Indexed In
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2024) Volume 15, Issue 6
Generation of Cultured Beef from Bovine Embryonic Stem Cells
Xue Rui1, Zehan Li1, Jiehuan Xu2, Jianjun Dai2, Xiaohui Zhang1, Xiao Jin1 and Yang Liu1*2Shanghai Municipal Key Laboratory of Agri-Genetics and Breeding, Institute of Animal Husbandry and Veterinary Science, Shanghai Academy of Agricultural Sciences, Shanghai, China
Received: 12-Nov-2024, Manuscript No. JCRB-24-27497; Editor assigned: 14-Nov-2024, Pre QC No. JCRB-24-27497 (PQ); Reviewed: 28-Nov-2024, QC No. JCRB-24-27497; Revised: 05-Dec-2024, Manuscript No. JCRB-24-27497 (R); Published: 12-Dec-2024, DOI: 10.35248/2155-9627.24.15.504
Abstract
Bovine Embryonic Stem Cells (bESCs) serve as an optimal source for generating muscle and fat tissues. However, systematic methodologies for creating cultured beef from bESCs have not yet been reported. Here, we successfully established a bESC line and formulated a specialized culture medium that supports cell pluripotency in both Two-Dimensional (2D) and Three-Dimensional (3D) cultures. Additionally, we have also developed serum-free and transgene-free protocols that efficiently differentiate bESCs into muscle and fat cells. These differentiated cells were validated at the transcriptional and protein levels. Furthermore, we devised a method using high moisture stretched plant-based protein scaffolds for incorporating differentiated muscle and fat cells derived from bESCs, producing an innovative plant-cell hybrid cultured beef. These advancements provide a solid foundation for future cultured beef production using pluripotent stem cells.
Keywords
Muscle; Fat; Hybrid; Cultured meat; Adipocytes
Abbreviations
bESCs: Bovine Embryonic Stem Cells; 2D: Two-Dimensional; 3D: Three-Dimensional; GMO: Genetically Modified Organisms; EB: Embryoid Body; SPI: Soy Protein Isolate; TPA: Texture Profile Analysis; ANOVA: Analysis of Variance; SEM: Standard Error of the Mean; MEF: Mouse Embryonic Fibroblast; PCA: Principal Component Analysis; bFBs: bovine Fibroblasts; MSC: Mesenchymal Stem Cell; PAD: Preadipocytes; AD1: Adipocytes 1; AD2: Adipocytes 2; NE: Neutralized Ectoderm; MPC: Muscle Progenitor Cell; MB: Myoglobin; iPSCs: Induced Pluripotent Stem Cells; MUFAs: Monounsaturated Fatty Acids; PUFAs: Polyunsaturated Fatty Acids; SFAs: Saturated Fatty Acids
Introduction
Cultured meat (also cultivated, cell-cultured, in vitro meat) uses tissue engineering to produce meat for food, offering a novel approach to meat production [1]. This innovation has attracted considerable interest for its capacity to mitigate challenges inherent in traditional livestock farming, such as environmental sustainability, animal welfare, public health and food security [2]. Consequently, cultured meat development emerges as a viable solution to the problems plaguing conventional meat production, presenting an avenue for a more sustainable, ethical and healthier food system. Nonetheless, substantial technological challenges must be addressed to fully harness its potential.
The advancement of cultured meat technology encounters numerous challenges, including the establishment of cell lines, development of cost-effective serum-free culture media, fabrication of textured scaffolds, replication of taste and nutritional profiles of commercial meats and establishment of scalable, automated bioproduction processes [3]. The generation of high-quality cell lines is fundamental to cultured meat production. During cell line development, factors such as proliferation rate, differentiation efficiency into specific cell types like muscle and fat and stability are critical. Current literature documents various cell lines for cultured meat, including primary adipose and muscle stem cells, alongside Genetically Modified Organisms (GMO) and spontaneously immortalized cell lines. Primary stem cells offer promising differentiation capabilities but are limited by their proliferation capacity in vitro, necessitating regular animal sourcing [4,5]. Conversely, GMO and spontaneously immortalized cell lines facilitate continuous proliferation in vitro but encounter regulatory and consumer acceptance hurdles, impacting the perceived safety of cultured meat [6,7]. Moreover, spontaneously immortalized cell lines have by far predominantly been explored in lower species such as chicken and fish [7,8]. Embryonic stem cells, not requiring genetic modification, exhibit indefinite and stable proliferation and versatile differentiation potential in vitro [9,10]. Establishing bESC lines has been a challenging task, with significant progress made only in recent years [11-13]. This advancement has paved the way for the application of ESC in cultured meat. However, the development of robust serum-free media for the expansion of ESC in feeder-free conditions, efficient protocols for inducing differentiation of bESCs into muscle and fat cells, as well as the development of cultured meat fabrication methods that are more aligned with commercial manufacturing scenarios, remain pressing issues that need to be addressed in the field [14,15].
In this study, we established a bESC line and introduced a novel, customer-made culture medium, termed NBFR+ medium, designed to facilitate the stable passage of these cells under feeder-free conditions. Importantly, our findings indicate that this medium effectively maintains the pluripotency of bovine pluripotent stem cells in both 2D and 3D culture systems. Furthermore, we developed transgene-free and serum-free protocols that enable the differentiation of bESCs into muscle and fat cells, with the characteristics of these differentiated cells validated at the transcriptional and protein levels. In a final step, we combined muscle and fat cells derived from bESCs with textured plant-based protein chunks, thereby creating a novel plant-cell hybrid cultured beef.
Materials and Methods
TrypLE Express (12604-013), DMEM/F-12 (11320033), Neurobasal (21103049), N-2 Supplement (17502001), B-27 Supplement (17504001), MEM NEAA (11140050), GlutaMAX (35050-061), Vitronectin (A14700), PluronicTM F-68 (24040032), α-MEM (12571063), UltraPure Water (10977015), Activin A (120-14E), Sodium Pyruvate (11360070) and CD Lip concentration (12531018) were purchased from Thermo Fisher Scientific. Tyrode′s Solution (T1788-100 ML), TritonTM X-100 (T8787), Bovine Serum Albumin (BSA, A1933), Mitomycin C (M5353), Dexamethasone (D4902) and Gelatin (G7041) were purchased from Sigma Aldrich. IWR- 1-endo (S7086), Y-27632 (S6390) and Givinostat (GIVI, 732302-99-7) were purchased from Selleck. Penicillin-Streptomycin- Amphotericin B Solution (PSA, 4408584), Oil Red O Staining Kit (C0157S), Enhanced Cell Counting Kit-8 (C0042), Hoechst 33342 Staining Solution (C1029), Alkaline Phosphatase Assay Kit (P0321S) and HEPES (C0217) were purchased from Beyotime. HiPure Total RNA Mini Kit (R4111-02) and BODIPY (MX5403) were purchased from MGBio. 4% Paraformaldehyde Fix Solution (4% PFA, F8011) was purchased from Adamas Life. Fetal Bovine Serum (FBS, FSP500) was purchased from ExCell. Pronase (10165921001) was purchased from Roche. Low fatty acid BSA (4483930) was purchased from MP Biomedicals. Recombinant Human FGFb (C751) was purchased from Novoprotein. Dulbecco's modified eagle medium (DMEM, SH30022.01) was purchased from Hyclone. Laminin (LN521-05) was purchased from BioLamina. TransScript® One-Step gDNA Removal and cDNA Synthesis SuperMix (AT311-03) was purchased from TransGen. ChamQ SYBR qPCR Master Mix (Q311-02) was purchased from Vazyme. SB-431542 (T1726) and 3-isobutyl-1-methylxanthine (IBMX, T1713) were purchased from TOPSCIENCE. Indomethacin (HY-14397) was purchased from MCE. Recombinant Human EGF (C029) was purchased from Novoprotein. TeSRTM-E6 (05946) and FreSR-STM (05859) were purchased from Stem Cell Technologies. Skeletal Muscle Cell Growth Medium-2 (SKGM, CC-3245) was purchased from LONZA. Defatty acid BSA (dBSA, S25762) was purchased from MedMol. MSC serum-free medium (S101-500/S102-025) was purchased from Baidi Biotechnology. Gluten from wheat, corn starch and Soy Protein Isolate (SPI) were purchased from Feng Rui Biotechnology, China. Beet root powder was purchased from Tong Ren Tang, China. Sodium alginate was purchased from Feng Rui Biotechnology, China.
