Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- CiteFactor
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2021) Volume 12, Issue 10
Gene Transformation of Alfalfa Plants (Medicago varia L.) Domestic Cultivar ‘Burgaltai’ for Increasing Leaf Size
Uuganzaya Myagmarjav1*, Enkhchimeg Vanjildorj2 and Altantsetseg Khajidsuren22School of Animal Science and Biotechnology, Mongolian University of Life Sciences, Mongolia
Received: 04-Aug-2021 Published: 28-Oct-2021, DOI: 10.35248/2157-7471.21.12.578
Abstract
‘Burgaltai’ cultivar of alfalfa is the most important forage in Mongolia and its yield had decreasing last decades due to cross pollination. This study was conducted for increasing leaf size of ‘Burgaltai’ cultivar through Agrobacterium mediated genetic transformation transferring growth regulating factor AtGRF2 gene of Arabidopsis thaliana. In vitro regeneration was derived from seven day old hypocotyl and cotyledon explants for callus induction. Induced calli were used for shoot induction and proliferation. Proliferated shoots carried to root induction medium. The best calli induction medium was MSB5 (Murashige Skoog’s medium with Gamborg’s vitamin) medium supplemented with 2 mg/L 2,4-D, 0.2 mg/L BAP and 1 mg/L NAA, shoot induction and proliferation media from calli were MSB5 medium supplemented with 1mg/L BAP, 0.1 mg/L NAA, proliferated shoots were rooted on half strength MSB5 medium supplemented with 0.5 mg/L NAA. For the genetic transformation, hypocotyl explants used as an initial source. Totally 235 explants were tested to transformation and 30 explants (12.7%) were resistant to hygromycin antibiotics and regenerate plantlets. Plantlets were transferred to the soil and fresh young leaf used for GUS histochemical assay and RT-PCR for confirmation. Among them fully regenerated seven independent transgenic plantlets (T6, 8, 11, 12, 13, 15, 22) were confirmed. Transgenic ‘Burgaltai’ cv. of alfalfa leaf size was increased by 0.7 cm2 comparing to the wild type. T6 and 11 lines showed 3:1 segregation ratio in T1 progenies (X2=0.04-0.42, p ≤ 0.52-0.84).
Keywords
Medicago; Transformation; AtGRF2 gene; ‘Burgaltai’ cultivar; Alfalfa; Lucerne
Introduction
Alfalfa is the most important forage of its palatable and nutritional values in Mongolia. ‘Burgaltai’ cultivar of alfalfa is cultivated for hay production in the country. It had obtained by hybridization of Medicago sativa and Medicago falcata in the forest-steppe zone of Mongolia for increasing biomass and had approved as a cultivar on the feature of its high yield. It have been reported that alfalfa puts about 50-100 kg/ha biological fixed nitrogen into the soil and increasing the soil fertility. Moreover it’s suitable for cultivated pastures, hay production and improvement of vegetation [1]. But last decades there is lack of characteristic have been appeared in ‘Burgaltai’ cultivar, which is losing its biomass and low percentage of leaves in the yield (up to 45%) due to cross pollination. Consequently, nutritional value had decreased dramatically in development cycles. One way for improving fodder nutrition is using recombinant DNA technology with the objective of introducing or enhancing a desirable characteristic in the plants and seeds [2]. For example, the postruminal supply of sulfur-containing amino acids, methionine and cysteine, has been reported to be a major limitation to wool growth and Australian commercial alfalfa cultivar had improved its protein quality by introducing chimeric genes encoding a ruminally stable, sulfur amino acid-rich protein through Agrobacterium [3]. Growth-Regulating Factor genes (GRFs) encode plant-specific transcription factors, which play a critical role in regulation of plant growth and development [4-7]. Korean scientists had identified Growth Regulator Factors (GRF) gene family in 2003 form Arabidopsis thaliana which comprises nine members (AtGRF1-AtGRF9). Over-expression of AtGRF1 and AtGRF2 resulted in larger leaves and cotyledons by increasing cell expansion comparing to wild type [8]. In this study, we aim to increase ‘Burgaltai’ cultivar of alfalfa leaf using AtGRF2 gene through Agrobacterium-mediated genetic transformation.
