Indexed In
- Open J Gate
- Academic Keys
- RefSeek
- Hamdard University
- EBSCO A-Z
- Publons
- Geneva Foundation for Medical Education and Research
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Brief Report - (2024) Volume 12, Issue 3
Glucocorticoid Receptor Expression in Torus Tubarius Hypertrophy Tissue Causing Secretory Otitis Media in Children
Zhi-Pan Teng, Qi Li* and Xiao-Fei Shen*Received: 30-Sep-2024, Manuscript No. HCCR-24-27026; Editor assigned: 02-Oct-2024, Pre QC No. HCCR-24-27026 (PQ); Reviewed: 17-Oct-2024, QC No. HCCR-24-27026; Revised: 25-Oct-2024, Manuscript No. HCCR-24-27026 (R); Published: 01-Nov-2024, DOI: 10.35248/2375-4273.24.12.414
Abstract
Background: Torus Tubarius Hypertrophy (TTH) after adenoidectomy causing nasal obstruction, recurred snoring, sleep apnea, or mouth breathing has been reported more and more recently, partial resection of TTH conducted by a low-temperature plasma technique is a safe and effective therapy and some benefit from intranasal steroid therapy. However, the expression of Glucocorticoid Receptor (GCR) in TTH tissue is currently unknown and no paper reported secretory otitis media caused by TTH.
Objectives: To assess the expression patterns of the human GCR in TTH tissue in children and report secretory otitis media caused by TTH.
Methods: 5 children from Jan to Jun 2024 were included in this study. All children underwent partial resection of TTH and TTH tissues were collected for immunofluorescence.
Results: GCR-α and GCR-β were both expressed in TTH tissues in 5 children, the expression of GCR-α was significantly higher than GCR-β. Since it has been reported that the high expression of GCR-α is correlated to glucocorticoid therapy sensitivity, the glucocorticoid therapy may be curative to TTH in children.
Conclusion: TTH after adenoidectomy were reported more often in recently, partial resection of TTH operation conducted by a low-temperature plasma technique under general anesthesia is a safe and effective therapy, intranasal corticosteroids could be an alternative solution of TTH. However, the function of GCR in children with TTH still requires further investigation.
Keywords
Torus Tubarius Hypertrophy (TTH); Glucocorticoid Receptor (GCR); Children; Glucocorticoid therapy; Adenoidectomy; Corticosteroids; Anesthesia
Introduction
Adenoidectomy conducted by a low-temperature plasma technique is one of the most curative operations in children with adenoid hypertrophy causing sleep-disordered breathing symptoms (nasal obstruction, snoring, sleep apnea, or mouth breathing) [1,2]. TTH after adenoidectomy is reported more often in recently, partial resection of TTH conducted by a low- temperature plasma technique is a curative therapy for TTH, whereas some benefit from intranasal steroid therapy [3-5]. However, the expression of GCR in TTH is currently unknown. This study is to assess the expression patterns of the human GCR in TTH in children with adenoidectomy and reported TTH could cause secretory otitis media in children.
Materials and Methods
Tissue preparation
This study was approved by the Ethics Committee of Children’s Hospital of Nanjing Medical University. Informed consents were obtained from the guardians of participants. From Jan to Jun 2024, 5 children with TTH identified by nasal endoscopy at our institution, TTH caused recurred obstructive sleep apnea in 2 cases, secretory otitis media in 1 cases and both in 2 children (Figure 1). All 5 cases underwent partial resection of TTH performed by a low-temperature plasma technique under general anesthesia, 3 children underwent auripuncture or grommet insertion and TTH tissues were collected and fixed in 4% formalin, then stored at 4°C for processing.
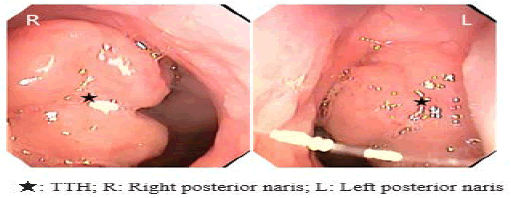
Figure 1: The images of nasopharynx by nasal endoscopy. Note: TTH blocks posterior naris and ostium pharyngeum tubae auditivae.