Isolation of MEF
Mouse embryos at E12.5 were carefully harvested from the uterus of pregnant mice. After the fetal head, limbs, tail and internal organs were removed, the torsos were finely minced with sterilized surgical scissors until achieving uniform and small pieces (diameter <3 mm). These minced torsos were subsequently digested with 0.25% Trypsin-EDTA at 37°C for 10 min. The trypsinization process was terminated by the addition of MEF medium containing DMEM, 10% FBS and 1% PSA. The resulted cells were cultured in a 5% CO2, 37°C incubator. MEF cells (P3) intended for use as a feeder layer were treated with 10 µg/mL mitomycin C for 3 h. These treated MEFs could either be frozen and stored in liquid nitrogen or used as feeder cells by placing them in 0.2% gelatin-coated petri dishes or well plates at a density of 60,000 cells/cm2.
bESCs establishment from embryos
Holstein embryos at E5-7 developmental stage were sourced from the Shanghai Academy of Agricultural Sciences. Thawed bovine blastocysts were carefully transferred to Tyrode's acid solution using a mouth pipette. Subsequently, the blastocysts were transferred to Pronase drops and observed under a stereomicroscope (HSZ300), when most of the zona pellucida disappeared. The Pronase treatment (2-lasting 2-3 min, facilitated the removal of zona pellucida. Afterwards, the blastocysts were rinsed in NBFR medium to eliminate any residual Pronase and then plated onto mitotically inactivated MEF feeders. Blastocysts failed to adhere to the MEFs were gently pressed to the bottom of 12-well plate using a 22G needle for attachment. The growth of ESC colonies was monitored daily, with typically evident growth observed within 1-2 weeks.
Culturing, passaging and freezing of bESCs
When reaching ~70% confluency in the MEF feeder system, colonies were enzymatically detached using TrypLE. Afterwards, the cells were resuspended in NBFR medium containing 10 µM Y-27632 and passaged at a ratio of 1:16. The cells were then inoculated onto mitomycin C-treated MEF feeder cells and feed daily. The cell culture and passage protocol in E8+ (TeSRTM-E6, 20 ng/mL FGFb, 2.5 µM IWR-1-endo, 1% PSA and 20 ng/mL Activin A) within the feeder system closely parallels that of NBFR medium. When reaching ~80% confluency in the feeder-free system, the colonies were enzymatically detached using TrypLE, resuspended in feeder-free medium containing 10 µM Y-27632 and passaged at a ratio of 1:8 in 0.5 µg/cm2 Laminin-coated 6-well plates with daily feeding. Of note, the feeder-free medium comprised of 50% DMEM/F-12, 50% Neurobasal, 1% low fatty acid BSA, 20 ng/mL FGFb, 5 µM IWR-1-endo, 20 ng/mL Activin A, 1% N-2 Supplement, 2% B-27 Supplement, 1% MEMNEAA, 1% GlutaMAX, 50 µg/mL Vitamin C, 1 µM CHIR-99021, 0.25 mM Sodium Pyruvate, 0.3 µM WH- 4-023, 1% PSA and 10 ng/mL LIF. For cryopreservation, cells were frozen using FreSR-S in liquid nitrogen, with each cryovial containing 1 million cells suspended in 1 mL freezing solution.
Embryoid Body (EB) differentiation
The bESCs were employed for EB formation. When reaching 70%-80% confluency, the cells were detached using TryPLE and seeded in ultralow-adhesion petri dishes (Thermo Fisher Scientific) containing feeder-free medium supplemented with 1 µM Rocki. Half of the medium was replaced with fresh DMEM medium containing 10% FBS every other day. After 7 days, the EBs were transferred to gelatin-coated plates and cultured in DMEM medium containing 10% FBS, with feeding every other day. On day 7, cells migrating from the EBs were harvested for RNA isolation and gene expression analysis.
3D suspension culture
The bESCs were detached with TryPLE, when reaching 70%-80% confluency. Subsequently, the cells were seeded in a 50 mL Glass Spinner Flask (CytoNiche) in feeder-free medium containing 1% F68 at a concentration of 1 × 106 cells/mL. Half of the medium was replaced daily. Samples were collected every two days for live/dead staining and cell counting to monitor cell viability and proliferation.
Alkaline phosphatase staining
The bESCs were fixed with 4% PFA for 15 min at room temperature. The staining was conducted according to the Alkaline Phosphatase Assay Kit procedures. After the staining, the cells were immersed in PBS and imaged with a Nikon ECLIPSE Ts2 inverted microscope.
Karyotype analysis
The bESCs were cultured in medium containing 75 ng/mL colcemid for 2 h at 38.5°C. Afterwards, when reaching confluency, the cells were detached with TryPLE and then treated with 0.075 M KCl at room temperature for 20 min. Following this, the cells were fixed with methanol/acetic acid (3:1, v/v), centrifuged at 1000 rpm for 5 min, transferred to slides and air-dried overnight. The chromosome number was determined from stained chromosome using 5% Giemsa for 10 min. A total of 50 cells in metaphase were used for counting. When a predominant number of cells exhibited an identical chromosome count, it was acknowledged as the modal chromosome number.
Immunofluorescence staining
The cells were fixed with 4% PFA for 15 min, permeabilized with 0.1% Triton X-100 in PBS (0.1% PBST) for 15 min and then blocked with 1% BSA in PBS for 1 h at room temperature. Subsequently, cells were incubated overnight with primary antibodies listed in Supplementary Table 1 in 0.1% PBST at 4°C. The next day, cells were rinsed in DPBS and incubated with secondary antibodies (Supplementary Table 1) at a dilution of 1:500 in 0.1% PBST for 1 h at room temperature. Next, cells were rinsed in DPBS three times and stained with DAPI for 10 min. The stained cells were imaged using Nikon ECLIPSE Ts2 inverted microscope, Olympus CKX 53 inverted microscope, or Confocal microscope (Leica STELLARIS 5).
Oil Red O (ORO) staining
The bESCs were fixed with 4% PFA for 15 min at room temperature and then stained following the Oil Red O staining kit procedures. Finally, cells were rinsed in wash buffer twice, stored in PBS and imaged using a Nikon Ts2 inverted microscope.
Adipogenic differentiation of bESCs
Initially, bESCs were thawed and seeded in a matrix-coated 6-well plate at a density of 1 × 105 cells/well in feeder-free medium supplemented with 10 µM Y-27632. When reaching 100% confluency, the cells were switched to adipogenic induction medium consisting of 50% Neurobasal, 50% DMEM/F12 medium, 1% N2, 2% B27, 3 µM CHIR-99021 and 10 µM SB-431542. During the first stage of differentiation, the cells were rinsed in DPBS prior to being cultured in neural ectoderm medium for up to 5 days, with feeding every other day. Subsequently, the cells were transitioned to commercial MSC serum-free medium for the second stage of differentiation, aiming for PAD formation after 4 days. When reaching ~90% confluency, the PADs were switched to our self-developed medium for 5 days of lipid accumulation in a 5% CO2, 38.5°C incubator, with feeding every other day. The cells were rinsed gently twice in DPBS to remove any residual MSC serum-free medium. During feeding, great care was taken to handle the cells gently to prevent lift-off of lipid-laden adipocytes. Tiny lipid droplets were observed within 2 days. Our self-developed differentiation medium consisting of 16 components, including DMEM/F12, dBSA, Hepes and CD Lip concentration, while the conventional medium comprised of DMEM, 10% FBS, 1 µM dexamethasone, 0.5 mM IBMX, 0.2 mM indomethacin and 10 µM insulin.
