Brianza, Italy
8Research Director, Economic Evaluation and HTA (EEHTA) CEIS, Faculty of Economics, University of Rome "Tor Vergata", Institute for Leadership and Management in Health, Kingston University, London, UK
9Direttore, Dipartimento di Prevenzione, ASL, Taranto, Italy
10Professore Associato di Endocrinologia, Docente di Endocrinologia, Medicina della Riproduzione e Metodologia Clinica, Dipartimento di Medicina Sperimentale, University La Sapienza, Roma, Italy
11Responsabile, HPV-UNIT, Istituto Nazionale Tumori Regina Elena, Roma, Italy
12Dipartimento di Malattie Infettive Parassitarie e Immunomediate, Istituto Superiore di Sanità, Roma, Italy
Received: 25-Feb-2019 Published: 21-Mar-2019
Abstract
The aim of this review is to evaluate the complex interaction between HPV and human body, in order to contextualize its role in the development of infective-related disease and neoplastic transformation.
While the clinical value of vaccination on the field of gynecology has been widely investigated, the growing introduction of universal coverage (both males and females) leads to the need to extend investigations to adjunctive areas of interest. This is the rationale to extend and combine in a single review the main emerging areas where the HPV vaccination could induce an effective immunological response: HPV-related upper aerodigestive tract (UADT) pathologies, and HPV-related alterations of reproductive processes in males and females.
There is no doubt that universal vaccination against HPV (9vHPV) is demonstrating its effectiveness in the prevention of most HPV-related conditions and worldwide prevalence data allow us to consider HPV vaccination from adolescence to late adulthood.
We can conclude that: i) HPV infection is still very prevalent in sexually active population and may be associated to cervical, oropharyngeal, penile and anal cancers; ii) due to HPV vaccination and screening for cervical cancer, the incidence of cervical carcinoma is strongly decreased; iii) the rate of oropharyngeal cancer is growing worldwide and since 2010 it represents in males the most frequent HPV related cancer among U.S. population; iv) HPV semen infection, has been related to impaired sperm parameters, higher miscarriage rate and, in general, to couple infertility both by natural and assisted conception; v) recent efforts toward a universal vaccination against HPV have demonstrated its effectiveness in the prevention of most HPV-related conditions; vi) growing evidences suggest positive effects of adjuvant vaccination in patients with HPV related cancer and in HPV infected infertile couples.
Introduction
More than 630,000 cancer cases each year (4.5% of all cancers) are attributable to the Human Papilloma Virus (HPV), which represents a significant threat to global public health [1]. HPV-related tumors account for 8.6% of all tumor cases in females (N=570,000) and 0.8% of cases (N=60,000) in males. Most of HPV-related tumors are localized in the uterine cervix (N=528,000), followed by those of the head and neck region (N=37,500), anus (N=35,000), penis (N=13,000), vagina (N=12,000) and vulva (N=8,500). HPV-16 is the most commonly identified genotype in HPV-related tumors, while the combination of HPV 16/18 genotypes (included in 2vHPV) and HPV 6/11/16/18/31/33/45/52/58 genotypes (included in 9vHPV) account for 73% (N=460,000) and 90% (N=570,000) of cases, respectively.
Cervical cancer remains the second most common cancer in women living in less developed regions, with about 445,000 new cases in 2012 and approximately 230,000 deaths. On the other hand, oropharyngeal carcinoma (OPC) represents a growing threat in developed countries.
Currently, more than 200 types of HPV have been identified and about 15 types (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, 82) have demonstrated tumorigenic potential. However, it is important to remember that Human Papilloma Viruses are ubiquitous. The estimated worldwide HPV-DNA point prevalence is approximately 10%. The highest estimates were found in Africa and Latin America (20–30%), and the lowest in southern Europe and South East Asia (6– 7%).
HPV infections are transmitted mainly through direct skin-to-skin or skin-to-mucosa contact, and the highest incidence of anogenital infection occurs in teens and young adults. Increasing age is linked to a decrease in the acquisition of anogenital HPV infection as a corollary of fewer new partners and, possibly, immunity to previously cleared infections. Non-sexual routes account for a tiny minority of HPV infections, and include perinatal transmission and, possibly, transmission by medical procedures and fomites.
The 2vHPV vaccine containing genotypes 16 and 18 and the 4vHPV vaccine containing genotypes 16, 18, 6 and 11 have been introduced in Europe and United States since many years. Furthermore, in June 2015 the EMA (European Medicines Agency) [2] has authorized the commercialization of the 9-valent HPV vaccine (9vHPV) in the European Union. In line with the new European public health target for HPV vaccination, the objective is to offer adolescents of both genders maximum protection against all HPV-related pathologies.
This literary review is aimed at assessing new evidences and developments in the field of HPV-related diseases, giving a comprehensive and updated view on the impact of novel vaccination strategies.
HPV Virion or Virus: Infection or Cancer
In 1983 Bândea [3] proposed the hypothesis that within HPV infected cells, HPV could be represented by virus particles, or by spores (or virions), that are reproductive forms. Low-risk HPVs may have evolved a life cycle that is optimized to rapidly produce copious amounts of progeny virus and readily form large productive lesions to maximize transmission of the virus to a new host. Conversely, highrisk HPV types tend to favor integration in the infected cell, altering cell regulation and promoting neoplastic transformation through expression of E6 and E7 (Figure 1). Such a model may predict that different HPVs may infect distinct target cells and that there may be differences in the persistence of viral genomes in infected host cells [4].
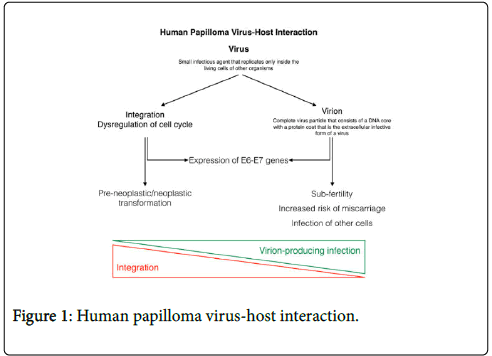
Figure 1. Human papilloma virus-host interaction.