Immunofluorescence
The following sections detail the key steps of the immuno- fluorescence protocol applied to TTH tissue samples:
Frozen section fixation: Frozen section is baked at 37º C for 10-20 min and fixed in fixed solution for 30 min, washed in Phosphate Buffered Saline (PBS) (pH 7.4) on decolorizing shaking table for 3 times, 5 min each time.
Antigen repair: During the repair process, the buffer should be prevented from excessive evaporation. After repair, cool naturally. The slide was placed in PBS (pH 7.4) and washed by shaking on the decolorizing shaker for 3 times, 5 min each time.
Sealing with serum in a circle: After the section is slightly dried, add blocking solution and seal it for 30 min.
Add the mixed reagents of the first and second primary antibodies: The mixed configurations of the two different sources of primary antibodies are added to the prepared primary antibodies and the slices are placed flat in a wet box at 4º C and incubated overnight.
Plus secondary antibody: Add the corresponding secondary antibody and incubate at room temperature for 50 min away from light.
DAPI restaining nuclei: Add 4,6-Diamidino-2-Phenylindole (DAPI) dye solution and incubate at room temperature away from light for 10 min.
Quenched tissue autofluorescence: Add quenching agent B solution for 5 min and rinse with water for 10 min.
Sealing tablets: anti-fluorescence quenching sealing tablets.
Image acquisition: DAPI excitation wavelength 330-380 nm, emission wavelength 420 nm; The excitation wavelength of 488 is 465-495 nm and the emission wavelength is 515-555 nm. The excitation wavelength of CY3 is 510-560 nm and the emission wavelength is 590 nm. The excitation wavelength of CY5 is 608-648 nm and the emission wavelength is 672-712 nm.
Software
The software of Aipathwell by Servicebio was used to analyse the results of immunofluorescence.
Results
Demographics
Of the 5 patients with TTH, 4 boys and 1 girl. The age of TTH ranged from 64-138 months and the age of first operation ranged from 28-78 months, only 1 child was diagnosed with Allergic Rhinitis (AR), 3 children underwent adenoidectomy and 2 underwent adenoidectomy along with tonsillectomy.
Immunofluorescence
DAPI channel nuclei are blue, channel 488 positive is green, CY3 positive is red and CY5 positive is pink. GCR-α is red and GCR-β is green. The colocalization red or green mean density were summarized in Table 1 and the images of TTH immunofluorescence were shown in Figure 2.
| Items | First operation age (months) | Sex | Allergic Rhinitis (AR) | Colocalization red mean density | Colocalization green mean density |
|---|---|---|---|---|---|
| 1 | 70 | M | N | 0.6802 | 0.0752 |
| 2 | 49 | F | N | 0.6458 | 0.0749 |
| 3 | 28 | M | N | 0.6926 | 0.1119 |
| 4 | 60 | M | N | 0.539 | 0.0912 |
| 5 | 78 | M | Y | 0.5061 | 0.1048 |
Table 1: Summary of results of the colocalization red or green mean density.
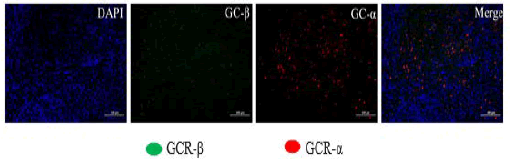
Figure 2: The immunofluorescence of GCR-α and GCR-β in colocalization of TTH. Note: The images of immunofluorescence in colocalization.