Myogenic differentiation of bESCs
Initially, bESCs were thawed and seeded in a matrix-coated 6-well plate at a density of 6 × 105 cells/well in a 5% CO2 , 38.5°C incubator. After 24 h, the cells were switched to differentiation medium-1, consisting of E6 (as the basal medium supplemented with 10 µM CHIR99021) for 2 days. Following this, the cells were switched to differentiation medium-2, consisting of E6 supplemented with 100 nM GIVI for 5 days to complete the initial differentiation. Next, the cells were cultured in E6 for 7 days with daily feeding. The resulting MPCs exhibited sustained expansion capacity, as evidenced by their ability to undergo passage for up to 10 generations in SKGM medium. After reaching ~90% confluency, the MPCs were switched to Neuro-2 medium, consisting of DMEM/F12 supplemented with1% ITS, 1% N2 and 1% GlutaMAX (differentiation medium) for 7 days with feeding every other day.
qRT-PCR analysis
RNA was extracted and purified using the HiPure Total RNA Mini Kit according to the manufacturer's instructions. The concentration and purity of RNA were assessed using Thermo Scientific NanoDrop Microvolume Spectrophotometers. Subsequently, cDNA was synthesized using the cDNA SuperMix and Thermal Cycler T100 following the manufacturer's instructions. The primers (Supplementary Table 2), SYBR FAST Universal 2 × qPCR Master Mix and LightCycler® 480 Instrument II were used for the analysis of gene expression. The transcription levels of genes were determined using the ΔCt method and normalized to GAPDH.
RNA sequencing (RNA-seq) analysis
RNA was extracted from bESCs and bovine fibroblasts using HiPure Total RNA Mini Kit according to the manufacturer’s directions. Library preparation and RNA sequencing were performed by Shanghai Biochip Co. Ltd. Library construction was conducted using VAHTS Stranded mRNA-seq Library Prep Kit (NR602,Vazyme) and sequenced on Illumina HiSeqX with paired-end, 150 base pair (bp). Sequencing reads were mapped to the U.S. Department of Agriculture, Agricultural Research Service Bovine transcriptome (genome build ARS-UCD1.3) using hisat2 version 2.2.0 and expression levels of all genes were quantified using htseq-count version 0.13.5. htseq-count yield an expression matrix of inferred gene counts. Normalized reads count, PCA and differential expression analysis was performed using R package edgeR with default parameters.
Graphic display of sequencing data
R studio (https://www.rstudio.com/) was used to perform principal component analysis (R package FactoMineR, factoextra, ggsci, ggpubr). Hierarchical clustering and heat maps were created by R package pheatmap.
Manufacturing of cultured beef
The manufacturing process of cultured beef involved a two-step procedure. Initial, the edible plant-based protein scaffold was fabricated utilizing a modified mechanical elongation method [1]. The preparatory process for the scaffolds is depicted in Supplementary Figure 1, while the comprehensive information on the formulation of each component is provided in Supplementary Tables 3 and 4. Adipocyte-like and myocyte-like cells underwent purification through washing in DPBS, followed by preservation at -20°C until their utilization. In the subsequent phase, cultured fat and muscle cells were introduced into the scaffold, constituting 20% of the total weight (with 10% attributed to the fat component and an additional 10% allocated to the muscle component), together with beet root powder, corn starch and gluten. The resulting mixture was shaped as designed, such as a burger and subsequently stored at 4°C for further characterization. Empty scaffold and commercial animal beef were employed as negative and positive controls, respectively.
Texture Profile Analysis (TPA)
TPA tests were conducted employing a Universal TA Texture Analyzer (Shanghai Tengba Instrument Technology Co. Ltd., China) at room temperature. All samples were standardized to the same dimensions (25 mm in diameter and 5 mm in height) for consistency. A stainless plunger with a diameter of 36 mm was employed as the compressive probe. Consistent experimental conditions prevailed across all measurements. The pre-test speed, test speed and post-test speed were all calibrated to 1 mm/s based on the preliminary test results. The compression ratio was standardized at 30%, with a trigger force of 8 g and a 5 sec interval between successive compressions. Upon completion of the second compression cycle, the probe returned to the trigger point and then to its initial position.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 9. The specific analyses utilized are detailed in the text and comprise Analysis of Variance (ANOVA) with Tukey’s post-hoc tests, t-tests with Welch’s correction, principal component analyses and chi- square tests. Error bars and ± ranges denote standard deviations unless otherwise specified with SEM (Standard Error of the Mean). The p-value of 0.05 served as the threshold for statistical significance.
Results
Establishment of bESCs suitable for development of cultured meat
Embryonic stem cells, characterized by their infinite self-renewal capability and pluripotent differentiation potential, are considered ideal seed cells for the cultured meat. Our initial focus was on the feasibility of establishing bESC lines from the blastocysts of Chinese Holstein dairy cows.
Employing the NBFR culture system, we dissolved the zona pellucida from intact embryos at embryonic day 6-7 and inoculated the isolated bovine blastocysts onto Mouse Embryonic Fibroblast (MEF) feeder layers (Figure 1A), [16]. Significant outgrowth was observed by the fourth day post inoculation. Subsequent passages involved removing trophoblast cells, resulting in densely cellular colonies with sharp edges. Totally, we successfully isolated 10 bESC lines from 18 Holstein cow embryos. These cell lines have been continuously passaged, now reaching over 50 generations. Further validation demonstrated that our bESCs exhibited the capability to be passaged as either individual cells or small clusters, with positive results in alkaline phosphatase assays (Figure 1B). Throughout longterm passaging, bESCs maintained a stable doubling time of approximately 24 h (Figure 1C) and a normal karyotype (2n=60) (Figure 1D). Immunofluorescence staining confirmed the expression of pluripotency markers such as NANOG, SOX2, OCT4 and SSEA4 (Figure 1E). Gene expression analysis via qPCR further verified the high expression and maintenance of pluripotency markers over time (Figures 1F and 1G). To assess differentiation capabilities, we cultured Embryonic Bodies (EBs) in ultra-low attachment plates, which spontaneously differentiated into three germ layers, as evidenced by specific marker expressions (Figures 1H and 1I). Principal Component Analysis (PCA) of RNA- seq data conclusively demonstrates the distinct differentiation between bESCs and bovine Fibroblasts (bFBs) (Figure 1J). Consistent with prior assumptions, RNA-seq confirms the expression of unique classical biomarkers in each cell type (Figure 1K). We next evaluated the applicability of NBFR medium for feeder-free culture of the established Chinese Holstein dairy cow ESC. Culturing these cells without a feeder layer led to significant cell death, with surviving cells diverging from clonal growth and exhibiting signs of differentiation and reduced proliferation (Figure 1L). Through iterative refinement, we developed a new culture medium, designated NBFR+, which notably supported dense clonal formation under feeder-free conditions (Figure 1L).