In the everyday practice HPV, DNA testing can detect both virus and virion through the expression of relevant genes or proteins, considering the necessity of the expression of E6 and E7 genes to maintaining the malignant growth of cervical cancer cells, specifically by inhibiting the tumor suppressors p53 [5]. Therefore, conventional DNA-based HPV tests that measure by E6 or E7 are well suitable for detection of HPV in clonal transformed cells that can lead to cancer but will also detect the E6/E7 DNA part present inside virions. When the detected viral HPV-DNA originates from a dividing cell, the detected HPV-DNA is never infectious (dividing cells do not support virion production) and does not affect fertility, but the viral DNA can transform the dividing cell it resides in, which could in time lead to pre-cancer and cancer [6].
The above mentioned concepts are opening a new questioned approach on the effect of the HPV virion producing pathway. In fact, in non-dividing desquamating cells HPV virions can cause temporal subfertility and the documentation shows that an infection with HPV during pregnancy is associated with the risk of spontaneous abortion [6].
Nevertheless, detection of HPV-DNA in women does not preclude from achieving a pregnancy. It only diminishes the chances with increasing amount of free virions being able to bind or to enter the oocyte. Different is expected to be the evaluation if the detected oncogenic HPV-DNA could originate from clonal HPV transformed cell population that could develop into cancer. Women with HPV induced cervical cancer and without simultaneously occurring HPV virion inducing infection would not be at increased risk for miscarriages or reduced chances of achieving a pregnancy. The explanation of this would be related to the adherence of HPV virions because the detected HPV-DNA is confined to the nucleus of the dividing HPV transformed cells.
Many reports suggested that the presence of circulating HPV-DNA could be a triggering event for virus diffusion into bloodstream, even in the absence of clinically detectable HPV related diseases. The presence of papillomavirus in peripheral blood leukocytes was reported by experimental data from animal models, showing that BPV (Bovine Papilloma Virus) DNA and transcripts can be found in blood cells a few days after intradermal inoculation of horses with BPV1 [7]. In humans, different types of HPV (genotypes 6,11,16,19) were reported to be present in peripheral blood mononuclear cells (PBMC) from female patients affected by HPV infections of the genito-urinary tract [8]. Moreover, in a study performed on HIV patients, about 20% of blood samples were tested positive for HPV-DNA type 16 [9]. Again, in female patients with cervical cancers Rombaldi et al. demonstrated that HPV-DNA can be detected in peripheral blood cells [10]. In this study, authors concluded that HPV detected in blood cells of cancer patients was not-only subtended by metastatic cells but was also related to PBMC. In another report performed in Australia, authors demonstrated the presence of HPV-DNA in peripheral leukocytes from a cohort of healthy blood donors (8% of subjects) [11].
However, the vast majority of these studies did not show HPV inside blood cells but its presence on the outside surface of cells. A more recent study, documented the presence of human papillomavirus markers in peripheral blood of 25% of patients with HPVgenitourinary infection. Both HPV-16 E6 and L1 proteins co-localized with the viral DNA [12]. In that study, authors reported that cells displaying molecular hallmarks of HPV were frequently found to be CD20+ B-lymphocytes and CD56+ natural killer-like (NK-like) cells, two leukocyte populations recruited during viral infection. Taken together, these findings rises major concerns regarding the risk of developing cancers to distal organs.
The Role of HPV Seminal Infection
Seminal infection represents the consequences of a predominantly virion-producing differentiation of the HPV “life-cycle”. Transmission of the infection may be horizontal (by genital-genital, manual-genital, or oral-genital contact) or vertical (from mother to fetus). The risk factors that aid the establishment of this infection are: sexual intercourse at a young age, multiple sexual partners, prolonged use of oral contraceptives, high degree of parity, smoking, immunosuppression, co-infection with HIV and other sexually transmitted agents [13].
Several studies have now shown the presence of HPV in the semen of some subjects and its correlation with infertility. In fact, a higher prevalence of HPV infection has been reported in semen from infertile patients, independently of the presence of risk factors for HPV [14].
A systematic review and meta-analysis by Laprise et al. [15] reported a prevalence of HPV in the seminal fluid of 16% and 10% in the infertile and general population, respectively. Similarly, in a subsequent meta-analysis, Foresta et al. described a prevalence ranging from 2% to 31% in the general population, rising to 10%-36% in men with infertility of unknown origin [16]. Finally, in 2017, Lyu et al. published a meta-analysis on the relationship between HPV seminal infection and clinically treated infertility [17]. Evaluating a total of 31 studies, including 5,194 males, they observed an 11.4% prevalence of seminal infection in the general population (n=2.122) and of 20.4% in subjects afferent to assisted reproduction clinics (n=3.072). HPV 16 was the most common genotype, with a prevalence of 4.8% in the general population and 6% in fertility treatment candidates. Also in this case, a significantly increased risk of infertility was reported in infected males, suggesting that HPV at the seminal level could represent a risk factor for infertility in males. This has been confirmed by a further meta-analysis published in 2018 [18].
The prevalence of HPV in the sperm of infertile males therefore appears to be higher than that of the general population. Furthermore, data from the literature suggest that the presence of HPV could be able to reduce motility, induce the presence of antibodies and modify the seminal pH [19-22]. In a recent study, Boeri et al. showed that the presence of high-risk HPV in semen of infertile men is associated even with higher sperm DNA fragmentation [23].
However, there are only few data about the presence and significance of HPV in sperm. In particular, the exact localization and mechanism of infection by HPV in sperm, as well as the role of infected sperm cells as a transmission vector for the virus to the oocyte and possible interference with fertilization and embryo development are little known. Beside natural fertility, this is also of crucial importance in relation to in vitro fertilization techniques because of the possibility that sperm infected with HPV injected in the oocyte cytoplasm could interfere with fertilization, implantation, embryo development, premature abortion, and definitively with outcome and safety of these techniques. An in vitro study performed in 2011 [24], shed new light in these aspects. The authors showed that sperm infection takes place through the binding between the viral capsid of HPV and the protein syndecan-1 on the sperm head. Moreover, they demonstrated that infected sperm are able to transfer the capsid protein L1 and E6/E7 viral genes to the oocyte. Finally, they showed that HPV genome released by sperm was actively transcribed by the penetrated oocyte. These findings would explain the growing number of evidences concurred to identify the impairment of human reproduction as a consequence of sperm HPV infection [25,26,16]. In these articles, authors clearly demonstrated that couples undergoing assisted reproduction more frequently experience reduced fertilization, implantation and birth rate when the male partner has HPV semen infection. In particular, the presence of HPV-DNA bound to sperm resulted as the best predictive factor of early miscarriage.