Discussion
The torus tubarius is the projecting posterior lip of the pharyngeal opening of the Eustachian tube, which forms the lateral border of nasopharynx [6]. Kim et al., first reported TTH after adenoidectomy in children [4]. Yang et al., and Teng et al., shared experiences of surgical treatment of TTH [3,5]. They all believed that TTH was more likely to happen in male children and in patients who took adenoidectomy before 5 years old [3-5]. All of them reported TTH causing sleep-disordered breathing symptoms, TTH could block the ostium pharyngeum tubae auditivae causing conductive hearing loss, we were first time to report secretory otitis media caused by TTH. Yang et al., and Teng et al., also introduced intranasal steroid therapy for TTH, but both could not give reasonable evidences [3,5].
Under normal physiological conditions, GCR is primarily localized in the cytoplasm and exists as GCR-α isoform and the GCR-β isoform. Glucocorticoids exert their biological effects primarily through binding to GCR-α [7,8]. GCR-β does not bind glucocorticoids and mainly interacts with DNA. However, recent studies have shown that GCR-β has antagonistic effects on GCR- α [9]. GCR expression is important for glucocorticoids to exert their effects. Many clinical studies have indicated that the density of GCR-α and its affinity for glucocorticoids is positively correlated with glucocorticoid therapy efficacy [8,10]. Previous studies have shown that the expression level of GCR-α in adenoid tissues of Obstructive Sleep Apnea Hypopnea Syndrome (OSAHS) children was significantly higher than that in non- OSAHS children [11,12]. And some articles found that intranasal steroid therapy would reduce the adenoid mass to improve the symptoms of obstructive sleep apnea [13-17]. In our study, the expression of GCR-α in TTH was significant high than GCR-β, the ratio of GCR-α and GCR-β was 5:1 to 9:1, high expression of GCR-α was a potential evidence for glucocorticoid therapy for TTH.
Conclusion
GCR-α and GCR-β were both expressed in TTH tissues in children and the high expression level of GCR-α may be an important factor affecting the sensitivity of TTH to glucocorticoid therapy in children. The limitation of the study is we could not obtain torus tubarius tissues from normal children for obvious ethical reasons, therefore we could not compare GCR expression levels between TTH tissue and normal torus tubarius tissue. Thus, randomized controlled trials of intranasal steroids should be conducted in children with symptomatic snoring caused by TTH to delineate better the role and optimal duration of this therapeutic modality.
Funding
This work was supported by Technology Development Fund projects of the Nanjing Medical University (NMUB20220017).
Ethics Statement
This study was approved by the Ethics Committee of Children’s Hospital of Nanjing Medical University. Informed consents were obtained from the guardians of participants.
Authors Contributions
Zhi-Pan Teng contributed to the acquisition and interpretation of data and drafted the manuscript.
Qi Li contributed to the design of the study, performed statistical analyses and critically revised the manuscript.
Xiao-Fei Shen contributed to the study design, provided critical feedback on the manuscript and supervision of the study.
Conflict of Interest
There is no potential conflict of interests of this manuscript.
Data Availability Statement
The data are available from the corresponding author on reasonable request.
References
- Passali D, de Benedetto M, Lauriello M, Passàli FM. Influence of Waldeyer’s ring hypertrophy on snoring and sleep apnea. Adv Otorhinolaryngol. 2011;72: 132-135.
[Crossref] [Google Scholar] [PubMed]
- Redline S, Amin R, Beebe D, Chervin RD, Garetz SL, Giordani B, et al. The Childhood Adenotonsillectomy Trial (CHAT): Rationale, design and challenges of a randomized controlled trial evaluating a standard surgical procedure in a pediatric population. Sleep. 2011;34(11):1509-1517.
[Crossref] [Google Scholar] [PubMed]
- Yang SZ, Zhou CY, Wang ZL, Han BC, Sun WH, Wan Y, et al. Preliminary experience of surgical treatment for torus tubarius hypertrophy in children. Chinese J Otorhinolaryngol Head Neck Surg. 2022;57(4):505-509.
[Crossref] [Google Scholar] [PubMed]
- Kim JW, Rhee CS, Jung HJ. Partial resection of hypertrophic torus tubarius for recurred snoring: Case series. Medicine. 2020;99(10):e19329.