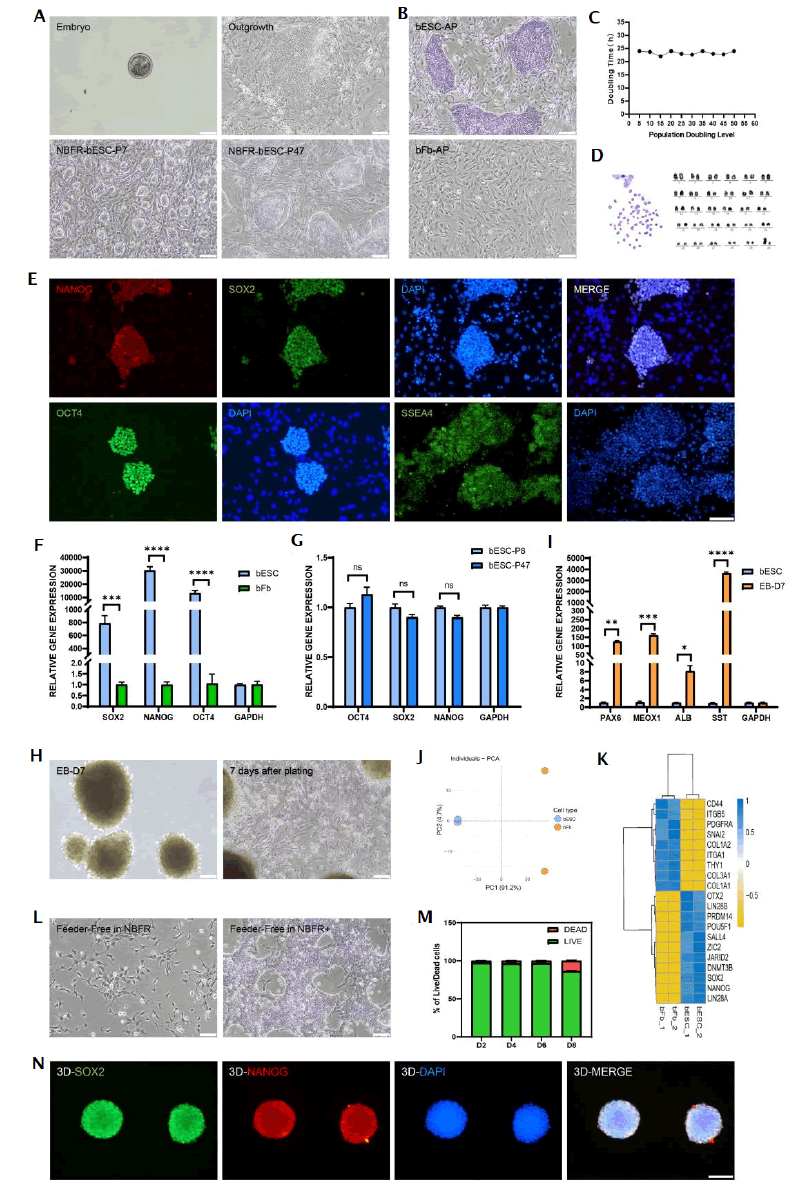
Figure 1: Establishment, characterization and culture medium optimization of bESCs. Note: ns: No Significance; p-values are labelled and indicates p>0.05; *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001.
Comprehensive analysis, including immunofluorescence staining and qPCR, verified the sustained expression of stemness genes in cells cultured with feeder-free NBFR+ system (Supplementary Figure 2). Encouraged by NBFR+’s efficacy in maintaining stem cells properties without feeder layers, we explored its utility in suspension cultures to facilitate large-scale 3D cell expansion, inoculating bESCs into a glass spinner flask. We continuously monitored the cell status over 8 days. We found suspension cultured bESCs formed spheroids, maintaining over 96% viability by day 6 (Figure 1M and Supplementary Figure 3), while continuing to express pluripotent genes on day 8 (Figure 1N). Overall, we successfully established the bESC line, which exhibited self-renewal capabilities and potential for multi-lineage differentiation. Moreover, we developed a new serum-free culture medium that support the maintenance of stemness under feeder-free culture conditions (Figure 1).
In Figure 1, A) Representative bright field images of bESCs derived from the whole bovine blastocysts at various stages. These images represented a selected E5 embryo for bESC derivation, outgrowth in culture, typical colony morphology of bESCs at P7 and P47 when cultured in NBFR medium. B) Comparison of Alkaline Phosphatase (AP) activity of bESC and bFb. C) The doubling time of bESCs, stabilized at 23 ± 2 h within 50 generations. D) Karyotype analysis of bESCs (P10), maintained normal chromosome numbers (2n=60). E) Immunofluorescence staining of pluripotency markers (NANOG, SOX2, OCT4) and live staining of cell morphology marker (SSEA4) of bESCs. DAPI was used for nucleus staining. F) Comparison of gene expression related to pluripotency markers (SOX2, NANOG, OCT4) between bESC and bFb. G) Gene expression related to pluripotency of bESCs at P8 and P47. H) Gene expression of ectoderm markers (PAX6, SST), mesoderm maker (MEOX1) and endoderm marker (ALB) of spontaneous three germ layer differentiation for 7 days. I) EB formation after 3D suspension culture and cell morphology after adherent differentiation on day 7 day. J) Principal Component Analysis (PCA) plot of RNA-seq from bESC and bFb. K) Heatmap of differentially expressed gene of bESCs and bFb. L) Feeder-free bESCs cultured in NBFR medium. M) Percentage of live and dead bESCs on day 2, 4, 6 and 8 during 3D suspension culture, which were calculated from the of results of live/dead staining images. N) Immunofluorescence staining of pluripotency markers (SOX2, NANOG) of bESC spheroids after 3D suspension culture on day 8. DAPI was used for nucleus staining. P refers to passage. Scale bars equal 100 µm. Data are expressed as mean plus standard error of the mean (n=3).
A serum-free approach for efficient differentiation of bESCs into adipocytes
The aroma of meat originates from the oxidation of fats. Therefore, we explored whether bESCs could be efficiently differentiated into adipocytes. Previous research in humans has shown that the induction of the neuroectoderm can effectively differentiate pluripotent stem cells into mesenchymal stem cells, which are adipogenic precursors with the capacity to generate fat [17]. Thus, we hypothesized that the similar approach might guide the differentiation of bESCs into adipocytes.
To induce bESCs into adipogenic precursors, we initially added 10 µM SB431542 and 3 µM CHIR99021 to the culture medium to induce the formation of neuroectoderm. After 5 days, the cells exhibited a neural-like morphology and spread out from each other (Figure 2A). qPCR analysis indicated an elevated expression of neuroectodermal markers such as P75 and ZIC1 post-induction (Figure 2B). To further induce these neuroectodermal cells into adipogenic precursors, the cells were switched to a serum- free Mesenchymal Stem Cell (MSC) medium. After 4 days, the cells adopted a spindle-shaped morphology (Figure 2A). qPCR analysis revealed that cells at this stage expressed classic adipogenic precursor markers such as NEIF3K, CD90 and CD105 (Figure 2C). Notably, these cells could proliferate in this serum-free medium, with markers such as ITGA5 exhibiting an increase from passage 0 to passage 2 (Figure 2C). A classic adipocyte maturation medium was employed to further mature the adipocytes [18]. However, we found limited efficiency of this medium in differentiation of bovine cells to adipocytes (Figure 2D). Consequently, we developed the adipogenic maturation serum-free medium (self-developed medium). We found adipogenic precursors derived from bESCs were efficiently differentiated into mature adipocytes within 5 days using our self-developed medium (Figure 2D). qPCR further verified a notable augmentation in mature adipocyte markers in cells (Figure 2E). Remarkably, adipocyte precursor cells cultured in our self-developed medium displayed visible lipid accumulation within 7 days (Figure 2F). Moreover, combining the harvested fat cells with sodium alginate hygrogel resulted in a macroscale construct that resembled bovine adipose tissue (Figure 2G). We next performed lipidomic analysis and obtained quantifiable comparison of cultured fat with native bovine subcutaneous fat tissues. Interestingly, cultured fat contained lower levels of palmitic acid (C16:0) and higher levels of oleic acid (C18:1) (Figure 2H), compared to commercial beef fat, which has been reported to correlate positively with palatability [19]. Indeed, the proportion of monounsaturated fatty acids and polyunsaturated fatty acids increased, while saturated fatty acids reduced in cultured fat (Figure 2I). Taken together, these results demonstrated that we were able to efficiently induce bESCs into mature fat cells with a serum-free and transgene-free approach (Figure 2).