Recent data suggested that HPV prophylactic vaccination is effective in reducing mean clearance time in patients featured by HPV semen infection. This evidence may have a break through impact on the clinical approach to patients with long-lasting HPV semen infection and in particular for those infertile patients eligible for ART (assisted reproduction technique) [27]. On this basis, a retrospective study was conducted to compare the natural reproductive outcome in vaccinated and unvaccinated patients [28]. The authors included 151 infertile couples with partners suffering from HPV seminal infection. Among the partners, 79 had received the vaccine while 72 had refused it. In the group receiving vaccination, in parallel to a higher rate of recovery, an improvement in the seminal parameters and a higher percentage of natural pregnancies were found (36% vs. 6%). Furthermore, among couples where the partner had received the vaccine, the rate of abortions was significantly reduced compared to that observed in the unvaccinated ones.
In summary, the finding of HPV in the sperm seems to be strongly predictive of the negative outcome of pregnancy. In this context, adjuvant vaccination seems to be associated with a higher eradication of HPV in the seminal fluid and with a significant increase in the rate of natural pregnancies and live births.
The Impact of Human Papilloma Virus in the Upper Aerodigestive Tract
The recently increasing prevalence of HPV infection in OPC has led to a radical change in the clinical and epidemiological characteristics of this disease, with a growing interest in the role of HPV in head and neck oncology. During the period 1999-2015, the CDC (Centers for Disease Control and Prevention) analyzed data from cancer registries covering 97.8% of the U.S. population [29]. In this report, cervical cancer rates decreased 1.6% per year while OPC rates increased among both men (2.7%) and women (0.8%). Incredibly in 2010, as a result of cervical cancer screening [30], the incidence of OPC in males resulted for the first time higher than that of cervical cancer. During 2015, the CDC reported 11,788 cases of cervical carcinoma and 18,917 cases of OPC, including 15,479 (82%) among men and 3,438 (18%) among women.
Conventional non-HPV-related OPC have comparable characteristics to the wide family of head and neck tumors. These tumors are typically characterized by a peak of incidence in the advanced age and a close relationship with classic risk factors, such as smoking and alcohol abuse. However, researchers have recently identified a progressive growth in a patient population with significantly different characteristics: younger age, lower prevalence of risk factors and favorable prognostic results [31]. This group of subjects, previously unidentified due to their lower prevalence, represents the cohort of patients affected by HPV-related OPC (HPVOPC). In fact, further studies demonstrated that HPV infection represents the main oncogenic drive in this subset of tumors.
Currently, the incidence of OPC is steadily growing throughout the world (particularly in North America and northern Europe), with a relative prevalence in males [32]. This phenomenon, investigated by various meta-analyses [33,34] is mainly linked to the increasing prevalence of oral infection from high-risk HPV genotypes and, consequently, to an increased incidence of HPV-OPC. The magnitude of this increase led to define this scenario as an actual HPV epidemic [35].
According to the study by Mehanna et al. [33] the global prevalence of HPV in the context of OPC has shifted from about 40% before 2000 to more than 70% after 2005. This growth appears to be rapid and progressive, with more recent data showing a prevalence above 90% in northern Europe [36].
Specifically, the HPV genotype most commonly associated with these tumors is the 16, responsible for about 84% of HPV-OPC [37-40].
Additional high-risk genotypes (mainly 33 and 18, also comprised in the 9-valent vaccine) are identifiable in approximately 10% of cases. This represents a significant advantage for potential vaccination coverage. In fact, the 9-valent vaccine has the potential to prevent infection by almost all genotypes associated with this disease.
In general, the prevalence of oral/oropharyngeal infection from high-risk HPV genotypes is highly variable in the population: ranging from 0% to about 6% [41-43], with peaks of 18% when more accurate analyses are performed [44]. This variability is mainly related to the geographic area analyzed, the genotypes included in the evaluation and the technique employed for determination. However, it is possible to observe a large group of individuals characterized by silent infection, without the immediate development of benign or neoplastic lesions, for whom the mechanism of initiation and neoplastic progression is not yet clear.
The number of sexual partners (especially as regards oral sex) was found to be the primary modifiable risk factor for this infection [45,43]. In addition, males showed a prevalence of infection three times higher than females. It has been suggested that the recent increase of incidence in HPV-OPC may be connected to the progressive modification of sexual habits, leading to a greater risk of infection [46].
In general, HPV-related OPC represents a separate neoplasm from conventional head and neck cancers and its differences can be observed in a variety of aspects: epidemiology, genetic and molecular mechanisms, clinical presentation, response to therapy and prognosis. Although the different epidemiological characteristics have already been mentioned, it is essential to underline how they often lead to a different patient management in the daily clinical practice. In fact, patients are frequently younger, belonging to a higher socio-economic class, with a healthier lifestyle, lower comorbidities and a lower rate of tobacco consumption [45]. These differences encouraged the search for a better balance in their therapeutic approach. In the face of a higher life expectancy and greater social and work commitment, the treatment outcomes (long-term complications and sequelae) assume a predominant value in view of the improved prognosis. The main objective is to obtain the same survival results while reducing treatment invasiveness and sequelae.
For this reason, it has been possible to observe a rise in minimally invasive surgical techniques and non-surgical therapeutic regimens that may reduce post-treatment sequelae [47-49]. Currently, several clinical trials aimed at identifying the right balance between therapeutic efficacy and side effects are ongoing [47-49]. However, given the very recent development of these techniques, there is still no sufficient data to modify the current treatment indications.