Materials and Methods
Materials
The seeds of “Burgaltai” cultivar of Medicago varia Marthz was kindly provided Dr. Batsukh Sharav (Research Institute of Animal Husbandry, Mongolia). The binary plasmid pCAMBIA1300 contains GUS gene to confirm detection, and hygromycin resistance gene (hpt) for selection and interesting AtGRF2 (Arabidopsis thaliana Growth Regulating Factor 2) gene leaf increasing. All of expression cassettes were under the control of the cauliflower mosaic virus 35S promotor and the NOS3 terminator and introduced into Agrobacterium tumefaciens strain EHA105 via electroporation. Bacterial strain with the plasmid was kindly provided by Dr. Kim J.H (Cheju Univ, Korea)
Methods for in vitro regeneration
Sterilized seeds were germinated on MSB5 (Murashige and Skoog, 1962 with Gamborg B5 vitamin) medium for a week. Seven day-old hypocotyls were used for the source of initial explants and cultured on solid MSB5 and different combination and concentration of plant growth regulators, including BAP (0, 0.5, 1.0 and 2.0 mg/L) or kinetin (0, 0.5, 1.0 and 2.0 mg/L) in the presence or absence of NAA (0 and 0.5 mg/L) for shoot organogenesis. Elongated shoots were excised and transferred half-strength MSB5 medium supplemented with different concentration of IAA (0.5 and 1.0 mg/L) NAA (0.5 and 1.0 mg/L) and IBA (0.5 and 1.0 mg/L). The plant growth regulators were filter sterilized (0.22 μmol/L, Milipore, USA) and added to cooled autoclaved basal medium.
Methods for genetic transformation
Seven day-old hypocotyl explants were infected with Agrobacterium suspension (OD600 was 1.0 in log phase and adjusted 0.5-0.6) for 30 min, and co-cultivated 72 hrs under the dark at 28°C. Then explants were washed double distilled water with 500 mg/L cefotaxime and air dried and cultivated on solid MSB5 selection medium supplemented with 1 mg/L BAP, 0.1 mg/L NAA, 25 mg/L hygromycin, 250 mg/L cefotaxime for shoot induction. All cultures described above were maintained at 28°C under a 16/8 photoperiod. The antibiotics were sterilized (0.45 μmol/L, Milipore, USA) and added cooled autoclaved medium.
Analysis of transgene expression
Methods for GUS assay: The sample, transient plant tissue/leaves were placed in a 1.5 ml plastic tube. Added X-gluc solution to the tube to float the sample and placed the tube in a 37°C growth chamber for overnight. Preparing X-Gluc solution: 1 mM X-Gluc (5-bromo- 4-chloro-3-indolyl) β-D-glucuronic acid in 50 mM Na2HPO4, pH 7, and 0.1% Triton X-100 [9]. The screening of antibiotic resistant plantlets tested on β-glucuronidase (GUS) histochemical assay first and transgenic alfalfa had confirmed by RT-PCR. RNA for RTPCR was extracted according to EasyScript RNA kit instruction from fresh leaves of transgenic alfalfa. The coding region-AtGRF2 gene was amplified using the sets of primers: AtGRF2 gene (upper) 5′-GGGAATTCCCTGTGGAAGTCCAATCT-3′, and (lower) 5′-GGGAGATCTCAGTTTTCAGGTTGTGTAATGAAA-3′. Total volume of RT-PCR was 20 ml, including 7.5 ml RNAse free water, 10 ml reaction mixture, 0.5 ml enzyme mixture, 0.5 ml each primer and 1 ml RNA. Cycling parameters began with an initial hot start at 94°C for 2 min, then 35 cycles of denaturation 94°C for 1 min, annealing at 57°C for 1 min and extension at 72°C for 1 min, followed by a final extension of 3 min at 72°C. PCR amplification products were analysed by electrophoresis in 1.0% agarose gel and stained with Ethidium Bromide (EtBr) and visualized with UV.
Measurement of leaves
The leaf area was calculated by its width and length measurements at 2-4 apical leaves of wild and transgenic plants.
Self-pollinated seeds (T1) of transgenic plants were placed on segregation medium comprising MSB5 with 50 mg/l hygromycin. The treatment was carried out in triplicate (10 seeds per dish) and was repeated three times in each transgenic line. After 2 weeks, hygromycin-resistant plants developing true leaves were counted, and data were analysed by the X2 test at p ≤ 0.05.
Statistical analysis
The experiment was performed with three replicates with 18-20 explants in each treatment. Analysis of variance (ANOVA) were evaluated using SAS software package (version 9.2, SAS Institute Inc, Cary, NC) and Duncan’s Multiple Range Test were performed to analyse the means for significant differences at p≤0.05.