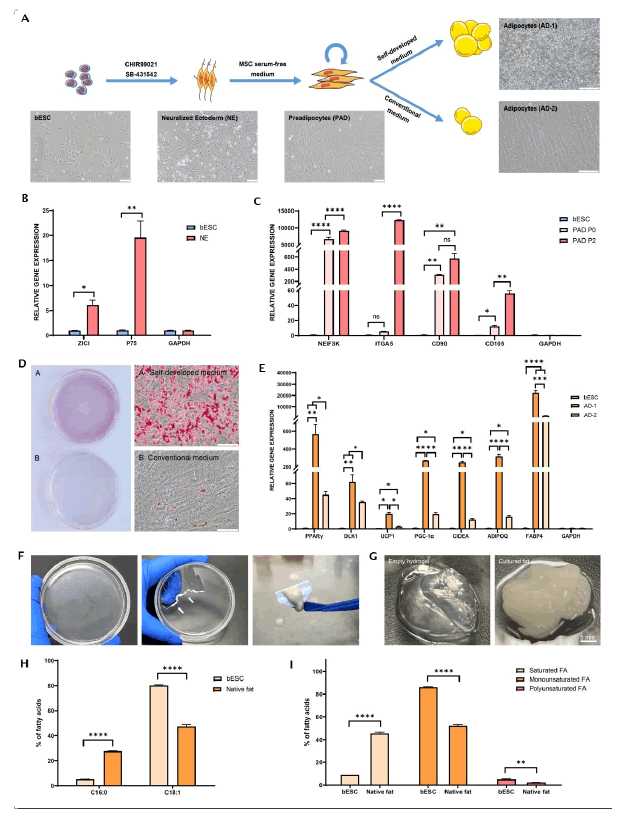
Figure 2: Evaluation and application of adipocyte differentiation in vitro. Note: ns: No Significance; p-values are labelled and indicates p>0.05; *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001.
In Figure 2, A) Schematic illustration of directional differentiation of bESCs to bovine adipocyte using our self-developed and conventional medium. B) Quantitative PCR (qPCR) analysis of neuroectoderm gene expression from bESCs and Neuralized Ectoderm (NE). C) Gene expression of preadipocytes (PAD, P0, P2) and bESCs after post-adipogenic induction. D) Oil red O staining of adipocytes cultured with self-developed (a) Conventional (b) medium for 5 days. E) Gene expression of Adipocyte 1 (AD1) cultured with self-developed medium compared to Adipocyte 2 (AD2) cultured with conventional medium. F) Representative bright filed images of accumulated adipocytes, rendered opaque on the bottom of the petri dish (left), with white arrows indicating the scraped adipocytes (middle) and mechanically collected adipocytes using a cell scraper (right) after 7 days of differentiation. G) Formation of cultured fat by combining adipocytes with empty alginate hydrogel. 1.5% (w/v) alginate solution was prepared by adding alginate powder to DI water, fully hydrated at 50°C with continuously stirring for 1-2 h and centrifuged to remove any impurities. The scraped adipocytes were mixed with 1.5% (w/v) alginate at a 1:1 (volumetric) ratio. For cultured bovine fat, 50 mM calcium chloride was mixed with alginate/adipocytes at a 1:1 (volumetric) ratio to induce the gelation. H) Comparison of percentages of fatty acid species between bESCs after 7 days of differentiation and native fat tissue. I) Comparison of percentages of saturated, monounsaturated and polyunsaturated fatty acids between bESCs after 7 days of differentiation and commercial beef fat tissue. P refers to passage. Scale bars equal 100 µm. Data are expressed as mean plus standard error of the mean (n=3).
Direct differentiation of bESCs into skeletal muscle cells
Muscle cells, alongside fat cells, constitute another critically important cell type in meat. Leveraging the pluripotent differentiation potential of bESC, we investigated whether bESCs could be efficiently differentiated into muscle cells. We initially used a serum-free medium containing 10 µM CHIR99021 to direct bESC towards mesodermal differentiation (Figure 3A). After 2 days, the cells underwent a significant epithelial-to-mesenchymal transition (Figure 3A). The mesodermal marker T and early muscle cell marker PAX3 were highly expressed in the cells at this differentiation stage, while pluripotency markers such as OCT4 and SOX2 were significantly downregulated (Figure 3B). To further induce MPC formation, we subsequently treated the cells with 100 nM Givinostat (a histone deacetylase inhibitor) in E6 medium for 5 days, followed by a week in basal E6 medium, leading to elongated, spindle-shaped cells (Figure 3A). The MPCs expressed significantly higher levels of myogenic transcription factors, including MYOG and MYOD, compared to bESCs (Figure 3C). Culturing MPCs in SKGM muscle stem cell expansion medium (LONZA) allowed for further propagation, partially maintaining muscle stem cell marker expression (Figure 3D). The MPCs were incubated in N2 medium, led to more elongated and aligned cells after one week. We found increased expression of mature myogenic markers such as CAV3, MYHC and MYOSIN (Figures 3E and 3F). Remarkably, Myoglobin (MB) gene expression significantly increased in myotubes (Figure 3E), which is in contrast with prior studies that suggested limited MB expression in myotubes derived from adult muscle stem cells [20]. Myoglobin is significant in the color and flavor of meat underscores the potential advantages of using muscle cells differentiated from bESCs. These results present a novel method for the efficient differentiation of bESCs into muscle cells (Figure 3).
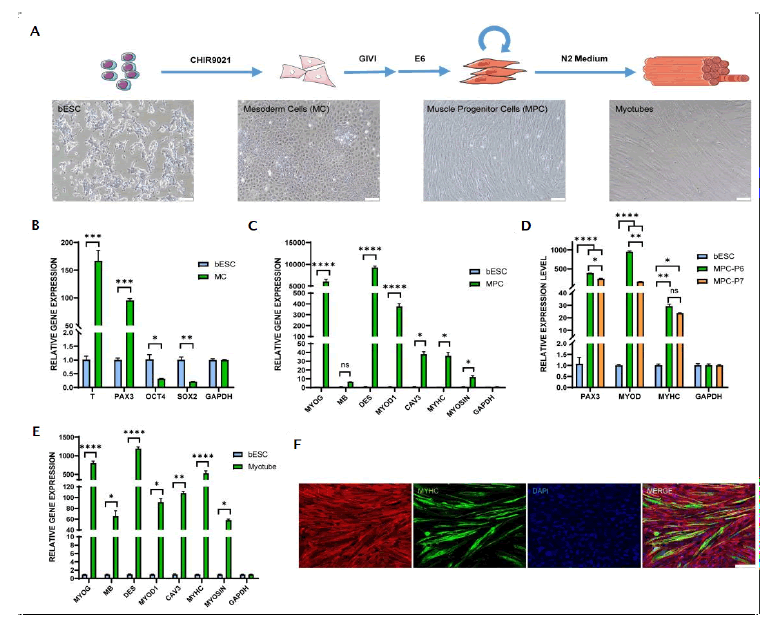
Figure 3: Evaluation of myocyte differentiation in vitro. Note: ns: No Significance; p-values are labelled and indicates p>0.05; *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001.
In Figure 3, A) Schematic illustration of directional differentiation of bESCs to bovine myocytes and myotubes, along with cell morphology at various stages. B) Gene expression of mesoderm markers (T, PAX3) and pluripotency markers (OCT4, SOX2) in myocytes compared to bESCs. C) Analysis of myogenesis markers (MYOG, MB, MYOD1, CAV3, MYHC, MYOSIN) expression in MPC (P6) compared to bESCs. D) Analysis of myogenesis markers (PAX3, MYOD, MYHC) expression in MPC (P6, P7) compared to bESCs. E) Analysis of myogenesis markers (MYOG, MB, DES, MYOD1, CAV3, MYHC, MYOSIN) expression in myotubes compared to bESCs. F) Immunofluorescence staining of myotubes (MF20, green), cellular actin (Phalloidin, red) and nuclei (DAPI, blue) from MPC after differentiation for 7 days. P refers to passage. Scale bars equal 100 µm. Data are expressed as mean plus standard error of the mean (n=3).