From a genetic and molecular point of view, HPV-OPC is radically different from its HPV-negative counterpart. The Cancer Genome Atlas (TCGA) [50] provided essential genetic characterization data for hundreds of head and neck squamous cell carcinomas (including HPV-OPC). Therefore, it was possible to confirm the substantial difference of the tumors driven by HPV infection, which are based on molecular activation mechanisms that are separate from other neoplasms. This is mainly due to the influence of the E6 and E7 proteins (an integral component of HPV) on the genomic regulation network.
These differences in the constitutive mechanisms of HPV-related OPC result in a different biological behavior. In fact, these tumors frequently present as early lesions (small and with minimal deep infiltration), but with significant nodal involvement [31]. In some cases, this imbalance between local extension (T category) and nodal burden (N category) can result in the impossibility of identifying the tumor of origin in the presence of metastatic lateral neck lymph nodes positive for HPV. This scenario, also characterized by an increasing incidence in the last decade, is defined as "carcinoma of unknown primary" [51]. Finally, it is crucial to underline how the different biological characteristics of HPV-OPC translate into prognostic results that are significantly improved when compared with those of the HPVnegative counterpart, regardless of the stage of disease. This has been widely demonstrated by several researches, including the rigorous study by Ang and co-workers [52] assessing a homogeneous cohort of 323 patients.
These clinical and prognostic differences have led to a change in the TNM staging system, which introduced a different prognostic classification for HPV-OPC and non-HPV-related carcinomas in the transition from the 7th to the 8th edition [53].
Indeed, this is the first official staging system differentiated by a molecular factor in head and neck oncology.
Once the critical role of HPV in the oncologic field has been clarified, it is necessary to mention its impact on other diseases of the upper aerodigestive tract (UADT). In fact, vaccination can have an equally significant effect on these affections. The most frequent are the squamous papilloma, the condyloma acuminatum, and the verruca vulgaris. Although the effect on individual heath is not as significant as that of OPC, the impact of these frequent diseases on general healthcare is non-negligible.
HPV infection (genotype 6 and 11) is also closely related to the recurrent respiratory papillomatosis, a rare disease which prevalence is about 2:100,000 in adults and 4:100,000 in children. This affection is characterized by the development of numerous papillomas involving the larynx or contiguous regions of the respiratory tract, especially in the pediatric age, due to maternal-fetal transmission. These papillomas often interfere with breathing and phonation and can frequently relapse, requiring various surgical procedures throughout the patient's life. Without treatment, the disorder is potentially fatal due to the progressive obstruction of the airways.
Thus, in the head and neck region, vaccination for HPV may potentially influence this complex array of diseases, characterized by a wide range of incidence and clinical impact. The main reason why the current HPV vaccines are not licensed for prevention of head and neck cancers is that clinical trials were only able to show an effect against cervical and other anogenital premalignant lesions [54-58].
Interestingly, various randomized phase III clinical trials have shown a significant reduction in the incidence of anogenital infections, genital warts and cervical or anal pre-neoplastic HPV 16/18 lesions following the implementation of vaccination programs [59-62].
This provides a first indirect proof of the possible effect of this preventive measure also at the level of the UADT [63]. Furthermore, additional studies have shown the production of a high titre of HPVspecific antibodies in oral mucosa fluids (saliva and mucosal transudate) in vaccinated subjects [64,65].
A study published in 2018 by Chaturvedi et al. [66] showed the first direct results of vaccination on the prevalence of oral/oropharyngeal HPV infection in 2,627 young adults. The comparison between unvaccinated and vaccinated subjects showed an 88% reduction in the rate of oral/oropharyngeal infection of the 4 genotypes included in the vaccine employed in the American population (6, 11, 16 and 18), shifting from a prevalence of 1.61% to 0.11%. These data confirm the preliminary results obtained by Herrero et al. [67] that observed a 93% reduction in the prevalence of HPV 16 and 18, although the analyzed population was less generalizable compared to that of Chaturvedi et al. However, although with favorable perspectives, high-quality data confirming the effectiveness of vaccination in preventing HPV-OPC is still lacking. In fact, the wide latency interval between HPV oropharyngeal infection and the development of HPV-related tumors, as well as their relatively advanced age of onset, do not yet allow to evaluate the effects of recent vaccination campaigns. In the future, it will be possible to observe the direct influence of this preventive measure on the global incidence of OPC.
Finally, tertiary prevention (reduction of relapses) represents a further potential for HPV vaccination in the field of UADT pathologies. This is particularly true when considering both recurrent respiratory papillomatosis and OPC, for which several there are several studies showing a reduction in the relapse rate and an increase in the disease-free interval in affected patients who were treated with the quadrivalent vaccine [68,69].
Conclusions
Only a few years ago attention for HPV vaccination was paid almost entirely to the prevention of genital cancers and of cervical cancer. In particular where, along with cervical cancer screening programs, it helped lowering its incidence. Vaccination programs were aimed almost exclusively at young girls and women, with very few exceptions (e.g. Australia). In the meantime, the rate of OPC was growing worldwide and since 2010 it represents the most frequent HPV related cancer in the U.S.: data that helped paying attention to male HPV vaccination. This was even more evident when the first data about presence of the virus in the seminal fluid, bound to sperm’s head, were published, making it clear that infection could lead to impaired sperm parameters, higher miscarriage rate and in general to couple infertility both by natural and assisted conception (Tables 1 and 2).
| Does the presence of the virus in the individual have only a meaning of sexual infection, due to sexual actions between individuals? In the majority of cases the presence of HPV is due to a sexual transmission of the virus. Infants, children, and adults can acquire both high-risk and low-risk infections by birth or by close contact. HPV DNA detection in amniotic fluid, fetal membranes, cord blood and placental trophoblastic cells all suggest HPV infection in utero, i.e. prenatal transmission. The mother seems to be the main transmitter of HPV to her newborn, but subsequent HPV infections are acquired horizontally via saliva or other contacts. (20% of cases?). The possibility of transmitting infection via breastfeeding is debated, but to date there is not enough evidence to limit the practice of natural breastfeeding in HPV-positive women. Infection can also happen through auto or hetero-inoculation, through the contact with the fingers of subjects with genital lesions due to HPV strains by uninfected individuals. There is some evidence that infection can also occur through prolonged exposure to contaminated clothing, because the virus can persist on contaminated objects [82-85]. |
Table 1: Focus on the Context.
| Can the presence of the HPV virus be seen as evidence of a parasitic strain (such as from cutaneous or intestinal microbiota)? An alteration in the bacterial microbiota composition is described, even if there is not enough evidence on how the bacterial microbiota alterations can influence and predispose the HPV infection. The association between HPV infection and alterations in the composition of the oral bacterial microbiota in breast-fed infants is described It is believed that the composition of the vaginal microbiome can influence the innate immune response, the susceptibility to infection and the development of cervical pathology [86,87]. |
Table 2: Focus on the New.