Results and Discussion
In vitro regeneration
Two weeks old fresh calli, derived from hypocotyls explants were chosen for shoot organogenesis. Our experiment result suggested that the MSB5 medium supplemented with BAP in the presence of NAA might have assisting the shoot production from calli (84.4%), but also showed that there is no significant difference when compared to the MS medium containing the same concentration of BAP in the absence of NAA (82.2%). Elongated/proliferated shoots were transferred to half-strength MSB5 medium with auxins including NAA, IBA, and IAA to promote rooting. Elongated shoots were rooted in the range of 90-96.7% and the highest frequency of root induction (96.7) with the maximum number of roots (7.2) obtained on half-strength MSB5 medium supplemented with 0.5 mg/l NAA. The best callus induction medium from both of hypocotyl and cotyledon explants was MSB5 medium supplemented with 2 mg/L 2,4-D, 0.2 mg/L BAP and 1 mg/L NAA, shoot induction and proliferation medium from calli were MSB5 medium supplemented with 1 mg/L BAP, 0.1 mg/L NAA, proliferated shoots were rooted on half strength MSB5 medium supplemented with 0.5 mg/L NAA (Figure 1). Calli were derived from explant in 2 weeks, shoots were induced in 5-6 weeks, and roots were induced in 2-3 weeks. According to our result, the regeneration frequency was 72.2%, from initial explants to entire plants generate period were 7-11 weeks. Comparing with the alfalfa cultivars our result taken shorter period to regenerate entire plant but lower regeneration rate [10].
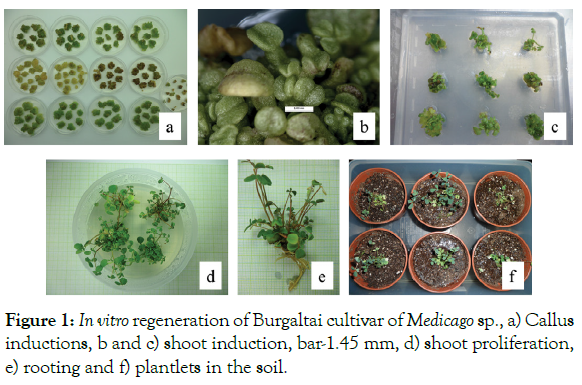
Figure 1: In vitro regeneration of Burgaltai cultivar of Medicago sp., a) Callus inductions, b and c) shoot induction, bar-1.45 mm, d) shoot proliferation, e) rooting and f) plantlets in the soil.
Result of genetic transformation
Initial explants of ‘Burgaltai’ cultivar of alfalfa was used for Agrobacterium-mediated genetic transformation. Totally 235 explants were tested to transformation and 30 explants (12.7%) were resistant to hygromycin antibiotics and regenerate plantlets (Table 1) within three months. Plantlets were transferred to the soil and fresh young leaf used for GUS-histochemical assay. Rooted small seven independent plantlets (T6, 8, 11, 12, 13, 15, 22) were confirmed by RT-PCR (Figures 2 and 3) analysis for transcription of AtGRF2 in transgenic. Fully regenerated seven plantlets were transferred to soil and morphologically indistinguishable from seed-grown plants and flowered.
| Agrobacterium Strain | Total Explant |
Hygromycin (hpt) resistant Plantlets |
Transgenic ‘Burgaltai’ | ||
|---|---|---|---|---|---|
| Number | % | Number | % | ||
| EHA105 | 235 | 30 | 12.7 | 7 | 3.0 |
Table 1: Transformation efficiency of ‘Burgaltai’ cultivar.
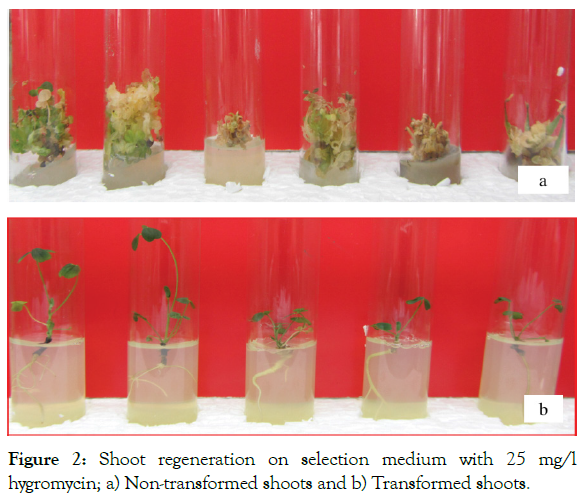
Figure 2: Shoot regeneration on selection medium with 25 mg/l hygromycin; a) Non-transformed shoots and b) Transformed shoots.
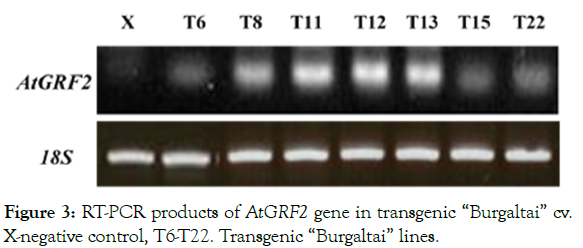
Figure 3: RT-PCR products of AtGRF2 gene in transgenic “Burgaltai” cv. X-negative control, T6-T22. Transgenic “Burgaltai” lines.