[Crossref] [Google Scholar] [PubMed]
- Teng ZP, Li Q, Shen XF. Torus tubarius hypertrophy after adenoidectomy. Ear Nose Throat J. 2023:01455613231199676.
[Crossref] [Google Scholar] [PubMed]
- Merati AL, Rieder AA. Normal endoscopic anatomy of the pharynx and larynx. Am J Med. 2003;115(3):10-14.
[Crossref] [Google Scholar] [PubMed]
- Bazsó A, Szappanos Á, Patócs A, Poór G, Shoenfeld Y, Kiss E. The importance of glucocorticoid receptors in systemic lupus erythaematosus: A systematic review. Autoimmun Rev. 2015;14(4):349-351.
[Crossref] [Google Scholar] [PubMed]
- Oakley RH, Cidlowski JA. The biology of the glucocorticoid receptor: New signaling mechanisms in health and disease. J Allergy Clin Immunol. 2013;132(5):1033-1044.
[Crossref] [Google Scholar] [PubMed]
- Fardet L, Fève B. Systemic glucocorticoid therapy: A review of its metabolic and cardiovascular adverse events. Drugs. 2014;74:1731-1745.
[Crossref] [Google Scholar] [PubMed]
- Caratti G, Matthews L, Poolman T, Kershaw S, Baxter M, Ray D. Glucocorticoid receptor function in health and disease. Clin Endocrinol. 2015;83(4):441-448.
[Crossref] [Google Scholar] [PubMed]
- Zhang XW, Li Y, Xie MQ, Zhang GH, Liu X, Wang ZL, et al. Glucocorticoid receptor subunit expression in adenoid tissue of children with obstructive sleep apnea hypopnea syndrome. Chinese J Otorhinolaryngol Head Neck Surg. 2007;42(2):109-112.
[Google Scholar] [PubMed]
- Goldbart AD, Veling MC, Goldman JL, Li RC, Brittian KR, Gozal D. Glucocorticoid receptor subunit expression in adenotonsillar tissue of children with obstructive sleep apnea. Pediatr Res. 2005;57(2):232-236.
[Crossref] [Google Scholar] [PubMed]
- Kheirandish-Gozal L, Gozal D. Intranasal budesonide treatment for children with mild obstructive sleep apnea syndrome. Pediatrics. 2008;122(1):e149-155.
[Crossref] [Google Scholar] [PubMed]
- Berlucchi M, Valetti L, Parrinello G, Nicolai P. Long-term follow-up of children undergoing topical intranasal steroid therapy for adenoidal hypertrophy. Int J Pediatr Otorhinolaryngol. 2008;72(8):1171-1175.
[Crossref] [Google Scholar] [PubMed]
- Zhang L, Mendoza‐Sassi RA, César JA, Chadha NK. Intranasal corticosteroids for nasal airway obstruction in children with moderate to severe adenoidal hypertrophy. Cochrane Database Syst Rev. 2008(3):CD006286.
[Crossref] [Google Scholar] [PubMed]
- Strickland I, Kisich K, Hauk PJ, Vottero A, Chrousos GP, Klemm DJ, et al. High constitutive glucocorticoid receptor β in human neutrophils enables them to reduce their spontaneous rate of cell death in response to corticosteroids. J Exp Med. 2001;193(5):585-594.
[Crossref] [Google Scholar] [PubMed]
- Kheirandish-Gozal L, Serpero LD, Dayyat E, Kim J, Goldman JL, Snow A, et al. Corticosteroids suppress in vitro tonsillar proliferation in children with obstructive sleep apnoea. Eur Respir J. 2009;33(5):1077-1084.
[Crossref] [Google Scholar] [PubMed]
Citation: Teng ZP, Li Q, Shen XF (2024). Glucocorticoid Receptor Expression in Torus Tubarius Hypertrophy Tissue Causing Secretory Otitis Media in Children. Health Care Curr Rev. 12:414.
Copyright: © 2024 Teng ZP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