The capacity of vaccination to elicit a powerful immune response has been used as an adjuvant in patients with HPV-related cancer both to prevent recurrence and in association with targeted therapy, as well as in HPV infected infertile couples. It is interesting to observe the implication of this ancestral virus in two apparently distant areas, the beginning of life – fertility – and one of the most destructive diseases – cancer. At present there is no doubt that universal vaccination against HPV has demonstrated its effectiveness in the prevention of most HPV-related conditions and worldwide prevalence data allow us to consider HPV vaccination from adolescence to late adulthood (Tables 1,2 and 3).
| After the introduction of the vaccine, the clinical information concerning the HPV changed considerably: At the time of its introduction, the HPV vaccine was mostly used for the prevention of cervical cancer in women. A few years later, the role of HPV as causative agent of gender-neutral cancers was proved, namely anal, oral and head and neck cancers. In 2015, a new version of the vaccine was introduced, active on a larger number of HPV strains causing malignancies. Switch to universal vaccination with the 9vHPV vaccine means: Further reduce the burden on public health of HPV-related cancers and diseases. May lead to near-elimination of related HPV cancers and warts. Reaching an important level of cost-effectiveness. To recover the necessary investment to finance the new vaccination strategy, freeing up resources to support the prevention of other infectious diseases. |
Table 3: The Economic Value of HPV Vaccination.
With this literature review, we can conclude that: i) HPV infection is still very prevalent in sexually active population and may be associated to cervical, genital, oropharyngeal, penile, and anal cancers; ii) in the past years, due to HPV vaccination and screening for cervical cancer, the incidence of cervical carcinoma is strongly decreased; iii) the rate of oropharyngeal cancer is growing worldwide and since 2010 it represents in males the most frequent HPV related cancer among U.S. population; iv) HPV semen infection, particularly when HPV is bound to sperm, has been related to impaired sperm parameters, higher miscarriage rate and in general to couple infertility both by natural and assisted conception; v) recent efforts toward a universal vaccination against HPV have demonstrated its effectiveness in the prevention of most HPV related conditions; vi) growing evidences suggest positive effects of adjuvant vaccination in patients with HPV related cancer and in HPV infected infertile couples.
HPV Infections, Immunity and Cancer
The immune system plays a central role in determining the outcome of HPV infection and the immune response essential for the clearance of HPV.
The HPV-16 E6 and E7 oncoproteins are constitutively expressed in cancer lesions as putative targets for the immune response against HPV [70].
As an aberration of virus infection, the constant activity of the viral proteins E6 and E7 leads to increasing genomic instability and accumulation of oncogene mutations, resulting in cancer formation [71,72,4]. Although the immune response is able to overcome the evasion mechanisms and clear infection in most cases, prolonged persistence of high-risk HPV types sometimes leads to malignancy.
Defense mechanisms include components of both the innate and the adaptive immune systems. The components of the innate immune response are activated complement ones, destroying virus infected cells and the virus itself. Specific antibodies for viral surface antigens block binding and/or fusion of virus, and coat the viral particles to facilitate receptor mediated phagocytosis and/or complemented mediated lysis of virus. Antibody production from the mucosal tissues prevents HPV infection in the mucosal surface. The majority of HPV infections are cleared before cancer formation begins. However, if the host immune system fails to eliminate the virus, persistent HPV infection occurs.
Recent therapeutic approaches have shown the great potential of the stimulation of innate immune mechanisms, generally increasing Th1 or cytotoxic cells activities at the final step or even, both. T-cell mediated immune responses against oncogenic HPV are believed to play a central role in HPV-associated cancers. Natural killer (NK) cells are important components of the innate immune system directly involved in the anti-viral immune response. Ligands for NK cell receptors are primary for viral immune evasion. A study field, which is receiving more attention currently, considers the importance of the cells adjacent to the HPV-related tumor and all the environment surrounding it, highlighting the stromal cells (e.g. fibroblasts) as well as the cytokines and chemokines they produce. These cells may be considered as co-factors for HPV-associated carcinogenesis due to the intense cross-talk between them and epithelial tumor or immune cells at the tumor microenvironment [73].
Numerous investigations have identified NF-kB as an important modulator in driving chronic inflammation to cancer. This transcription factor is indispensable for the malignant progression of transformed cells associated with various inflammatory cells and a network of signaling molecules. The expression and the function of numerous cytokines, chemokines, growth factors, and survival factors are NF-kB-dependent. It has been suggested that HPV oncogenemediated suppression of NF-kB activity contributes to HPV escape from the immune system [74-76].
Specifically, cytokines attract other immune cells to the HPVinfection or -tumor microenvironment, by inducing T cell activation or differentiation and, in case of NK cells, by killing HPV-infected cells. Several studies have demonstrated the great potential of these cells in tumor prevention and eradication [77,78].
In particular, it is highlighted the contribution of both cells with some immune properties (e.g., keratinocytes and stromal cells) and the immune cells themselves, such as the antigen presenting cells (APCs), NK cells, macrophages and neutrophils. Furthermore, a hypothesis of stromal cell-centered HPV-related carcinogenesis is presented. The major role of stromal cells in HPV-related carcinogenesis, however, is to induce a proinflammatory milieu, a condition for establishment of HPV transformed cells. In addition, stromal cells support virus immune evasion, matrix architectural change, proliferation, invasion, angiogenesis and are associated with chronic infection [79-81].