Engineered cultured meat by the assembly of bESC derived fat and muscle cells with plant-based edible scaffolds
Another challenge in cultured meat is how to integrate scaffolding technology with cultured cells and closely resembles conventional meat in appearance, nutrition and taste. Therefore, we developed a method for cultured beef in hamburger patties, integrating muscle and fat cells differentiated from bESCs with edible scaffolds containing gluten and soy protein isolate.
We first directed the differentiation of bESCs into muscle and fat cells (Figure 4A). In scaffold preparation, soy protein isolate and gluten were processed into scaffolds with specific textural characteristics through mechanical stretching methods to better simulate the texture of meat (Supplementary Figure 3). Cultured muscle and fat cells were then thoroughly mixed with the structural protein scaffolds. Optical images of both raw and fried cultured beef demonstrate that the meticulous blending and molding of these components yield a composition closely resembling the sensory attributes and appearances of commercial beef (Figure 4B). To further investigate the nutritional value of cultured beef, we conducted of its amino acid composition and content. All samples manifested the presence of nine essential amino acids, as anticipated with the inclusion of cultured cells and soy protein (Figure 4C and Supplementary Figure 4). Significantly, the cultured beef exhibited higher contents of Valine, Leucine, Isoleucine and Phenylalanine compared to animal beef in these amino acids (Figure 4C). Subsequently, the texture profile analysis was conducted to investigate the textural properties of cultured beef, empty scaffold and commercial beef (Figure 4D). The values for chewiness and hardness of the empty scaffold significantly surpassed both cultured and commercial beef, possibly attributed to its textured structure and preserved intrinsic properties. In contrast to the empty scaffold, cultured beef demonstrated mechanical properties more closely resembling those of commercial beef, signifying a heighted similarity in texture between cultured and commercial beef. Overall, we successfully created a novel plant-cell hybrid cultured beef through combining muscle and fat cells derived from bESCs with textured scaffolds (Figure 4).
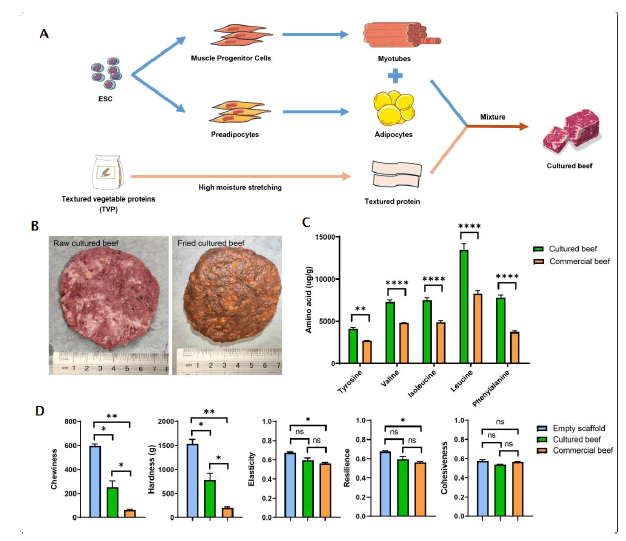
Figure 4: Manufacturing of cultured beef. Note: ns: No Significance; p-values are labelled and indicates p>0.05; *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001.
In Figure 4, A) A schematic illustration of the directional differentiation of bESCs to bovine adipocytes and myocytes and subsequently combined with plant-based edible scaffolds. The edible scaffolds were sourced from soy protein isolate and gluten from wheat, underwent a systematic blending and hydration procedure, followed by meticulous mechanical elongation. Simultaneously, cells underwent cultivation and expansion processes to augment the overall biomass. The resultant cultured cells were seamlessly incorporated into the scaffold, resulted in cultured beef. B) Comparison of raw (left) and fried (right) cultured beef. C) Comparison of Tyrosine, Valine, Isoleucine, Leucine and Phenylalanine contents of cultured and commercial beef. D) Texture profile analysis of empty scaffold, cultured beef and commercial beef.
Discussion
The production of cultured meat necessitates a reliable supply of muscle and fat cells. Currently used cell lines include primary, genetically modified and spontaneously immortalized cell lines, each facing significant challenges. Primary cells require frequent animal slaughter for tissue acquisition due to limited proliferative ability, contradicting cultured meat's ethical premise. Both genetically modified and spontaneously immortalized cell lines present food safety and regulatory hurdles and inadequate differentiation into muscle and fat cells. Spontaneously immortalized cell lines depend on selecting spontaneously mutated cells, a process feasible in lower species like fish and chicken but improbable in large mammals such as cattle [21]. Recently, induced Pluripotent Stem Cells (iPSCs) have emerged as a promising technology in regenerative medicine, although their reprogramming involves exogenous gene transfection, prompting safety concerns in food production. Advances in non-integrating reprogramming technologies such as mRNA show potential in positioning iPSCs as a vital source for cultured meat from livestock cells. Our work utilized non-genetically modified bESCs derived from fertilized and in vivo-developed blastocysts. We found these cells can be cultured in a serum-free and feeder-free culture system, demonstrating rapid and stable proliferation, as well as maintaining self-renewal and pluripotency across continuous passages. Cultured meat technology aims to leverage suspension culture methods for enhanced cell proliferation and differentiation. Our bESC lines sustain pluripotency in a microcarrier-free 3D suspension culture, highlighting the potential of augmenting suspension culture scale and density through biotechnological advancements. Research on human pluripotent stem cells demonstrate that modifications in culture apparatus, continuous perfusion techniques, or thermoreversible hydrogels significantly increase the cell density [22-24].
Fat and muscle cells are pivotal to the cultured meat, necessitating efficient differentiation from seed cells. Challenges in differentiating primary adult stem cells include prolonged differentiation cycles and diminished capacity with increased passage numbers [4,25]. Previous studies on differentiation of pluripotent stem cells focus on human and mouse models, adopting two primary strategies: One is a step-by-step induction of pluripotent stem cells into specific cell types via defined signaling pathways, plagued by lengthy cycles, complex steps and partial dependence on animal serum; the other one employs genetic modification to hasten differentiation, raising food safety and regulatory concerns [26-29]. Our work developed a rapid, serum-free and transgene-free protocol for differentiating bESCs into muscle and fat cells. We utilized a novel pathway inducing preadipocytes from the neuroectoderm, capable of in vitro proliferation and further rapid maturation using our self-developed adipogenesis medium. This method yielded mature adipocytes with a higher unsaturated fatty acid content compared to subcutaneous fat in cattle. Thus, our findings highlight the health advantages of unsaturated fats, particularly MUFAs and PUFAs, known for their antioxidant and anticarcinogenic properties, while SFAs are associated with cardiovascular risks [30]. We established a more straightforward and efficient myotube differentiation protocol. qPCR verified the myoglobin expression in myotubes, distinguishing them from those derived from adult muscle stem cells [20]. Myoglobin is significant for beef's color, taste and nutritional value [31]. Further work will focus on transitioning these differentiation techniques from 2D cultures to 3D suspension systems, a method already explored in human pluripotent stem cell research for differentiation into various cell types, including cardiomyocytes, hepatocytes, endothelial cells and MSC [32-35].
Plant-based proteins have received considerable attention in the food industry in respond to the increasing consumer preferences for ethical and eco-friendly alternatives to animal proteins [36]. Ideally, scaffolds derived from plant-based proteins should be cost-effective, thermally stable during cooking, non-toxic and non-allergenic, exemplified by soy, pea, chickpea, zein and wheat gluten. However, these individual plant-based proteins are often considered nutritionally incomplete, lacking some essential amino acids necessary for human consumption [37]. Consequently, a combination of proteins is necessary to achieve a balanced amino acid profile. To address the nutritional deficiencies, a blend of diverse plant-based proteins and incorporation of alternative proteins, such as cultured cells, in hybrid models, are being considered. Furthermore, hybrid meats, incorporating cultured cells, exhibit lower content of saturated fat and cholesterol along with fewer calories while maintaining protein richness compared to commercial meats. Various cell/scaffolding systems have been explored for hybrid meat production [38-40]. For example, the utilization of textured soy protein, an edible plant-based protein, in the cultivation of bovine satellite cells has demonstrated potential for high-quality cultured meat production [39]. However, these methods present challenges due to their complexity, cost, time consumption and stringent scaffold requirements, restricting the scalability of cultured meat. In contrast, our approach involving the separate culturing of fat and muscle cells without scaffold involvement in the culture media, offering advantages in taste, sensory experience, texture, health, convenience and scalability compared to methods involving scaffolds during culturing.