The highest transformation rate in alfalfa plants 7% in Medicago sativa [11] using vortex-mediated seedling transformation method. In our result, the transformation rate was 3% based on the in vitro regeneration system. In heterologous system, BnGRF2a gene were transferred to Arabidopsis thalina. Transgenic Arabidopsis showed that overexpressed BnGRF2a gene resulted more than 20% enlargement of leaves comparing to wild type. In our result, the leaf of transgenic ‘Burgaltai’ had increased 0.7 cm2 areas comparing with wild plant. The seed of wild ‘Burgaltai’ cultivar germination was totally restricted on MSB5 medium supplemented with 50 mg/L hygromycin antibiotic. Transgenic two lines (T6 and T11) were tested to segregation on basal MSB5 medium containing 50 mg/L. Those lines showed 3:1 segregation ratio in T1 progenies (Table 2).
| Transgenic Lines | Hygromycin Tolerant (R) | Hygromycin Intolerant (S) | Ratio (R:S) |
Hypothesis | X2 value | p value |
|---|---|---|---|---|---|---|
| T6 | 9.7 ± 0.6 | 2 ± 0.8 | 4.9:1 | 3:1 | 0.42 | 0.52 |
| T11 | 8.7 ± 1.0 | 3.3 ± 0.9 | 2.6:1 | 3:1 | 0.04 | 0.84 |
Table 2: Ratio of segregation of AtGRF2 gene in ‘Burgaltai’ cv. of alfalfa.
Conclusion
We had carried out this experiment at the first time in our laboratory. Plant regeneration and genetic transformation has been extensively practiced in alfalfa an effort to improve the economic value of this species. In this study, an efficient regeneration and transformation protocol for Medicago varia L. was established. Using this method, the development of environmental stress-resistant transgenic Medicago varia L with the desired genes via Agrobacterium-mediated transformation with hypocotyls explants are in progress.
Acknowledgements
This study is supported by Asian Development Bank and Ministry of Education of Mongolia (L2766-MON). We thank to Professors team of Agricultural Biotechnology at Mongolian University of Life Sciences.
Conflict of Interest
The authors declare no conflict of interest.
REFERENCES
- Jigjidsuren S, Johnson AD. Forage plants in Mongolia. Admon press, Ulaanbaatar, Mongolia. 2003.
- Bimrew A. Biotechnological advances for animal nutrition and feed improvement. World J Agric Res. 2014;2(3):115-118.
- Tabe LM, Wardly-Richardson M, Ceriotti A, Aryan A, McNabb W, Moore A, et al. A biotechnological approach to improving the nutritive value of alfalfa. J Anim Sci. 1995;73(9):2752-2758.
- Kim JH, Tsukaya H. Regulation of plant growth and development by the growth-regulating factor and GRF-interacting factor duo. J Exp Bot. 2015;66:6093-6107.
- Liu J, Hua W, Yang H L, Zhan GM, Li RJ, Wnag XF, et al. The BnGRF2 gene (GRF2-like gene from Brassica napus) enhances seed oil production through regulating cell number and plant photosynthesis. J Exp Bot. 2012;63(10):3727-3740
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473-497.
- Omidbakhshfard MA, Proost S, Fujikura U, Mueller-Roeber B. Growth-Regulating Factors (GRFs): A small transcription factor family with important functions in plant biology. Mol Plant. 2015;8:998-1010.
- Susan JK. Blue plants: Transgenic plants with the GUS reporter gene. Processing of the 23rd workshop/conference of the Association for biology laboratory Education. 2002;29-42.
- Kim J H, Choi D, Kende H. The AtGRF family putative transcription factors are involved in leaf and cotyledon growth in Arabidopsis. Plant J. 2003;36:94-104.
- Zhang H, Huang Q, Su J. Development of alfalfa (Medicago sativa L) regeneration system and Agrobacterium-Mediated genetic transformation. Agric Sci China. 2010;9(2):170-178.
- Troy JW, Jingsong Y, Caius MR. Development of an in planta for transformation of alfalfa (Medicago sativa). Transgenic Res. 2008;17:587-597.
Citation: Myagmarjav U, Vanjildorj E, Khajidsuren A (2021) Gene Transformation of Alfalfa Plants (Medicago varia L.) Domestic Cultivar ‘Burgaltai’ for Increasing Leaf Size. J Plant Pathol Microbiol 12:578.
Copyright: © 2021 Myagmarjav U, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