In summary, the causal relationship between chronic inflammation and cancer is widely accepted. Therefore, there is a strong association between tumor viruses and development of cancers due to viral immune evasion.
REFERENCES
- Serrano B, Brotons M, Bosch FX, Bruni L. Epidemiology and burden of HPV-related disease. Best Pract Res Clin Obstet Gynaecol 2018;47:14-26.
- European Medical Agency. European public assessment report (EPAR) for Gardasil 2019;9.
- Bândea CI. A new theory on the origin and the nature of viruses. J Theor Biol 1983;105:591-602.
- Münger K, Baldwin A, Edwards KM, Hayakawa H, Nguyen CL, Owens M,et al. Mechanisms of human papillomavirus-induced oncogenesis. J Virol 2004;78:11451-11460.
- Zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer 2002;2:342-350.
- Depuydt CE, Beert J, Bosmans E, Salembier G. Human Papillomavirus (HPV) virion induced cancer and subfertility, two sides of the same coin. Facts Views Vis Obgyn 2016;8:211-222.
- Hartl B, Hainisch EK, Shafti-Keramat S, Kirnbauer R, Corteggio A, Borzacchiello G, et al. Inoculation of young horses with bovine papillomavirus type 1 virions leads to early infection of PBMCs prior to pseudo-sarcoid formation. J Gen Virol 2011;92:2437-2445.
- Pao CC, Lin SS, Lin CY, Maa JS, Lai CH, Hsieh TT. Identification of human papillomavirus DNA sequences in peripheral blood mononuclear cells. Am J Clin Pathol 1991;95:540-546.
- Bodaghi S, Wood LV, Roby G, Ryder C, Steinberg SM, Zheng ZM. Could human papillomaviruses be spread through blood? J Clin Microbiol 2005;43:5428-5434.
- Rombaldi RL, Serafini EP, Mandelli J, Zimmermann E, Losquiavo KP. Perinatal transmission of human papilomavirus DNA. Virol J 2009;6:83.
- Chen AC, Keleher A, Kedda MA, Spurdle AB, McMillan NA, Antonsson A. Human papillomavirus DNA detected in peripheral blood samples from healthy Australian male blood donors. J Med Virol 2009;81:1792-1796.
- Foresta C, Bertoldo A, Garolla A, Pizzol D, Mason S, Lenzi A, et al. Human papillomavirus proteins are found in peripheral blood and semen Cd20+ and Cd56+cells during HPV-16 semen infection. BMC Infect Dis 2013;13:593.
- Foresta C, Garolla A, Zuccarello D, Pizzol D, Moretti A, Barzon L, et al. Human papillomavirus found in sperm head of young adult males affects the progressive motility. Fertility and Sterility 2010;93:802- 806.
- Garolla A, Pizzol D, Bertoldo A, Menegazzo M, Barzon L, Foresta C . Sperm viral infection and male infertility: focus on HBV, HCV, HIV, HPV, HSV, HCMV, and AAV. J Reprod Immunol 2013;100:20-29.
- Laprise C, Trottier H, Monnier P, Coutlee F, Mayrand MH (2014) Prevalence of human papillomaviruses in semen: a systematic review and metaanalysis. Hum Reprod 2014;29:640-651.
- Foresta C, Noventa M, De Toni L, Gizzo S, Garolla A.HPV DNA sperm infection and infertility: from a systematic literature review to a possible clinical management proposal. Andrology 2015;3:163-173.
- Lyu Z, Feng X, Li N, Zhao W, Wei L, Chen Y, et al. Human papillomavirus in semen and the risk for male infertility: a systematic review and meta-analysis. BMC Infectious Diseases 2017;17:714.
- Xiong YQ, Chen YX, Cheng MJ, He WQ, Chen Q. The risk of human papillomavirus infection for male fertility abnormality: a meta-analysis. Asian J Androl 2018;2:493-497.
- Garolla A, Lenzi A, Palù G, Pizzol D, Bertoldo A, Toni DL,et al. Human papillomavirus sperm infection and assisted reproduction: a dangerous hazard with a possible safe solution. Hum Reprod 2012;27:967-973.
- Rintala MA, Grénman SE, Pöllänen PP, Suominen JJ, Syrjänen SM. Detection of high risk HPV DNA in semen and its association with the quality of semen. Int J STD AIDS 2044;15:740-743.
- Lee CA, Huang CT, King A, Chan PJ. Differential effects of human papillomavirus DNA types on p53 tumor suppressor gene apoptosis in sperm. Gynecol Oncol 2002;85:511-516.
- Souho T, Benlemlih M, Bennani B. Human papillomavirus infection and fertility alteration: a systematic review. PloS One 2015;10:e0126936.
- Boeri L, Capogrosso P, Ventimiglia E, Pederzoli F, Cazzaniga W, Chierigo F, et al. High-risk human papillomavirus in semen is associated with poor sperm progressive motility and a high sperm DNA fragmentation index in infertile men. Hum Reprod 2019;34:209-217.
- Foresta C, Patassini C, Bertoldo A, Menegazzo M, Francavilla F, Barzon L,et al. Mechanism of human papillomavirus binding to human spermatozoa and fertilizing ability of infected spermatozoa. PLoS One 2011;6:e15036.
- Perino A, Giovannelli L, Schillaci R, Ruvolo G, Fiorentino FP, Alimondi P, et al. Human papillomavirus infection in couples undergoing in vitro fertilization procedures: impact on reproductive outcomes. Fertil Steril 2011;95:1845-1848.
- Garolla A, Engl B, Pizzol D, Ghezzi M, Bertoldo A, Bottacin A, et al. Spontaneous fertility and in vitro fertilization outcome: New evidence of human papillomavirus sperm infection. Fertil Steril 2016;105:65-72.
- Foresta C, Garolla A, Parisi S, Ghezzi M, Bertoldo A, Nisio AD,et al. HPV prophylactic vaccination in males improves the clearance of semen infection. EBioMedicine 2015;2:1487-1493.