Cultured beef production requires a consistent cell line supply, with bESCs emerging as a promising source due to their limitless replicative capacity. The persistent pluripotency and genetic stability of our bESC lines form a major foundation for the consistent replication of cultured meat production. This not only ensures reproducibility but also eliminates the need for repeated animal biopsies, reducing costs and enhancing food safety. Additionally, the easy transportability of bESC vials minimizes logistical challenges and lowers the carbon footprint, marking a substantial step toward bolstering the sustainability of our cultured meat production process. Notably, cultured beef demonstrates cost competitiveness against commercial beef by addressing primary cost drivers, such as growth factors, basal media and recombinant proteins [41-43]. For instance, Specht suggested aligning growth factor production with established enzyme production practices for optimization [44]. Integrating hybrid meat, combining plant-based ingredients with cultured cells, offers a near-term opportunity for cost reduction and a more authentic meat-eating experience. Moreover, technological advancements, including specialized cultivators, automated meat production and improved growth factor production methods, show promise in substantially decreasing the cost of cultured meat [45]. The utilization of perfusion bioreactors is notable for generating high-density cultures, facilitating continuous cell growth. These insights underscore the economic significance of addressing cost drivers and optimizing production processes in cultured beef.
Conclusion
In this study, we established a stable and continuously passaged bovine Embryonic Stem Cell (bESC) line, developed a serum-free expansion medium suitable for this cell line and demonstrated the feasibility of suspending expansion cultures of this cell line within this system. Furthermore, we developed an efficient method to differentiate this cell line into muscle and fat cells, using a differentiation system that does not rely on transgenes or serum- containing media. Finally, utilizing tissue engineering techniques, we combined muscle and fat cells derived from pluripotent stem cells with plant-based proteins to establish a method for producing hybrid cultured meat. Our goal is to create an efficient and robust production process for generating high-quality cultured meat products. Through comprehensive visual and sensory analyses, our results show a significant similarity between the cultured meat and commercial beef. We have demonstrated that cultured fat and muscle cells hold promise as viable alternatives to conventional meat sources. This work represents a pioneering initiative that may enhance the role of bESCs in cultured meat production.
Ethics and Guidelines Statement
All procedures in the manuscript were reviewed in advance by the Laboratory Animal Ethics Committee of Shanghai Academy of Agricultural Sciences and also met the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Authors Contributions
Xue Rui, Zehan Li, Jiehuan Xu, Xiaohui Zhang and Xiao Jin performed experiments. Jianjun Dai donated the blastocysts of cattle. Xue Rui, Zehan Li and Yang Liu drafted the manuscript. Yang Liu funded and supervised the entire work.
Competing Interests
This work was sponsored by C Future Biotechnology Co., Ltd. (C Future). Yang Liu is the CEO and shareholder in C Future. Xue Rui, Zehan Li, Xiaohui Zhang and Xiao Jin are employees of C Future. Jiehuan Xu and Jianjun Dai declare no competing interests.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We thank C Future for funding this work. Further, we thank Jiapei Wang, Xiaohong Chen and Mingqing Xu for technical support; Zhe Chen for concept discussion.
References
- Datar I, Betti M. Possibilities for an in vitro meat production system. Innov Food Sci Emerg Technol. 2010;11(1):13-22.
- Gaydhane MK, Mahanta U, Sharma CS, Khandelwal M, Ramakrishna S. Cultured meat: State of the art and future. Biomanuf Rev. 2018;3:1-10.
- Post MJ, Levenberg S, Kaplan DL, Genovese N, Fu J, Bryant CJ, et al. Scientific, sustainability and regulatory challenges of cultured meat. Nat Food. 2020;1(7):403-415.
- Ding S, Swennen GM, Messmer T, Gagliardi M, Molin DG, Li C, et al. Maintaining bovine satellite cells stemness through p38 pathway. Sci Rep. 2018;8(1):10808.
[Crossref] [Google Scholar] [PubMed]
- Dohmen RG, Hubalek S, Melke J, Messmer T, Cantoni F, Mei A, et al. Muscle-derived fibro-adipogenic progenitor cells for production of cultured bovine adipose tissue. NPJ Sci Food. 2022;6(1):6.
[Crossref] [Google Scholar] [PubMed]
- Stout AJ, Arnett MJ, Chai K, Guo T, Liao L, Mirliani AB, et al. Immortalized bovine satellite cells for cultured meat applications. ACS Synth Biol. 2023;12(5):1567-1573.
[Crossref] [Google Scholar] [PubMed]
- Pasitka L, Cohen M, Ehrlich A, Gildor B, Reuveni E, Ayyash M, et al. Spontaneous immortalization of chicken fibroblasts generates stable, high-yield cell lines for serum-free production of cultured meat. Nat Food. 2023;4(1):35-50.
[Crossref] [Google Scholar] [PubMed]
- Saad MK, Yuen JS, Joyce CM, Li X, Lim T, Wolfson TL, et al. Continuous fish muscle cell line with capacity for myogenic and adipogenic-like phenotypes. Sci Rep. 2023;13(1):5098. 2023.
[Crossref] [Google Scholar] [PubMed]
- Zhi M, Zhang J, Tang Q, Yu D, Gao S, Gao D, et al. Generation and characterization of stable pig pregastrulation epiblast stem cell lines. Cell Res. 2022;32(4):383-400.
[Crossref] [Google Scholar] [PubMed]
- Zhu G, Gao D, Li L, Yao Y, Wang Y, Zhi M, et al. Generation of three-dimensional meat-like tissue from stable pig epiblast stem cells. Nat Commun. 2023;14(1):8163.
[Crossref] [Google Scholar] [PubMed]
- Bogliotti YS, Wu J, Vilarino M, Okamura D, Soto DA, Zhong C, et al. Efficient derivation of stable primed pluripotent embryonic stem cells from bovine blastocysts. Proc Natl Acad Sci U S A. 2018;115(9):2090-2095.
[Crossref] [Google Scholar] [PubMed]
- Zhao L, Gao X, Zheng Y, Wang Z, Zhao G, Ren J, et al. Establishment of bovine expanded potential stem cells. Proc Natl Acad Sci U S A. 2021;118(15):e2018505118.
[Crossref] [Google Scholar] [PubMed]
- Kinoshita M, Kobayashi T, Planells B, Klisch D, Spindlow D, Masaki H, et al. Pluripotent stem cells related to embryonic disc exhibit common self-renewal requirements in diverse livestock species. Development. 2021;148(23):dev199901.
[Crossref] [Google Scholar] [PubMed]
- Martins B, Bister A, Dohmen RG, Gouveia MA, Hueber R, Melzener L, et al. Advances and challenges in cell biology for cultured meat. Annu Rev Anim Biosci. 2024;12(1):345-368.
[Crossref] [Google Scholar] [PubMed]
- Jara TC, Park K, Vahmani P, van Eenennaam AL, Smith LR, Denicol AC. Stem cell-based strategies and challenges for production of cultivated meat. Nat Food. 2023;4(10):841-853.
[Crossref] [Google Scholar] [PubMed]
- Soto DA, Navarro M, Zheng C, Halstead MM, Zhou C, Guiltinan C, et al. Simplification of culture conditions and feeder-free expansion of bovine embryonic stem cells. Sci Rep. 2021;11(1):11045.
[Crossref] [Google Scholar] [PubMed]
- Jin W, He Y, Li T, Long F, Qin X, Yuan Y, et al. Rapid and robust derivation of mesenchymal stem cells from human pluripotent stem cells via temporal induction of neuralized ectoderm. Cell Biosci. 2022;12(1):31.