- Garolla A, De Toni L, Bottacin A, Valente U, De Rocco Ponce M, Di Nisio A, et al. Human papillomavirus prophylactic vaccination improves reproductive outcome in infertile patients with HPV semen infection: a retrospective study. Sci Rep 2018;8:912.
- Van Dyne EA, Henley SJ, Saraiya M, Thomas CC, Markowitz LE, Benard VB, et al. Trends in human papillomavirus-associated cancers - United States, 1999-2015. MMWR Morb Mortal Wkly Rep 2018;6:918-924.
- Benard VB, Thomas CC, King J, Massetti GM, Doria-Rose VP, Saraiya M, et al. (2014) Vital signs: cervical cancer incidence, mortality, and screening—United States, 2007-2012. MMWR Morb Mortal Wkly Rep 2014;63:1004-1009.
- Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst 2008;100:261-269.
- Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol 2015;33:3235-3242.
- Mehanna H, Beech T, Nicholson T, El-Hariry I, McConkey C, Paleri V, et al. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer--systematic review and meta-analysis of trends by time and region. Head Neck 2013;35:747-755.
- Mourad M, Jetmore T, Jategaonkar AA, Moubayed S, Moshier E, Urken ML. Epidemiological trends of head and neck cancer in the United States: A SEER Population Study. J Oral Maxillofac Surg 2017;75:2562-2572.
- Dalianis T. Human papillomavirus and oropharyngeal cancer, the epidemics, and significance of additional clinical biomarkers for prediction of response to therapy (Review). Int J Oncol 2014;44:1799-1805.
- Nasman A, Attner P, Hammarstedt L, Du J, Eriksson M, Giraud G, et al. Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: an epidemic of viral-induced carcinoma? Int J Cancer 2009;125:362-366.
- de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010;11:1048-1056.
- de Sanjose S, Alemany L, Ordi J, Tous S, Alejo M, Bigby SM, et al. Worldwide human papillomavirus genotype attribution in over 2000 cases of intraepithelial and invasive lesions of the vulva. Eur J Cancer 2013;49:3450-3461.
- Alemany L, Saunier M, Tinoco L, Quiros B, Alvarado-Cabrero I, Alejo M, et al. Large contribution of human papillomavirus in vaginal neoplastic lesions: A worldwide study in 597 samples. Eur J Cancer 2014;50:2846-2854.
- Castellsague X, Alemany L, Quer M, Halec G, Quiros B, Tous S, et al. HPV involvement in head and neck cancers: comprehensive assessment of biomarkers in 3680 patients. J Natl Cancer Inst 2016;108:djv403.
- Ernster JA, Sciotto CG, O'Brien MM, Robinson LJ, Willson T. Prevalence of oncogenic human papillomavirus 16 and 18 in the palatine tonsils of the general adult population. Arch Otolaryngol Head Neck Surg 2009;135:554-557.
- Kreimer AR, Bhatia RK, Messeguer AL, Gonzalez P, Herrero R, Giuliano AR. Oral human papillomavirus in healthy individuals: a systematic review of the literature. Sex Transm Dis 2010;37:386-391.
- Orosco RK, Kedarisetty S, Hecht AS, Chang DC, Coffey CS, Weissbrod PA. Predictors of high-risk and low-risk oral HPV infection in the United States. Laryngoscope 2016;126:1365-1372.
- Bruni L, Albero G, Serrano B, Mena M, Gómez D, Muñoz J, et al. Human Papillomavirus and Related Diseases in the World. Summary Report 2019;22.
- D'Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med 2007;356:1944-1956.
- Herbenick D, Reece M, Schick V, Sanders S A, Dodge B, Fortenberry JD. Sexual behavior in the United States: results from a national probability sample of men and women ages 14-94. J Sex Med 2010;5:255-265.
- de Almeida JR, Byrd JK, Wu R, Stucken CL, Duvvuri U, Goldstein DP, et al. A systematic review of transoral robotic surgery and radiotherapy for early oropharynx cancer: a systematic review. Laryngoscope 2014;124:2096-2102.
- Cracchiolo JR, Baxi SS, Morris LG, Ganly I, Patel SG, Cohen MA, et al. Increase in primary surgical treatment of T1 and T2 oropharyngeal squamous cell carcinoma and rates of adverse pathologic features: National Cancer Data Base. Cancer 2016;122:1523-1532.
- Cracchiolo JR, Roman BR, Kutler DI, Kuhel WI, Cohen MA. Adoption of transoral robotic surgery compared with other surgical modalities for treatment of oropharyngeal squamous cell carcinoma. J Surg Oncol 2016;114:405-411.
- Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015;517:576-582.
- Strojan P, Ferlito A, Medina JE, Woolgar JA, Rinaldo A, Robbins KT, et al. Contemporary management of lymph node metastases from an unknown primary to the neck: I. A review of diagnostic approaches. Head Neck 2013;35:123-132.
- Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010;363:24-35.
- Brierley JD, Gospodarowicz MK, Wittekind C.TNM classification of malignant tumours. (8th edn.), West Sussex:Chichester Wiley-Blackwell; 2009:272.
- Ault KA, Future II Study Group. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet 2007;369:1861-1868.
- Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B. HPV Vaccine Study Group. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 2006;367:1247-1255.
- Lehtinen M, Paavonen J, Wheeler CM, Jaisamrarn U, Garland SM, Castellsagué X, et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 2012;13:89-99.
- Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. NEJM 2007;356:1928-1943.
- Giuliano AR, Palefsky JM, Goldstone S, Moreira ED, Jr, Penny ME, Aranda C,et al. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. NEJM 2011;364:401-411.
- Munoz N, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, et al. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J Natl Cancer Inst 2010;102:325-339.
- Kreimer AR, Gonzalez P, Katki HA, Porras C, Schiffman M, Rodriguez AC, et al. Efficacy of a bivalent HPV 16/18 vaccine against anal HPV 16/18 infection among young women: a nested analysis within the Costa Rica Vaccine Trial. Lancet Oncol 2011;12:862-870.