[Crossref] [Google Scholar] [PubMed]
- Pfützner A, Schipper D, Pansky A, Kleinfeld C, Roitzheim B, Tobiasch E. Mesenchymal stem cell differentiation into adipocytes is equally induced by insulin and proinsulin in vitro. Int J Stem Cells. 2017;10(2):154-159.
[Crossref] [Google Scholar] [PubMed]
- Hwang YH, Joo ST. Fatty acid profiles, meat quality, and sensory palatability of grain-fed and grass-fed beef from Hanwoo, American, and Australian crossbred cattle. Korean J Food Sci Anim Resour. 2017;37(2):153-161.
[Crossref] [Google Scholar] [PubMed]
- Simsa R, Yuen J, Stout A, Rubio N, Fogelstrand P, Kaplan DL. Extracellular heme proteins influence bovine myosatellite cell proliferation and the color of cell-based meat. Foods. 2019;8(10):521.
[Crossref] [Google Scholar] [PubMed]
- Soice E, Johnston J. Immortalizing cells for human consumption. Int J Mol Sci. 2021;22(21):11660.
[Crossref] [Google Scholar] [PubMed]
- Torizal FG, Lau QY, Ibuki M, Kawai Y, Horikawa M, Minami M, et al. A miniature dialysis-culture device allows high-density human-induced pluripotent stem cells expansion from growth factor accumulation. Commun Biol. 2021;4(1):1316.
[Crossref] [Google Scholar] [PubMed]
- Manstein F, Ullmann K, Kropp C, Halloin C, Triebert W, Franke A, et al. High density bioprocessing of human pluripotent stem cells by metabolic control and in silico modeling. Stem Cells Transl Med. 2021;10(7):1063-1080.
[Crossref] [Google Scholar] [PubMed]
- Lei Y, Schaffer DV. A fully defined and scalable 3D culture system for human pluripotent stem cell expansion and differentiation. Proc Natl Acad Sci U S A. 2013;110(52):E5039-E5048.
[Crossref] [Google Scholar] [PubMed]
- Yuen Jr JS, Saad MK, Xiang N, Barrick BM, DiCindio H, Li C, et al. Aggregating in vitro-grown adipocytes to produce macroscale cell-cultured fat tissue with tunable lipid compositions for food applications. Elife. 2023;12:e82120.
[Crossref] [Google Scholar] [PubMed]
- Chal J, Al Tanoury Z, Hestin M, Gobert B, Aivio S, Hick A, et al. Generation of human muscle fibers and satellite-like cells from human pluripotent stem cells in vitro. Nat Protoc. 2016;11(10):1833-1850.
[Crossref] [Google Scholar] [PubMed]
- Rao J, Djeffal Y, Chal J, Marchianò F, Wang CH, Al Tanoury Z, et al. Reconstructing human brown fat developmental trajectory in vitro. Dev Cell. 2023;58(21):2359-2375.
[Crossref] [Google Scholar] [PubMed]
- Pawlowski M, Ortmann D, Bertero A, Tavares JM, Pedersen RA, Vallier L, et al. Inducible and deterministic forward programming of human pluripotent stem cells into neurons, skeletal myocytes, and oligodendrocytes. Stem Cell Reports. 2017;8(4):803-812.
[Crossref] [Google Scholar] [PubMed]
- Ahfeldt T, Schinzel RT, Lee YK, Hendrickson D, Kaplan A, Lum DH, et al. Programming human pluripotent stem cells into white and brown adipocytes. Nat Cell Biol. 2012;14(2):209-219.
[Crossref] [Google Scholar] [PubMed]
- Webb EC. Cis/Trans-fatty acid content of red meats and the related effects on meat quality and human health. In Meat and Nutrition. 2021.
- Juárez M, Lam S, Bohrer BM, Dugan ME, Vahmani P, Aalhus J, et al. Enhancing the nutritional value of red meat through genetic and feeding strategies. Foods. 2021;10(4):872.
[Crossref] [Google Scholar] [PubMed]
- Kahn-Krell A, Pretorius D, Ou J, Fast VG, Litovsky S, Berry J, et al. Bioreactor suspension culture: Differentiation and production of cardiomyocyte spheroids from human induced pluripotent stem cells. Front Bioeng Biotechnol. 2021;9:674260.
[Crossref] [Google Scholar] [PubMed]
- Torizal FG, Utami T, Lau QY, Inamura K, Nishikawa M, Sakai Y. Dialysis based-culture medium conditioning improved the generation of human induced pluripotent stem cell derived-liver organoid in a high cell density. Sci Rep. 2022;12(1):20774.
[Crossref] [Google Scholar] [PubMed]
- Lin H, Du Q, Li Q, Wang O, Wang Z, Sahu N, et al. A scalable and efficient bioprocess for manufacturing human pluripotent stem cell-derived endothelial cells. Stem Cell Reports. 2018;11(2):454-469.
[Crossref] [Google Scholar] [PubMed]
- Yan L, Jiang B, Li E, Wang X, Ling Q, Zheng D, et al. Scalable generation of mesenchymal stem cells from human embryonic stem cells in 3D. Int J Biol Sci. 2018;14(10):1196-1210.
[Crossref] [Google Scholar] [PubMed]
- Sexton AE, Garnett T, Lorimer J. Framing the future of food: The contested promises of alternative proteins. Environ Plan E Nat Space. 2019;2(1):47-72.
[Crossref] [Google Scholar] [PubMed]
- Kumar M, Tomar M, Potkule J, Punia S, Dhakane-Lad J, Singh S, et al. Functional characterization of plant-based protein to determine its quality for food applications. Food Hydrocoll. 2022;123:106986.
- Yao Y, Yuen Jr JS, Sylvia R, Fennelly C, Cera L, Zhang KL, et al. Cultivated meat from aligned muscle layers and adipose layers formed from glutenin films. ACS Biomater Sci Eng. 2024;10(2):814-824.
[Crossref] [Google Scholar] [PubMed]
- Ben-Arye T, Shandalov Y, Ben-Shaul S, Landau S, Zagury Y, Ianovici I, et al. Textured soy protein scaffolds enable the generation of three-dimensional bovine skeletal muscle tissue for cell-based meat. Nat Food. 2020;1(4):210-220.
- Xiang N, Yao Y, Yuen Jr JS, Stout AJ, Fennelly C, Sylvia R, et al. Edible films for cultivated meat production. Biomaterials. 2022;287:121659.
[Crossref] [Google Scholar] [PubMed]
- Stout AJ, Mirliani AB, Rittenberg ML, Shub M, White EC, Yuen Jr JS, et al. Simple and effective serum-free medium for sustained expansion of bovine satellite cells for cell cultured meat. Commun Biol. 2022;5(1):466.
[Crossref] [Google Scholar] [PubMed]
- Kanayama T, Tanaka K, Shimada H, Ichiyama K, Higuchi S, Kawashima I. Improving the safety of cultured meat using basal medium prepared using food ingredients. bioRxiv. 2022;2022(3):486069.
- Lei Q, Ma J, Du G, Zhou J, Guan X. Efficient expression of a cytokine combination in Saccharomyces cerevisiae for cultured meat production. Food Res Int. 2023;170:113017.
[Crossref] [Google Scholar] [PubMed]
- Specht L. An analysis of culture medium costs and production volumes for cultivated meat. The Good Food Institute: Washington, DC, USA. 2020.
- Yang M, Wang Q, Zhu Y, Sheng K, Xiang N, Zhang X. Cell culture medium cycling in cultured meat: Key factors and potential strategies. Trends Food Sci Tech. 2023;138:564-576.
Citation: Rui X, Li Z, Xu J, Dai J, Zhang X, Jin X, et al. (2024). Generation of Cultured Beef from Bovine Embryonic Stem Cells. J Clin Res Bioeth. 15:504.
Copyright: © 2024 Rui X, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.