- Markowitz LE, Tsu V, Deeks SL, Cubie H, Wang SA, Vicari AS, et al. Human papillomavirus vaccine introduction--the first five years. Vaccine 2012;30:F139-F148.
- Lehtinen M, Eriksson T, Apter D, Hokkanen M, Natunen K, Paavonen J, et al. Safety of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine in adolescents aged 12-15 years: interim analysis of a large community-randomized controlled trial. Hum Vaccin Immunother 2016;12:3177-3185.
- Suzich J A, Ghim SJ, Palmer-Hill FJ, White WI, Tamura JK, Bell JA,et al. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc Natl Acad Sci USA 1995;92:11553-11557.
- Rowhani-Rahbar A, Carter JJ, Hawes SE, Hughes JP, Weiss NS, Galloway DA, et al. Antibody responses in oral fluid after administration of prophylactic human papillomavirus vaccines. J Infect Dis 20069;200:1452-1455.
- Handisurya A, Schellenbacher C, Haitel A, Senger T, Kirnbauer R. Human papillomavirus vaccination induces neutralising antibodies in oral mucosal fluids. Br J Cancer 2016;114:409-416.
- Chaturvedi AK, Graubard BI, Broutian T, Pickard RKL, Tong ZY, Xiao W, et al. Effect of prophylactic human papillomavirus (HPV) vaccination on oral HPV infections among young adults in the United States. J Clin Oncol 2018;3:262-267.
- Herrero R, Quint W, Hildesheim A, Gonzalez P, Struijk L, Katki HA, et al. Reduced prevalence of oral human papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomized clinical trial in Costa Rica. PLoS One 2013;8:e68329.
- Mauz PS, Schafer FA, Iftner T, Gonser P. HPV vaccination as preventive approach for recurrent respiratory papillomatosis - a 22-year retrospective clinical analysis. BMC Infect Dis 2018;18:343.
- Cheng MA, Farmer E, Huang C, Lin J, Hung CF, Wu TC. Therapeutic DNA vaccines for human papillomavirus and associated diseases. Hum Gene Ther 2018;29:971-976.
- Kadish AS, Ho GY, Burk RD, Wang Y, Romney SL, Ledwidge R, et al. Lymphoproliferative responses to human papillomavirus (HPV) type 16 proteins E6 and E7: outcome of HPV infection and associated neoplasia. J Natl Cancer Inst 1997;89:1285-1293.
- Senba M, Mori N, Wada A. Oncogenesis of human papillomavirus (HPV). New York:Nova Science Publishers 2009;75-102.
- Senba M, Mori N, Wada A.Oncogenesis of and the link between inflammation and cancer due to human papillomavirus (HPV) infection, and the forthcoming eradication for vaccination and the development of vaccine control strategies. New York:Nova Science Publishers 2011;85-115.
- Spurgeon M, Lambert P. Human papillomavirus and the stroma: bidirectional crosstalk during the virus life cycle and carcinogenesis. Viruses 2017;9:219.
- Nees M, Geoghegan JM, Hyman T, Frank S, Miller L, Woodworth CD. Papillomavirus type 16 oncogenes downregulate expression of interferon-responsive genes and upregulate proliferation-associated and NF-κB-responsive genes in cervical keratinocytes. J Virol 2001;75:4283-4296.
- Spitkovsky D, Hehner SP, Hofmann TG, Moller A, Schmitz L. The human papillomavirus oncoprotein E7 attenuates NF-κB activation by targeting the IκB kinase complex. J Biol Chem 2002;277:25576-25582.
- Mishra A, Bharti AC, Varghese P, Saluja D, Das BC. Differential expression and activation of NF-κB family proteins during oral carcinogenesis: Role of high risk human papillomavirus infection. Int J Cancer 2006;119:2840-2850.
- Langers I, Renoux V, Reschner A, Touzé A, Coursaget P, Boniver J,et al. Natural killer and dendritic cells collaborate in the immune response induced by the vaccine against uterine cervical cancer. Eur J Immunol 2014;44:3585-3595.
- Gras Navarro A, Björklund AT, Chekenya M. Therapeutic potential and challenges of natural killer cells in treatment of solid tumors. Front Immunol 2015;6:202.
- Erez N, Truitt M, Olson P, Hanahan D, Hanahan D. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-κB-dependent manner. Cancer Cell Int 2010;17:135-147.
- Woodby B, Scott M, Bodily J. The interaction between human papillomaviruses and the stromal microenvironment. Prog Mol Biol Transl Sci 2016;144:169-238.
- Fullár A, Dudás J, Oláh L, Hollósi P, Papp Z, Sobel G, et al. Remodeling of extracellular matrix by normal and tumor-associated fibroblasts promotes cervical cancer progression. BMC Cancer 2015;15:256.
- Sabeena S, Bhat P, Kamath V, Arunkumar G. Possible non-sexual modes of transmission of human papilloma virus. J Obstet Gynaecol Res 2017;43:429-435.
- Syrjänen S. Current concepts on human papillomavirus infections in children. APMIS 2010;118:494-509.
- Moscicki AB, Schiffman M, Burchell A, Albero G, Giuliano AR, Goodman MT, et al. Updating the natural history of human papillomavirus and anogenital cancers. Vaccine 2012;30:F24-F33.
- Ryndock EJ, Meyers C. A risk for non-sexual transmission of human papillomavirus? Expert Rev Anti Infect Ther 2014;12:1165-1170.
- Tuominen H, Rautava S, Syrjänen S, Collado MC, Rautava J. HPV infection and bacterial microbiota in the placenta, uterine cervix and oral mucosa. Sci Rep 2018;8:9787.
- Tuominen H, Rautava S, Collado MC, Syrjänen S, Rautava J. HPV infection and bacterial microbiota in breast milk and infant oral mucosa. PLoS One 2018;13:e0207016.
Citation: Paderno A, Garolla A, Pecorelli S, Lombardi A, Pinto C, Icardi G, et al. (2019) HPV Vaccination Concepts in the Reality of Today. J Vaccines Vaccin 10:399. doi: doi: 10.24105/2157-7560.10.399
Copyright: © 2019 Paderno A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

